The Effect of Exercise Training on Myocardial and Skeletal Muscle Metabolism by MR Spectroscopy in Rats with Heart Failure
Abstract
:1. Introduction
2. Results
2.1. Metabolic Profiles of Myocardial Tissue
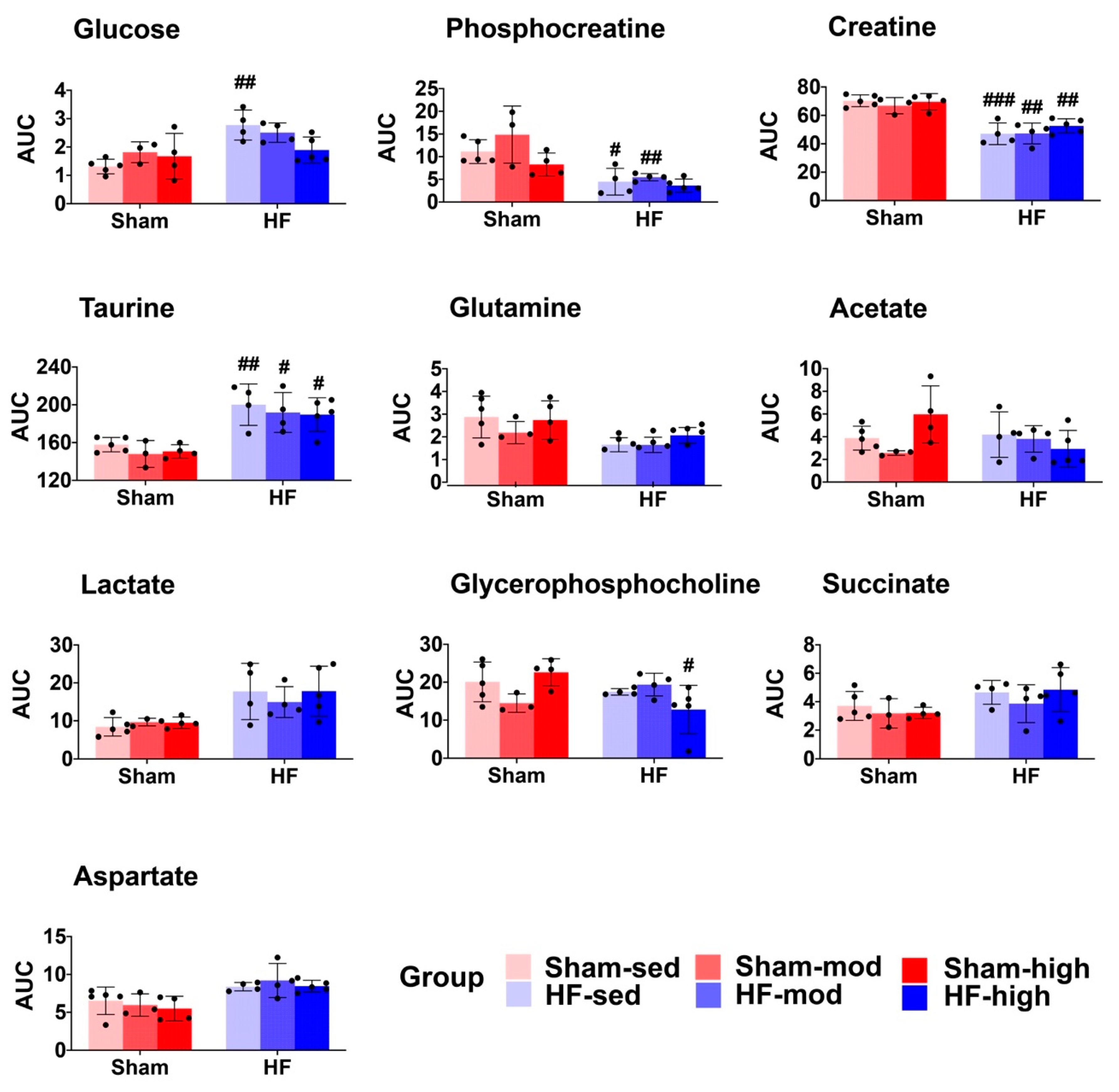
2.2. Metabolic Profiles of Skeletal Muscle
3. Discussion
3.1. Metabolic Changes in Myocardial Tissue
3.1.1. Creatine Metabolism
3.1.2. Aspartate Metabolism
3.1.3. Glutamine Metabolism
3.1.4. Glucose Metabolism
3.2. Metabolic Changes in Skeletal Muscle
3.2.1. Taurine Metabolism
3.2.2. Lysine Metabolism
3.2.3. Carnitine Metabolism
3.3. Limitations
4. Materials and Methods
4.1. Rat Heart Failure Model
4.2. Exercise Training and VO2max Testing
4.3. Tissue Extraction
4.4. Proton Magnetic Resonance Spectroscopy (MRS)
4.5. Spectral Processing and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, H.D.; Chew, D.P. Acute myocardial infarction. Lancet 2008, 372, 570–584. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Pfeffer, M.A. Heart failure. Lancet 2005, 365, 1877–1889. [Google Scholar] [CrossRef]
- James, S.; Barton, D.; O’Connell, E.; Voon, V.; Murtagh, G.; Watson, C.; Murphy, T.; Prendiville, B.; Brennan, D.; Hensey, M.; et al. Life expectancy for community-based patients with heart failure from time of diagnosis. Int. J. Cardiol. 2015, 178, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.A.; Shaw, A.O.; Miller, M.S.; VanBuren, P.; LeWinter, M.M.; Ades, P.A.; Toth, M.J. Effect of resistance training on physical disability in chronic heart failure. Med. Sci. Sports Exerc. 2011, 43, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Milner, K.; Gupta, A.; Lopaschuk, G.D. Myocardial Energy Substrate Metabolism in Heart Failure: From Pathways to Therapeutic Targets. Curr. Pharm. Des. 2015, 21, 3654–3664. [Google Scholar] [CrossRef]
- Sankaralingam, S.; Lopaschuk, G.D. Cardiac energy metabolic alterations in pressure overload-induced left and right heart failure (2013 Grover Conference Series). Pulm. Circ. 2015, 5, 15–28. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Myers, J.; Gullestad, L.; Vagelos, R.; Do, D.; Bellin, D.; Ross, H.; Fowler, M.B. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann. Intern. Med. 1998, 129, 286–293. [Google Scholar] [CrossRef]
- Bowen, T.S.; Rolim, N.P.; Fischer, T.; Baekkerud, F.H.; Medeiros, A.; Werner, S.; Bronstad, E.; Rognmo, O.; Mangner, N.; Linke, A.; et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur. J. Heart Fail. 2015, 17, 263–272. [Google Scholar] [CrossRef]
- Toth, M.J.; Ward, K.; van der Velden, J.; Miller, M.S.; Vanburen, P.; Lewinter, M.M.; Ades, P.A. Chronic heart failure reduces Akt phosphorylation in human skeletal muscle: relationship to muscle size and function. J. Appl. Physiol. (Bethesda, Md. 1985) 2011, 110, 892–900. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Nicklas, B.; Kraus, W.E.; Lyles, M.F.; Eggebeen, J.; Morgan, T.M.; Haykowsky, M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1364–H1370. [Google Scholar] [CrossRef] [Green Version]
- Schaufelberger, M.; Eriksson, B.O.; Grimby, G.; Held, P.; Swedberg, K. Skeletal muscle fiber composition and capillarization in patients with chronic heart failure: relation to exercise capacity and central hemodynamics. J. Card. Fail. 1995, 1, 267–272. [Google Scholar] [CrossRef]
- Rosca, M.G.; Hoppel, C.L. Mitochondrial dysfunction in heart failure. Heart Fail. Rev. 2013, 18, 607–622. [Google Scholar] [CrossRef]
- Bouchard, C.; Daw, E.W.; Rice, T.; Perusse, L.; Gagnon, J.; Province, M.A.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H. Familial resemblance for VO2max in the sedentary state: The HERITAGE family study. Med. Sci. Sports Exerc. 1998, 30, 252–258. [Google Scholar] [CrossRef]
- Frontera, W.R.; Hughes, V.A.; Lutz, K.J.; Evans, W.J. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J. Appl. Physiol. (Bethesda, Md. 1985) 1991, 71, 644–650. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; van Essen, M.; Wilkin, G.P.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef]
- Hagberg, J.M.; Graves, J.E.; Limacher, M.; Woods, D.R.; Leggett, S.H.; Cononie, C.; Gruber, J.J.; Pollock, M.L. Cardiovascular responses of 70- to 79-yr-old men and women to exercise training. J. Appl. Physiol. (Bethesda, Md. 1985) 1989, 66, 2589–2594. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Brubaker, P.H.; Morgan, T.M.; Stewart, K.P.; Little, W.C. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ. Heart Fail. 2010, 3, 659–667. [Google Scholar] [CrossRef]
- Prasad, A.; Popovic, Z.B.; Arbab-Zadeh, A.; Fu, Q.; Palmer, D.; Dijk, E.; Greenberg, N.L.; Garcia, M.J.; Thomas, J.D.; Levine, B.D. The effects of aging and physical activity on Doppler measures of diastolic function. Am. J. Cardiol. 2007, 99, 1629–1636. [Google Scholar] [CrossRef]
- Hirai, D.M.; Musch, T.I.; Poole, D.C. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1419–H1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santulli, G.; Ciccarelli, M.; Trimarco, B.; Iaccarino, G. Physical activity ameliorates cardiovascular health in elderly subjects: the functional role of the beta adrenergic system. Front. Physiol. 2013, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Libonati, J.R.; MacDonnell, S.M. Cardiac beta-adrenergic responsiveness with exercise. Eur. J. Appl. Physiol. 2011, 111, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Nascimben, L.; Friedrich, J.; Gwathmey, J.K.; Ingwall, J.S. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ. Res. 1996, 78, 893–902. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.M.; Yoshiyama, M.; Francis, G.S.; Ugurbil, K.; Cohn, J.N.; Zhang, J. Myocardial bioenergetic abnormalities in a canine model of left ventricular dysfunction. J. Am. Coll. Cardiol. 1994, 23, 786–793. [Google Scholar] [CrossRef]
- Neubauer, S.; Krahe, T.; Schindler, R.; Horn, M.; Hillenbrand, H.; Entzeroth, C.; Mader, H.; Kromer, E.P.; Riegger, G.A.; Lackner, K.; et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 1992, 86, 1810–1818. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Zubiri, I.; Maroto, A.S.; Gonzalez-Calero, L.; Posada-Ayala, M.; de la Cuesta, F.; Mourino-Alvarez, L.; Lopez-Almodovar, L.F.; Calvo-Bonacho, E.; Ruilope, L.M.; et al. KLK1 and ZG16B proteins and arginine–proline metabolism identified as novel targets to monitor atherosclerosis, acute coronary syndrome and recovery. Metabolomics 2015, 11, 2. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Alvarez, A.M.; Mukherjee, D. Liver Abnormalities in Cardiac Diseases and Heart Failure. Int. J. Angiol. Off. Publ. Int. Coll. Angiol. Inc 2011, 20, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Zhang, J.; Wen, J.; Ming, Q.; Zhang, J.; Xu, Y. Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int. J. Clin. Exp. Med. 2015, 8, 4399–4404. [Google Scholar]
- Weng, S.F.; Kai, J.; Guha, I.N.; Qureshi, N. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart 2015, 2, e000272. [Google Scholar] [CrossRef]
- Shahzad, K.; Chokshi, A.; Schulze, P.C. Supplementation of glutamine and omega-3 polyunsaturated fatty acids as a novel therapeutic intervention targeting metabolic dysfunction and exercise intolerance in patients with heart failure. Curr. Clin. Pharmacol. 2011, 6, 288–294. [Google Scholar] [CrossRef]
- Wu, C.; Kato, T.S.; Ji, R.; Zizola, C.; Brunjes, D.L.; Deng, Y.; Akashi, H.; Armstrong, H.F.; Kennel, P.J.; Thomas, T.; et al. Supplementation of l-Alanyl-l-Glutamine and Fish Oil Improves Body Composition and Quality of Life in Patients With Chronic Heart Failure. Circ. Heart Fail. 2015, 8, 1077–1087. [Google Scholar] [CrossRef]
- Khogali, S.E.; Pringle, S.D.; Weryk, B.V.; Rennie, M.J. Is glutamine beneficial in ischemic heart disease? Nutrition (Burbank, Los Angeles County, Calif.) 2002, 18, 123–126. [Google Scholar] [CrossRef]
- Davila-Roman, V.G.; Vedala, G.; Herrero, P.; de las Fuentes, L.; Rogers, J.G.; Kelly, D.P.; Gropler, R.J. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Neglia, D.; De Caterina, A.; Marraccini, P.; Natali, A.; Ciardetti, M.; Vecoli, C.; Gastaldelli, A.; Ciociaro, D.; Pellegrini, P.; Testa, R.; et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3270–H3278. [Google Scholar] [CrossRef] [Green Version]
- Yazaki, Y.; Isobe, M.; Takahashi, W.; Kitabayashi, H.; Nishiyama, O.; Sekiguchi, M.; Takemura, T. Assessment of myocardial fatty acid metabolic abnormalities in patients with idiopathic dilated cardiomyopathy using 123I BMIPP SPECT: correlation with clinicopathological findings and clinical course. Heart (Br. Card. Soc.) 1999, 81, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Chandler, M.P.; Kerner, J.; Huang, H.; Vazquez, E.; Reszko, A.; Martini, W.Z.; Hoppel, C.L.; Imai, M.; Rastogi, S.; Sabbah, H.N.; et al. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1538–H1543. [Google Scholar] [CrossRef] [Green Version]
- Osorio, J.C.; Stanley, W.C.; Linke, A.; Castellari, M.; Diep, Q.N.; Panchal, A.R.; Hintze, T.H.; Lopaschuk, G.D.; Recchia, F.A. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 2002, 106, 606–612. [Google Scholar] [CrossRef]
- Nagoshi, T.; Yoshimura, M.; Rosano, G.M.; Lopaschuk, G.D.; Mochizuki, S. Optimization of cardiac metabolism in heart failure. Curr. Pharm. Des. 2011, 17, 3846–3853. [Google Scholar] [CrossRef]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.J.; Berg, H.M.; Easton, C.J.; Bakker, A.J. The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Amino Acids 2006, 31, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Higgins, M.F.; Cox, V.M.; Duncan, M.J.; James, R.S. Does a physiological concentration of taurine increase acute muscle power output, time to fatigue, and recovery in isolated mouse soleus (slow) muscle with or without the presence of caffeine? Can. J. Physiol. Pharmacol. 2014, 92, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, S.W.; Jong, C.J.; Ramila, K.C.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17 (Suppl. 1), S2. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. Regulation of skeletal muscle protein degradation and synthesis by oral administration of lysine in rats. J. Nutr. Sci. Vitaminol. 2013, 59, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. Lysine suppresses myofibrillar protein degradation by regulating the autophagic-lysosomal system through phosphorylation of Akt in C2C12 cells. SpringerPlus 2014, 3, 584. [Google Scholar] [CrossRef] [Green Version]
- Montesano, A.; Senesi, P.; Luzi, L.; Benedini, S.; Terruzzi, I. Potential Therapeutic Role of L-Carnitine in Skeletal Muscle Oxidative Stress and Atrophy Conditions. Oxid. Med. Cell. Longev. 2015, 2015, 646171. [Google Scholar] [CrossRef]
- D’Antona, G.; Nabavi, S.M.; Micheletti, P.; Di Lorenzo, A.; Aquilani, R.; Nisoli, E.; Rondanelli, M.; Daglia, M. Creatine, L-carnitine, and omega3 polyunsaturated fatty acid supplementation from healthy to diseased skeletal muscle. Biomed Res. Int. 2014, 2014, 613890. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Demetrius, L. Caloric restriction, metabolic rate, and entropy. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, B902–B915. [Google Scholar] [CrossRef]
- Loennechen, J.P.; Stoylen, A.; Beisvag, V.; Wisloff, U.; Ellingsen, O. Regional expression of endothelin-1, ANP, IGF-1, and LV wall stress in the infarcted rat heart. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2902–H2910. [Google Scholar] [CrossRef]
- Johnsen, A.B.; Hoydal, M.; Rosbjorgen, R.; Stolen, T.; Wisloff, U. Aerobic interval training partly reverse contractile dysfunction and impaired Ca2+ handling in atrial myocytes from rats with post infarction heart failure. PLoS ONE 2013, 8, e66288. [Google Scholar] [CrossRef] [PubMed]
- Wisloff, U.; Helgerud, J.; Kemi, O.J.; Ellingsen, O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1301–H1310. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Randall, W.C.; Taylor, A.H.; Kappler, F.; Walker, M.; Brown, T.R.; Szwergold, B.S. Fructose-3-phosphate production and polyol pathway metabolism in diabetic rat hearts. Metab. Clin. Exp. 1997, 46, 1333–1338. [Google Scholar] [CrossRef]
- Nilsen, L.H.; Witter, M.P.; Sonnewald, U. Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2014, 34, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, G.; Savorani, F.; Engelsen, S.B. icoshift: An effective tool for the alignment of chromatographic data. J. Chromatogr. A 2011, 1218, 7832–7840. [Google Scholar] [CrossRef]
- Karaman, I. Preprocessing and Pretreatment of Metabolomics Data for Statistical Analysis. Adv. Exp. Med. Biol. 2017, 965, 145–161. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]





| Metabolites | Surgery | Exercise | Interaction |
|---|---|---|---|
| Glucose | <0.001 | - | 0.046 |
| Phosphocreatine | <0.001 | 0.050 | - |
| Creatine | <0.001 | - | - |
| Taurine | <0.001 | - | - |
| Glutamine | 0.0026 | - | - |
| Acetate | - | - | 0.037 |
| Lactate | <0.001 | - | - |
| Glycerophosphocholine | - | - | 0.012 |
| Succinate | 0.026 | - | - |
| Aspartate | <0.001 | - | - |
| Glycine | - | - | - |
| Glutamate | - | - | - |
| Lysine | - | - | - |
| Alanine | - | - | - |
| Metabolites | Surgery | Exercise | Interaction |
|---|---|---|---|
| Glycine | - | 0.0044 | 0.014 |
| Taurine | - | 0.0056 | <0.001 |
| Acetate | - | 0.0082 | - |
| Lysine | - | 0.0033 | 0.019 |
| Alanine | 0.043 | - | - |
| Lactate | - | 0.012 | - |
| Anserine | - | 0.032 | 0.010 |
| Glucose | - | - | - |
| Phosphocreatine | - | - | - |
| Creatine | - | - | - |
| Glutamine | - | - | - |
| Glutamate | - | - | - |
| Fumarate | - | - | - |
| Niacinamide | - | - | - |
| NAD | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Ellingsen, Ø.; Bathen, T.F.; Høydal, M.A.; Stølen, T.; Esmaeili, M. The Effect of Exercise Training on Myocardial and Skeletal Muscle Metabolism by MR Spectroscopy in Rats with Heart Failure. Metabolites 2019, 9, 53. https://doi.org/10.3390/metabo9030053
Shi M, Ellingsen Ø, Bathen TF, Høydal MA, Stølen T, Esmaeili M. The Effect of Exercise Training on Myocardial and Skeletal Muscle Metabolism by MR Spectroscopy in Rats with Heart Failure. Metabolites. 2019; 9(3):53. https://doi.org/10.3390/metabo9030053
Chicago/Turabian StyleShi, Mingshu, Øyvind Ellingsen, Tone Frost Bathen, Morten A. Høydal, Tomas Stølen, and Morteza Esmaeili. 2019. "The Effect of Exercise Training on Myocardial and Skeletal Muscle Metabolism by MR Spectroscopy in Rats with Heart Failure" Metabolites 9, no. 3: 53. https://doi.org/10.3390/metabo9030053
APA StyleShi, M., Ellingsen, Ø., Bathen, T. F., Høydal, M. A., Stølen, T., & Esmaeili, M. (2019). The Effect of Exercise Training on Myocardial and Skeletal Muscle Metabolism by MR Spectroscopy in Rats with Heart Failure. Metabolites, 9(3), 53. https://doi.org/10.3390/metabo9030053






