Abstract
Forty-one isomers of Al2C4H2 that lie within 50 kcal mol−1 are theoretically identified in this work using density functional theory. Among these, isomers 3 and 14 contain a planar tetracoordinate carbon (ptC) atom that lies at 3.3 and 16.9 kcal mol−1, respectively, and are above the global minimum geometry 1 at the ωB97XD/6-311++G(2d,2p) level of theory. The other ten isomers that also contain unique bonding features are isomers 4, 18, 20, 21, 22, 27, 28, 31, 34, and 40. Out of these isomers, 4, 18, 20, 22, 27, 28, and 34 contain planar tetracoordinate aluminum (ptAl) whereas isomers 31 and 40 contain both ptC and ptAl atoms. Chemical bonding characteristic features are thoroughly analyzed for all these eleven isomers with various bonding and topological quantum chemical tools, such as NBO, AdNDP, WBI, and ELF, except isomer 27 due to the observed elongated Al-Al bond length. The current results indicate that ptC isomer 3 is more stable than other isomers because electron delocalization is more prevalent and it also has double aromaticity as observed from the ELF, NICS, and AdNDP analysis. Further, the structural stability of these isomers is investigated through ab initio molecular dynamics (AIMD) simulation. Isomer 21 shows the planar pentacoordinate aluminum but it is observed as a kinetically unstable geometry from AIMD and, further, one could notice that it isomerizes to isomer 12.
1. Introduction
Planar tetracoordinate carbon (ptC) has made tremendous progress in the past five decades both theoretically [1,2,3,4,5,6,7,8,9,10] and experimentally [11,12,13,14,15,16,17,18,19,20]. This concept is a fundamental deviation from the conventional ideas of tetrahedral tetracoordinate carbon, which was independently postulated by van’t Hoff and Le Bel in 1874 [18,21,22]. For a century, van’t Hoff and Le Bel’s tetrahedral carbon model went unchallenged. Later, computational predictions and experimental observations led to the initial discovery of planar tetracoordinate or hypercoordinate compounds [4,23]. In 1968, H. J. Monkhorst first proposed the idea of ptC as a transition state for a non-dissociative racemization process [24]. Two years later, in 1970, Hoffmann et al. proposed two ways to stabilize ptC, which have served as a basic guideline for the design of ptC species [25] to date. First, the mechanical strategy was employed with transition metals, conjugative rings, or cages to induce the formation of ptC. On the other hand, the electronic strategy involved using strong σ-donor and π-acceptor ligands or aromatic delocalization to stabilize the lone pair present in the p-orbital on the ptC center. Based on the latter strategy, Schleyer and coworkers theoretically identified the first set of ptC molecules in 1976 but they were not global minimum geometries [26]. Since then, the chemistry of ptC has been extensively studied both theoretically and experimentally as well. Further, it has been extended to planar hypercoordinate carbon (phC) [4,27] as well as nanosized systems [28,29,30,31]. These ptC molecules not only challenge our current understanding of “chemical bonding,” but also have potential applications in materials science [31,32].
Only a few ptC compounds, either global or local minimum energy structures, have been experimentally identified to date [13,15,16,33] and other ptC molecules are elusive in the laboratory to date. In this direction, the identification of a ptC structure for a particular elemental composition leads to a high chance of theoretical viability and it delineates a new path to synthetic feasibility [16,33,34,35,36,37,38]. Dong et al. and Zhang et al. experimentally achieved the ptC clusters of C2Al4 [37] and C5Al5 [39], respectively, and were predicted to be the global minimum theoretically [40,41]. These two outstanding experimental achievements on aluminum clusters demonstrated that ptC structures are substantially feasible in the experimental laboratory.
Clusters of aluminum and carbon atoms have attracted a lot of attention because they can form non-classical and non-stoichiometric structures [4,42,43,44] different from most other metal carbide clusters with the cubic framework or layered structures. In 2022, Zhang and co-workers explored them both theoretically and experimentally and identified the most stable structure containing ptC in the carbon-doped aluminum clusters AlnC− (n = 6–5) [45]. In the past, Naumkin explored the flat structural motifs of small alumino-carbon clusters CnAlm (n = 2–3, m = 2–8) and identified unique clusters featuring ptC and phC [46]. Wu and co-workers identified the global minimum geometry of simple aluminum carbon clusters containing two ptCs [40]. Das et al. identified the global minimum ptC geometries of CSiGaAl2−/0 and CGeGaAl2−/0 systems, exhibiting both σ and π aromaticity [47].
The aluminum and carbon-based molecules could be used in cluster assembled materials [7,9], energy storage [48], aluminum nanoparticle protection, and two-dimensional donor materials in solar cells [49]. In 2021, the global minimum structures of aluminum-bearing planar tetracoordinate carbon isomers of CAl4Mg0/− [50] and planar tetracoordinate boron, as well as planar pentacoordinate boron isomers of BAl4Mg−/0/+ [51], were also reported. Dong et al. synthesized Al2CH3, Al2C3H3, Al3C3H2, Al3C4H2, Al4C3H, and Al4C4H clusters which have hydrogen storage properties [37]. Due to their ability to retain hydrogen and yield combustion products with minimal environmental impact, aluminum clusters have been the subject of several aspects of energy conversion materials development [8,52]. As said above, the exploration of aluminum, carbon, and hydrogen clusters with various molecular chemical compositions has been carried out in the recent past in both theoretical and experimental aspects and their potential applications have also been reported. Meanwhile, a lot of non-classical structure identification has also been carried out in this direction. They may have future potential as alternative fuel resources for overcoming one of the key challenges in developing a hydrogen economy [48] which is in line with one of the 17 UN Sustainable Development Goals [53]. Aluminum carbon clusters containing ptCs have moderate band gaps with wide applications in the areas of semiconductors, optoelectronics, and photovoltaics [28,49]. Al-doped carbon nanotubes have gained a lot of interest because of their nanocatalytic activity in reducing N2 to NH3 [54]. Al-doped single-walled carbon nanotubes are superior materials as sensors to detect air pollutants such as CO, NH3, NO2, and SO2 [55,56]. From this point of view, the Al2C4H2 system has yet to be explored in detail, hence the present work demonstrates its importance in this field.
On the other hand, aluminum has also been touted as having a significant potential for astrochemical interest [57]. Aluminum is a generously available metallic element in the interstellar medium (ISM) that has a cosmic abundance of∼3 × 10−6 [58]. AlCl [59,60], AlF [59,61,62], AlNC [63,64,65], AlO [66,67], and AlOH [62] are aluminum-bearing molecules that have been detected in the ISM. The depletion of aluminum in interstellar space due to the formation of interstellar dust was well reported [68] and the existence of aluminum in interstellar space is reported as carbonaceous grains [69]. From this point of view, the present work aimed to explore the possible isomers of the Al2C4H2 system theoretically and, in particular, the main focus is given to isomers that have ptC and ptAl isomers lying within 50 kcal mol−1.
2. Computational Methodology
The initial geometries of Al2C4H2 isomers are generated by chemical intuition. Density functional theory (DFT) was used to optimize the different possible geometries of Al2C4H2 isomers at the B3LYP/6-311++G(2d,2p) [70,71,72,73] and ωB97XD/6-311++G(2d,2p) [70,71,74] level of theoretical methods. All the investigated Al2C4H2 isomers considered here correspond to the singlet spin state. Harmonic vibrational frequencies are calculated for all the energy minimized geometries to ensure the stationary point on the potential energy surface is a minimum, a transition state, or a higher-order saddle point. Natural bond order (NBO) analysis [75], adaptive natural density partitioning (AdNDP) analysis [76,77], and Wiberg bond indices (WBIs) [78] were used to examine the nature of chemical bonding in the planar tetracoordinate isomers at the ωB97XD/6-311++G(2d,2p) level of theory. Nucleus-independent chemical shift (NICS) values were calculated to measure the π/σ dual aromaticity in isomer 3 [79]. Topological analysis of the electron localization function (ELF) [80] was carried out for the planar tetracoordinate isomers using the Multiwfn program [81]. The ab initio molecular dynamics (AIMD) simulation using the atom-centered density matrix propagation (ADMP) [82] method was performed for the planar tetracoordinate isomers to check their kinetic stability at the ωB97XD/6-311++G(2d,2p) level of theory. In-house Python code was used to generate other possible atomic re-arrangements in the isomers of 1, 2, 3, 4, 20, 22, and 40. All of these DFT calculations were carried out with the Gaussian suite of programs [83].
3. Results and Discussion
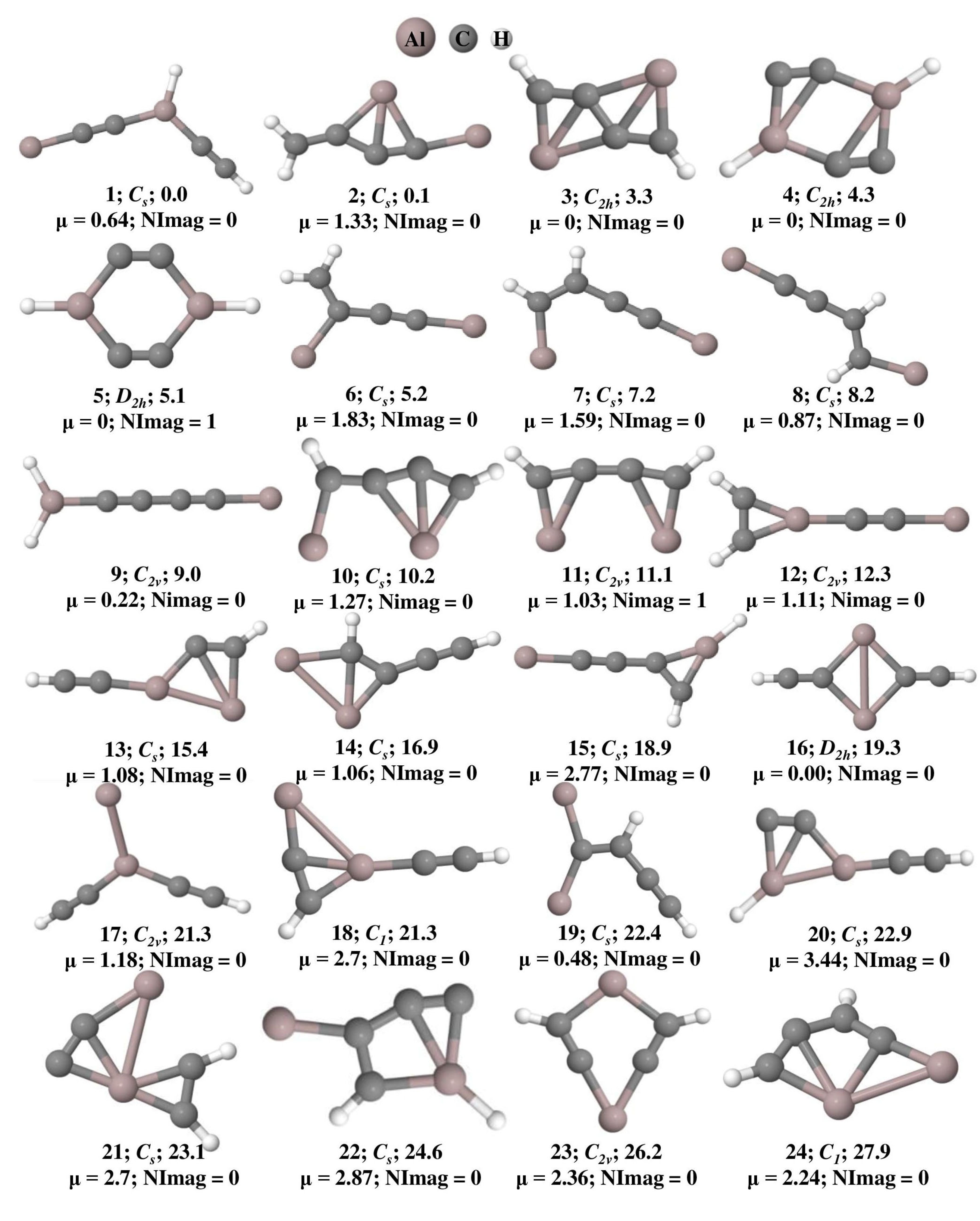
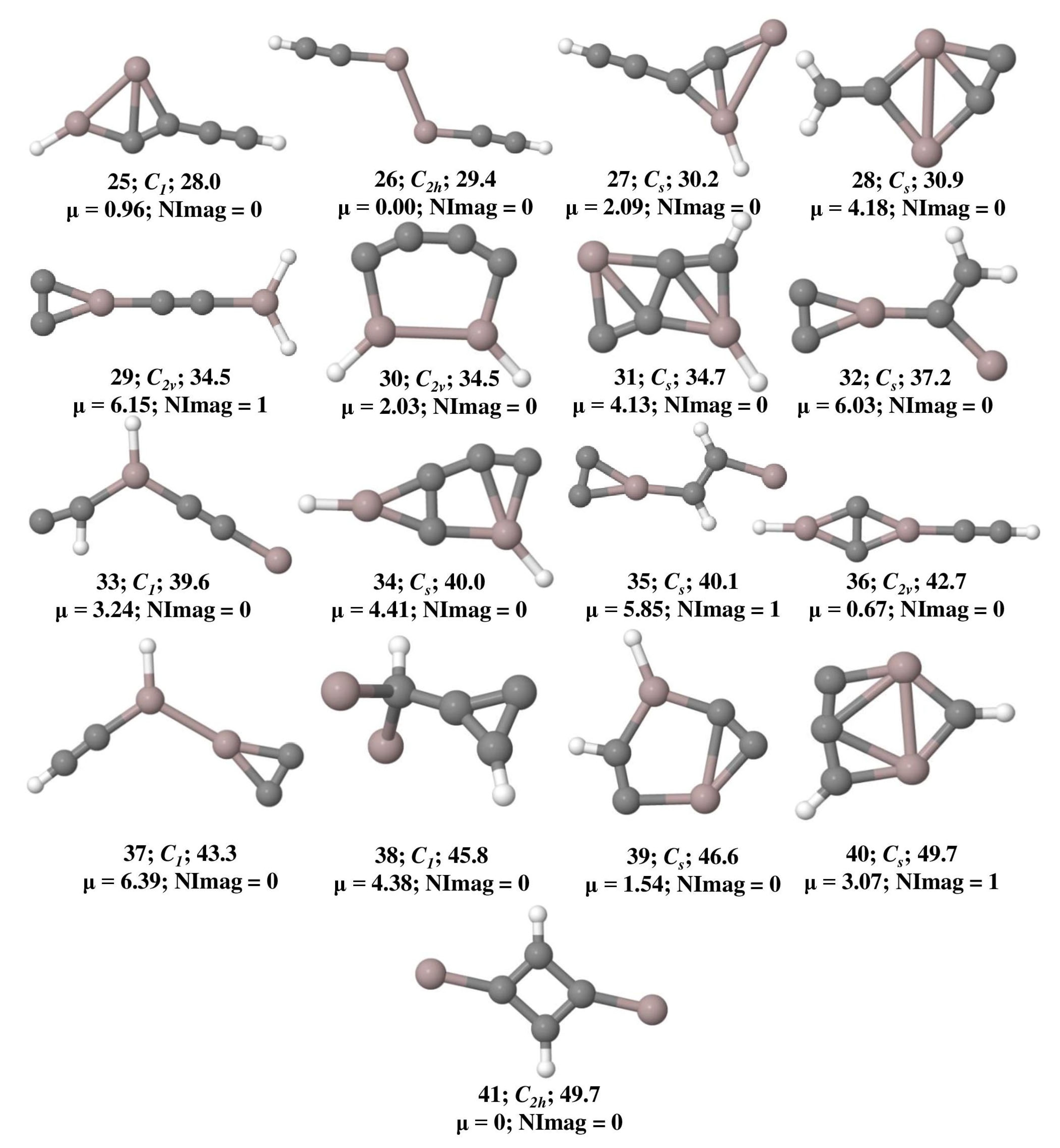
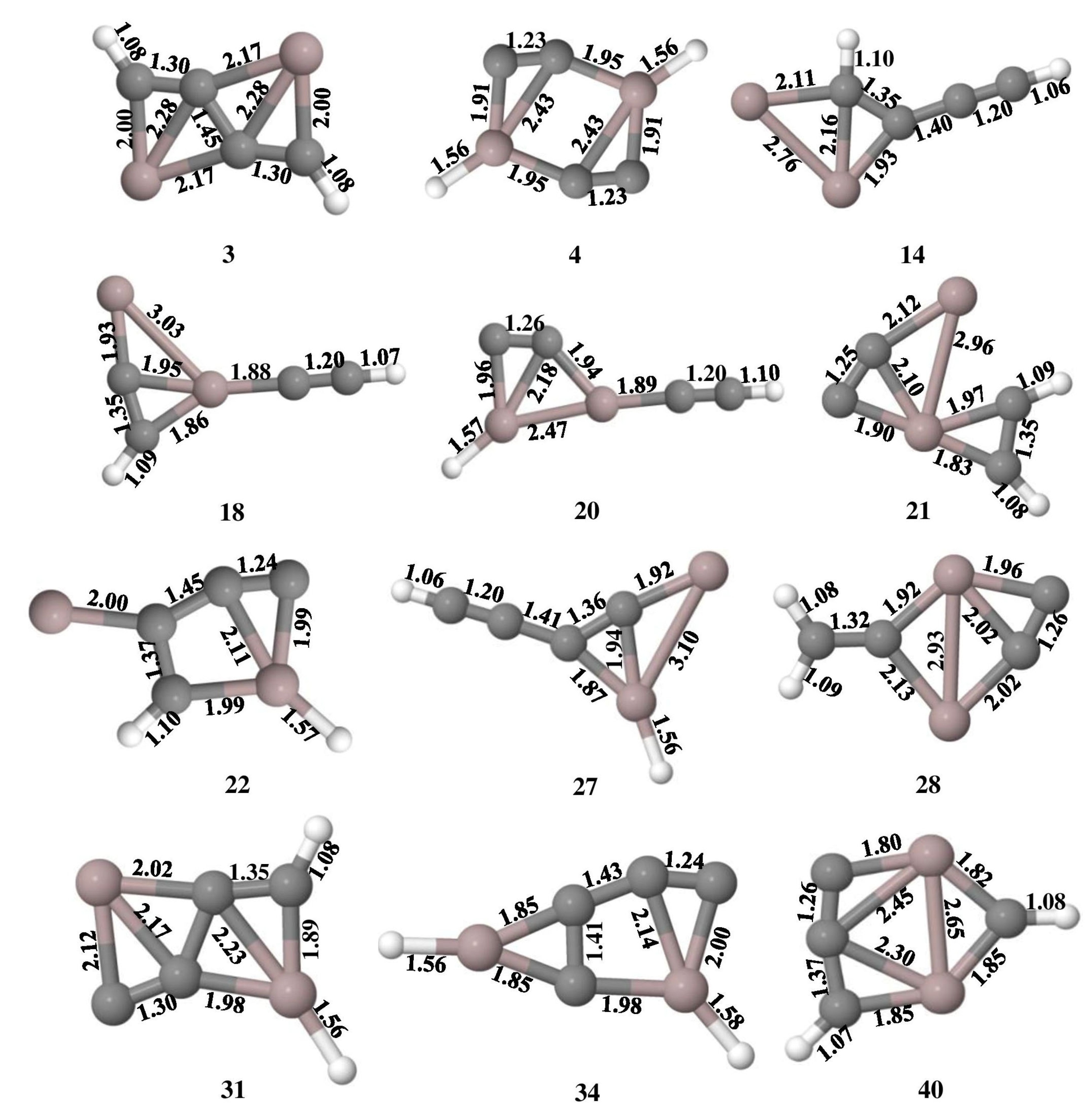
The optimized geometries of Al2C4H2 isomers that lie within 50 kcal mol−1 at the ωB97XD/6-311++G(2d,2p) level of theory are shown in Figure 1 and their total energy, ZPVE, and ZPVE corrected relative energies are listed out in Table S1. The B3LYP/6-311++G(2d,2p) level of theoretical results is provided in Table S2. Isomer 1 is a global minimum on the potential energy surface with a bent structure. Among these geometries, isomers 3, 4, 14, 18, 20, 22, 27, 28, 31, 34, and 40 are showing planar tetracoordinate structures that lie at 3.3, 4.3, 16.9, 21.3, 22.9, 24.6, 30.2, 30.9, 34.7, 40.0, and 49.6 kcal mol−1, respectively. Planar pentacoordinate aluminum (ppAl) is observed in isomer 21 which lies at 23.1 kcal mol−1 above isomer 1. All these planar tetracoordinate and pentacoordinate geometries with their bond lengths are shown in Figure 2. Among these, eleven isomers are local minima with zero imaginary frequencies except isomer 40, which is a first-order saddle point (transition state). Among all the isomers, only two isomers, 3 and 14, contain a ptC atom, while the other isomers such as 4, 18, 20, 22, 27, 28, and 34 contain ptAl. Isomers 3 and 4 have two planar tetracoordinate centers each. Isomer 3 contains two ptCs whereas isomer 4 contains two ptAls. Three planar tetracoordinate centers are present in isomers 31 and 40. In contrast to isomer 31, which has two ptCs and a ptAl, isomer 40 contains two ptAls and a ptC. Even though isomer 27 contains a ptAl center, because of the elongated Al-Al bond length of 3.10 Å, it is excluded from the bonding studies.
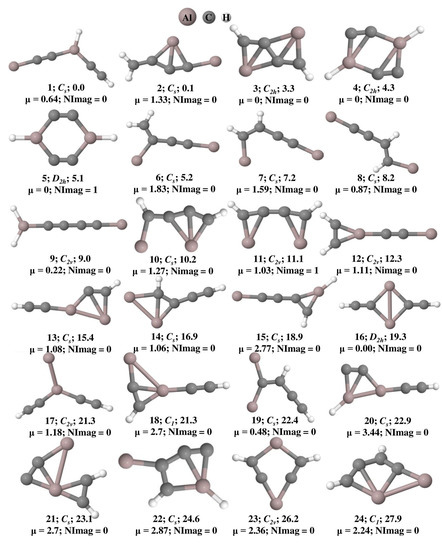
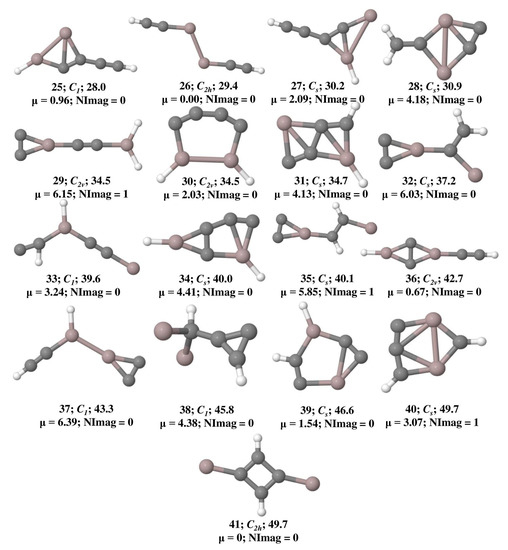
Figure 1.
Isomers of Al2C4H2 that lie within 50 kcal mol−1 and their zero-point vibrational energy (ZPVE) corrected relative energies (in kcal mol−1), dipole moments (in Debye), and the number of imaginary frequencies (NImag) obtained at the ωB97XD/6-311++G(2d,2p) level of theory.
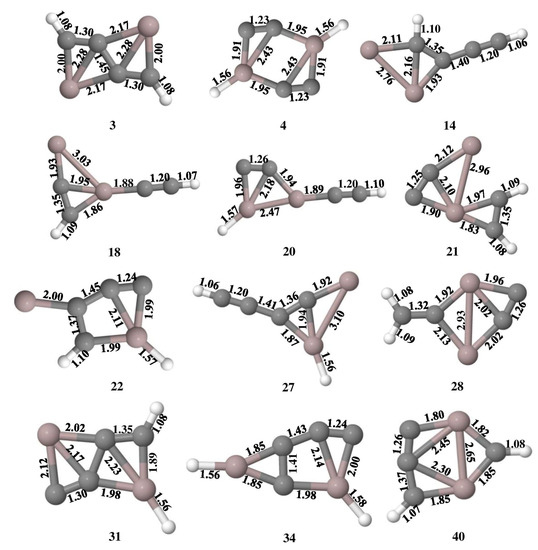
Figure 2.
Isomers of Al2C4H2 with the planar tetracoordinate carbon (ptC)/aluminum (ptAl) and planar pentacoordinate aluminum (ppAl) with their bond lengths in Å obtained at the ωB97XD/6-311++G(2d,2p) level of theory.
3.1. Wiberg Bond Indices
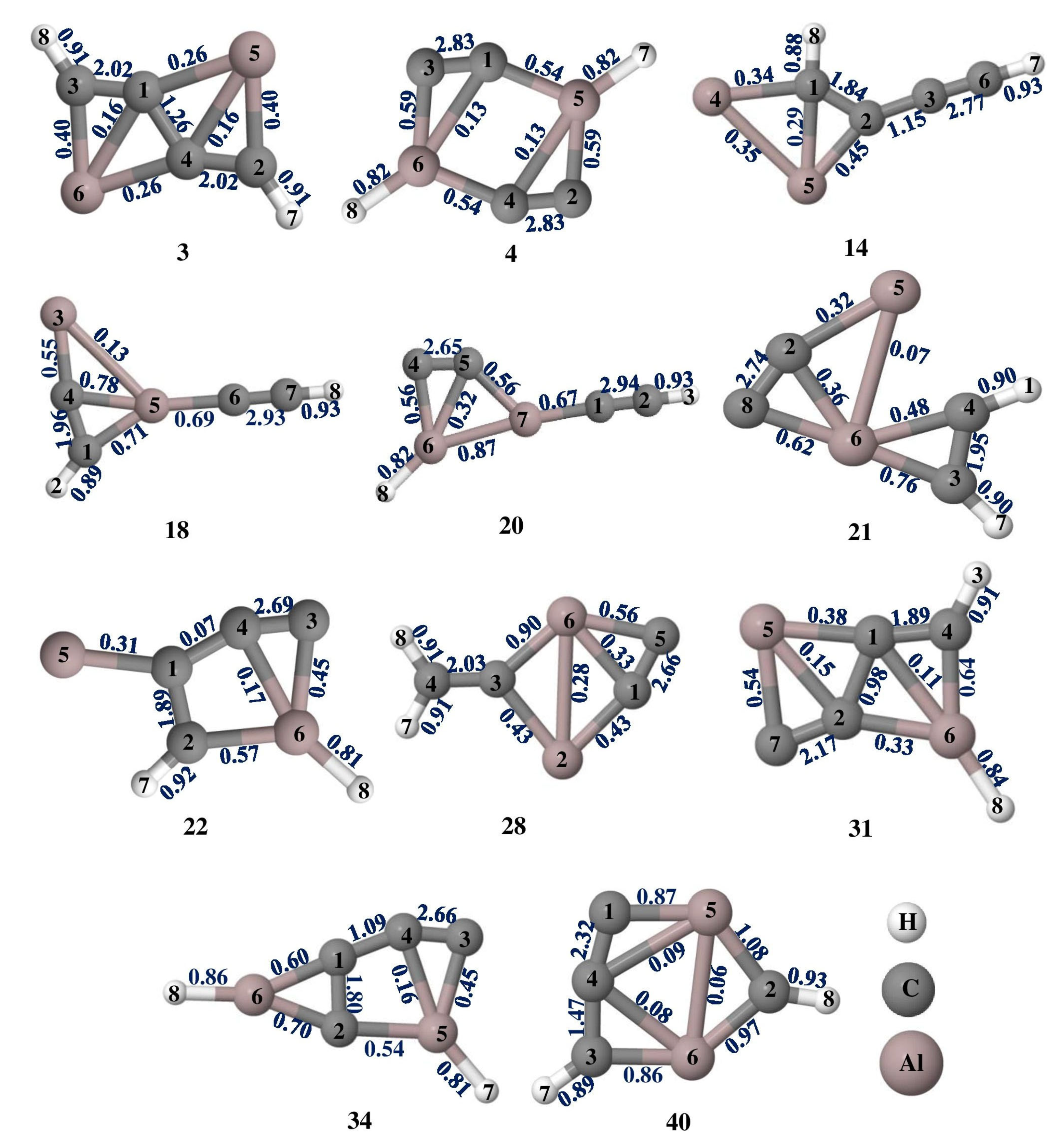
The Wiberg bond indices (WBIs) of isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 are shown in Figure 3, which are the measure of electronic parameters related to electron population overlap between two atoms. It indicates the bond order and the characteristic bonding features of these isomers. In isomer 3, the WBI values for C1–C3, C1–C4, and C2–C4 (numbering scheme followed as shown in Figure 3) are 2.02, 1.22, and 2.02, respectively, which indicates resonance stabilization with alternate double bonds along ptCs. Among the four bonds of the ptC in isomers 14, 31, and 40, one of the bonds has a WBI value close to or greater than two which indicates the π bonds between them. The ppAl–Al bond in isomer 21 has a WBI value of 0.07 which indicates the interaction between them is electrostatic, but all four C–ppAl bonds have WBI values in the range of 0.36 to 0.76 which indicates the single bonds between them. In contrast to isomers with ptAl, which only have four σ bonds, isomers with ptC have one π bond and four σ bonds.
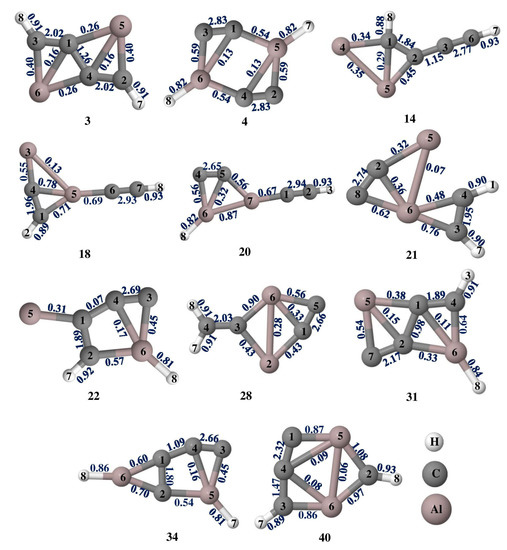
Figure 3.
Wiberg bond indices of Al2C4H2 isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 at the ωB97XD/6-311++G(2d,2p) level of theory.
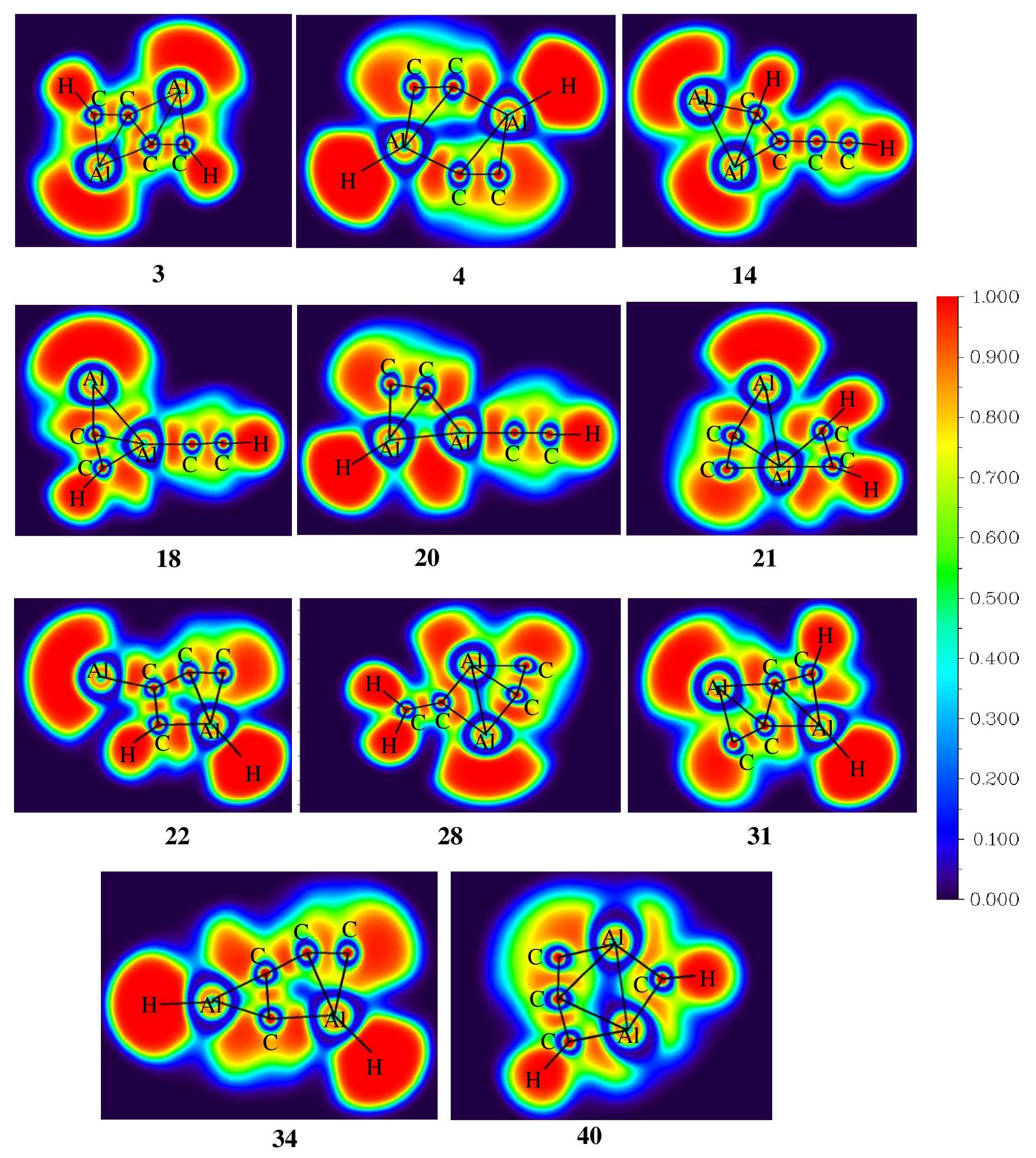
3.2. Topological Analysis
The electron localization function (ELF) analysis was carried out to measure the extent of electron delocalization in molecular systems. The color-filled ELF plots of isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 are shown in Figure 4. In isomer 3, the electron density is localized between the ptC–C bonds in the range of 0.80 to 1.00 which indicates strong localization of electrons, and also the electron density is delocalized through the ptC–C bonds, contributing to its stability. Furthermore, both of the aluminum atoms are involved in the delocalization of electrons, which enhances the stability of isomer 3. In isomers 14, 22, 31, and 34, the electron density is delocalized along the C–C bonds, contributing to their structural stability. In the case of isomers 4, 20, 22, 31, and 34, aluminum-attached hydrogen has electronegative values in the range of −0.345 to −0.378 |e| as listed in Table S3 due to the high electropositive nature of aluminum compared to carbon. The Al–Al bonds in isomers 21, 28, and 40 have ELF values in the range of 0.00 to 0.50 which indicates the poor localization of electrons between these atoms.
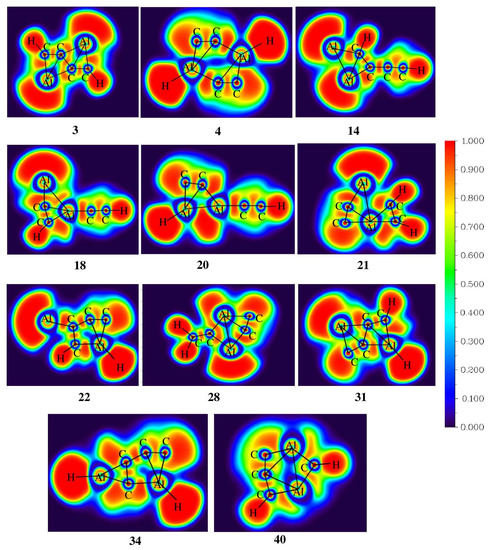
Figure 4.
Color-filled map of ELF for isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 obtained at the ωB97XD/6-311++G(2d,2p) level of theory.
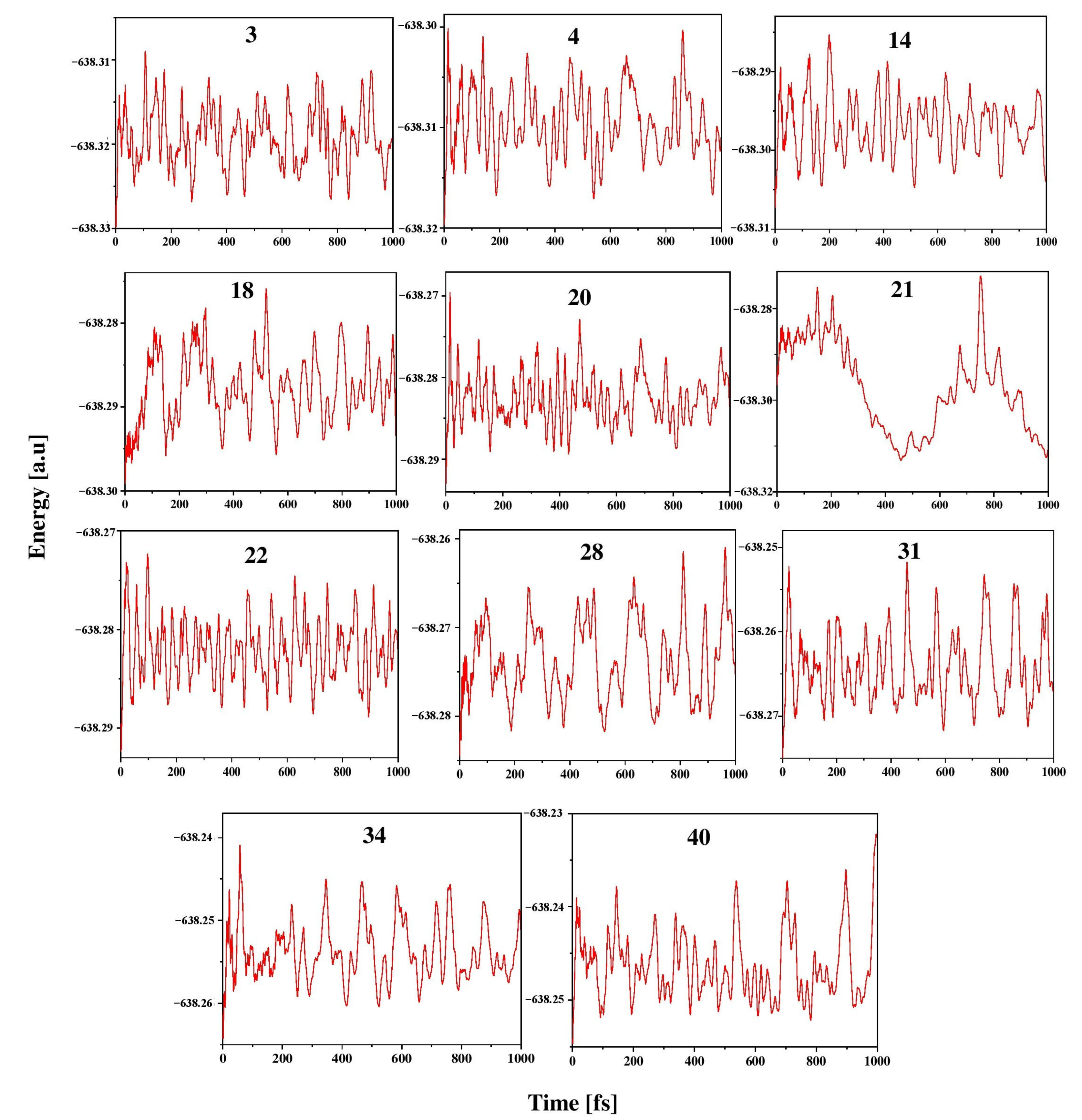
3.3. Kinetic Stability
To explore the kinetic stability of ptC and ptAl isomers in the Al2C4H2 system, ab initio molecular dynamics simulations were carried out using the ADMP approach. This study enables us to explore the structural variation through the atomic arrangement in the elemental composition. Isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 were considered for these simulations at 298 K and 1 atm pressure, and the simulations were carried out for up to 1000 fs. The structural stability in the time evolution of total energy for these isomers is given in Figure 5. The oscillations in the energy plots result from an increase in nuclear kinetic energy during the structural deformation of the systems throughout the simulation. These plots demonstrate well-balanced energy oscillations for isomers 3, 4, 14, 18, 20, 22, 31, 34, and 40. These isomers’ structural stability is well retained throughout the simulated time scale, and no isomerization or other structural modifications take place in these molecules and they are kinetically stable except for isomer 21. In the case of isomer 21, the geometry deforms by entirely shattering the geometry and isomerizing it into the low-energy isomer 12, confirming that it is kinetically unstable.
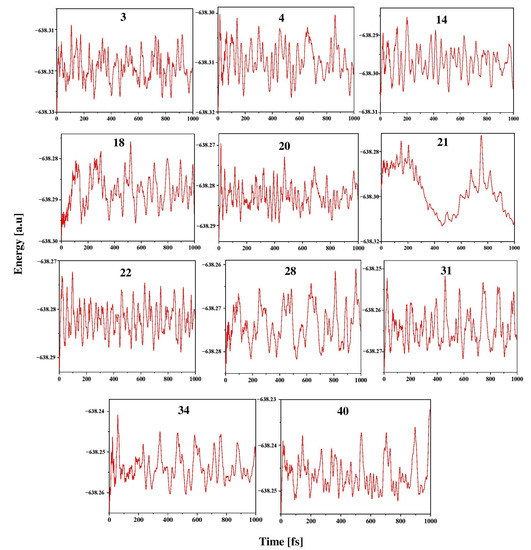
Figure 5.
Energy evolution of isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 at 298 K, 1 atm pressure for 1000 fs of time in the ADMP simulation performed at the ωB97XD/6311++G(2d,2p) level.
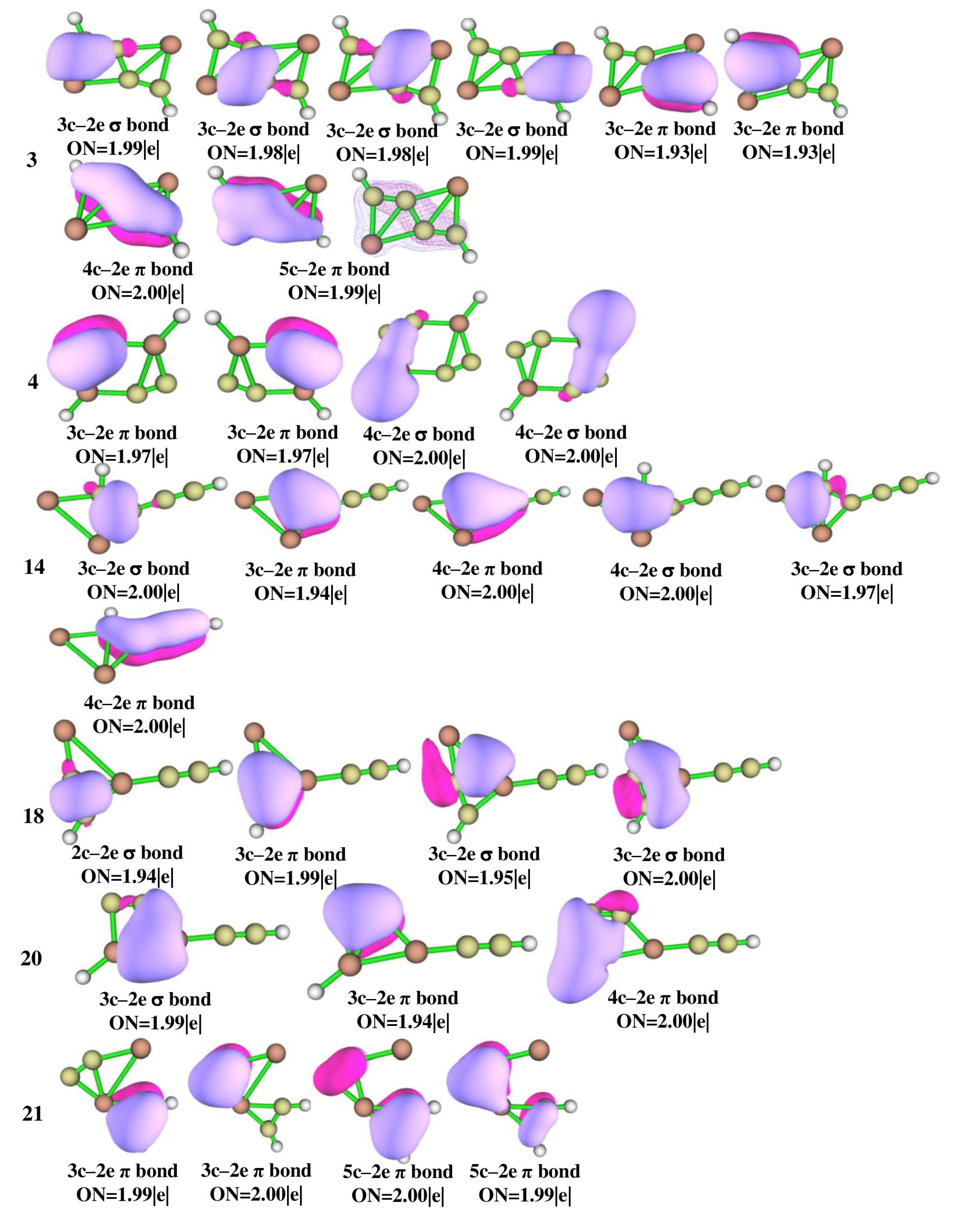
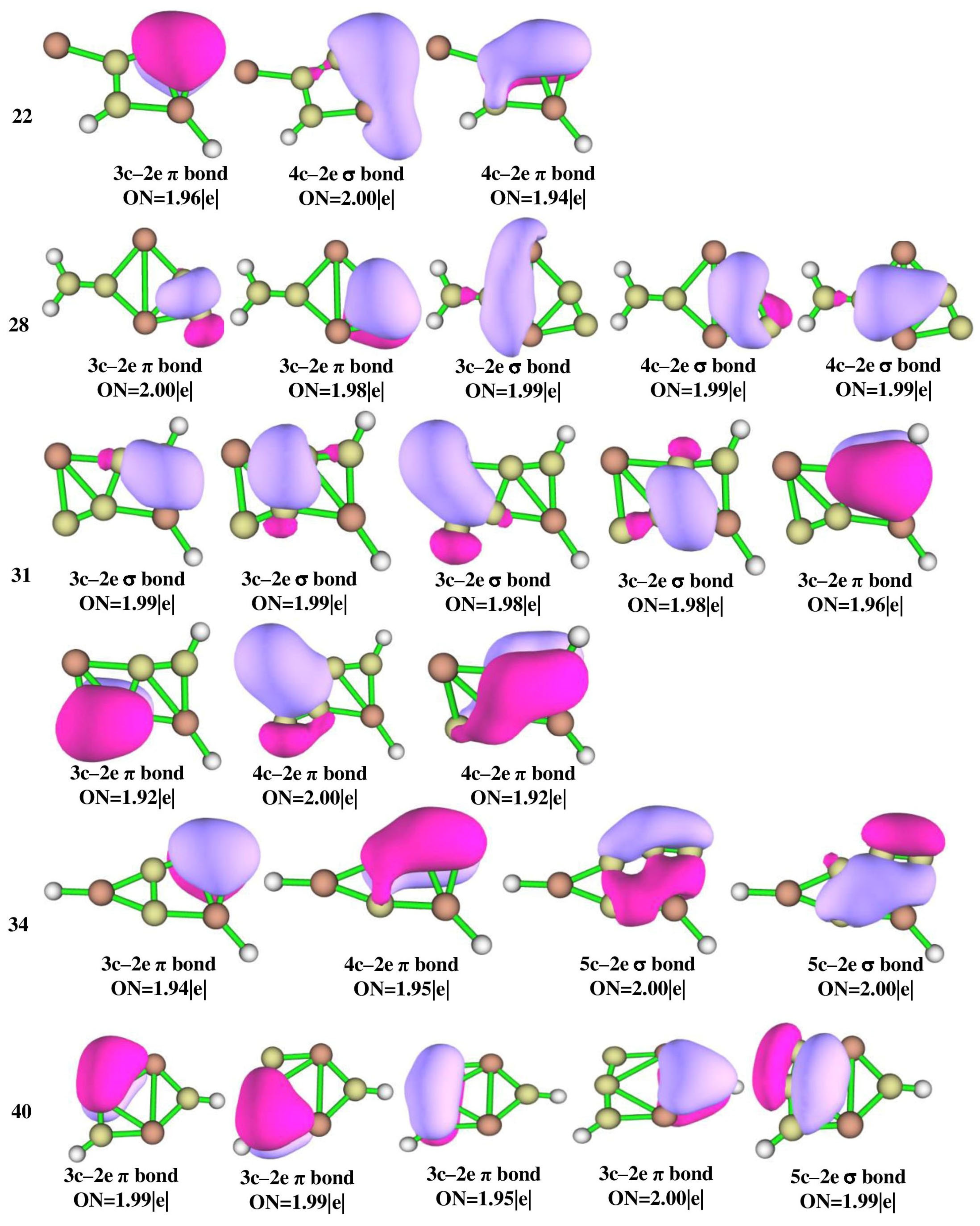
3.4. Adaptive Natural Density Partitioning and Natural Bond Order Analysis
The adaptive natural density partitioning (AdNDP) analysis was performed for isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 which are illustrated in Figure 6 for multi-center–2e bonds and Figure S2 for lone pairs and 2c–2e bonds. It gives insight into delocalized multi-center–2e bonds as well as Lewis bonding components. In a Lewis structure, the good electron pair should have an occupancy number (ON) greater than 1.90 |e|. The 2c–2e σ bonds in all of the isomers have an ON value above 1.90 |e| that dictates the electron density which is localized between the planar tetracoordinate centers and their adjacent atoms. The presence of multi-center–2e π bonds in isomers 3, 14, 22, 31, 34, and 40 shows how the electrons participate in delocalization along C-C carbon bonds. In isomer 3, four 3c–2e σ bonds with ON 1.99 and 1.98 |e|, two 3c–2e π bonds with ON 1.93 |e|, 4c–2e π bonds with ON 2.00 |e|, and a 5c–2e π bond with ON 1.99 |e| are observed and are in accord with the concept of double aromaticity in the molecule as shown by the negative values of NICS (0) and NICS (1) in Figure S3. Delocalization encompasses the whole system, making isomer 3 more stable than other isomers. All the other isomers also have multi-center–2e bonds with ON greater than 1.90 |e|. In isomer 3, the aluminum LP orbitals with low ON 1.80 |e|, as shown in Figure S2, confirm that the electrons are shared by aluminum atoms which is consistent with NBO and ELF analysis.
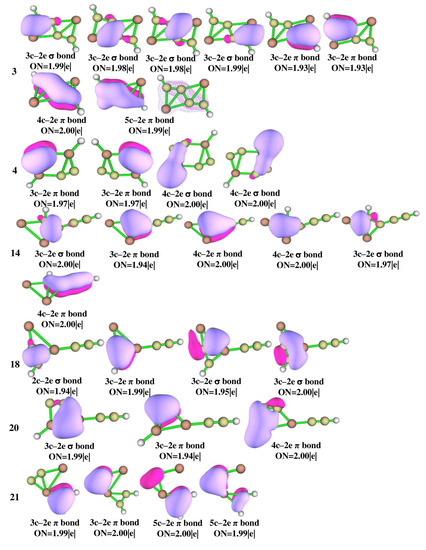
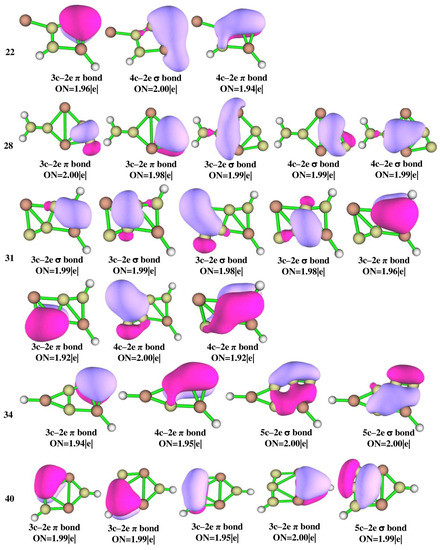
Figure 6.
AdNDP bonding patterns of Al2C4H2 isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 at the ωB97XD/6-311++G(2d,2p) level of theory.
The energies of donor–acceptor interaction of lone pairs of aluminum obtained for isomers 3, 4, 18, 21, 22, 28, and 31 with NBO analysis are presented in Table S4. The occupancies of lone pairs for isomers 3, 14, 18, 21, 22, 28, 31, and 40 from NBO analysis are given in Table S5. The E(2) is the hyperconjugative interaction between acceptor and donor which governs the stabilization energy. The LP(Al5) → π*(C1–C4) and LP(Al6) → π*(C1–C4) in isomer 3 have larger stabilization energy of 36.71 kcal mol−1, as compared to other isomers, which confirms that the electrons from both of the aluminum atoms are shared and accepted by surrounding atoms that is consistent with the delocalization nature of the structure obtained from ELF analysis.
4. Conclusions
In summary, the isomers of Al2C4H2 have been explored at the ωB97XD/6-311++G(2d,2p) level of theory. The isomers containing planar tetracoordinate carbon/aluminum that lie within 50 kcal mol−1 are subjected to chemical bonding analysis. Isomers 3 and 4 are planar tetracoordinate systems that are energetically close to global minimum geometry 1. The isomers that have the ptCs are energetically more stable than the isomers that contain ptAl because of the electron delocalization nature of ptC compared to ptAl. The isomers with ptC have one π bond and four σ bonds, while the isomers with ptAl have only four sigma bonds. Among the planar tetracoordinate isomers, isomer 3 is energetically stable due to its high delocalization nature and double aromaticity as observed in the ELF, NICS, and AdNDP analysis. So, the stability of isomer 3 is achieved by the tendency of the electron-donating nature of the aluminum atom. The ab initio molecular dynamics simulations revealed that all these planar tetracoordinate molecules are kinetically stable except isomer 21 which isomerizes to isomer 12. Exploring the electron density distribution and orbital orientation of these isomers provides insight into the nature of chemical bonding and may lead to potential applications in the future. The insights of this work would impart great interest to experimentalists to find Al2C4H2 isomers in the laboratory which remain elusive to date.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atoms10040112/s1. The optimized geometries of all the isomers are given in Figure S1, AdNDP for lone pairs and 2c–2e bonds of isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 are given in Figure S2, NICS for isomer 3 is given in Figure S3, total energies (in a.u), zero-point vibrational energies (ZPVEs; in a.u.), ZPVE corrected total energies (E+ZPVE; in a.u.), relative energies (ΔE+ZPVE; in kcal mol−1), and the number of imaginary frequencies (NImag) of Al2C4H2 isomers calculated at ωB97XD/6-311++G(2d,2p) and B3LYP/6-311++G(2d,2p) level are given in Tables S1 and S2, respectively, natural atomic charges of hydrogen in isomers 3, 4, 14, 18, 20, 21, 22, 28, 31, 34, and 40 are given in Table S3, the donor → acceptor orbitals of lone pairs of aluminum in isomers 3, 4, 18, 21, 22, 28, and 31 are given in Table S4, occupancies of lone pairs for isomers 3, 14, 18, 21, 22, 28, 31, and 40 from NBO analysis are given in Table S5, cartesian coordinates of all isomers at ωB97XD/6-311++G(2d,2p) and B3LYP/6-311++G(2d,2p) level are given in Tables S6 and S7.
Author Contributions
Conceptualization, V.S.T. and K.T.; Investigation, A.H.M., S.S. (Sony Sobinson), N.J., S.S. (Shilpa Shajan), S.P.M., V.S.T. and K.T.; Methodology, A.H.M., S.S. (Sony Sobinson), N.J., S.S. (Shilpa Shajan), S.P.M., V.S.T. and K.T.; Supervision, V.S.T. and K.T.; Visualization, A.H.M., N.J., S.S. (Shilpa Shajan), S.P.M. and K.T.; Writing – original draft, A.H.M.; Writing – review & editing, V.S.T. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the article or Supplementary Materials.
Acknowledgments
The computational facility provided at the VIT, Vellore, to carry out this work is gratefully acknowledged. Computational support provided at the SDSU (for VST) by DURIP Grant W911NF-10-1-0157 from the U.S. Department of Defense and by NSF CRIF Grant CHE-0947087 is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AdNDP | Adaptive Natural Density Partitioning |
| ADMP | Atom-centered Density Matrix Propagation |
| DFT | Density Functional Theory |
| ELF | Electron Localization Function |
| NBO | Natural Bond Order |
| ON | Occupation Number |
| ptC | Planar Tetracoordinate Carbon |
| phC | Planar Hypercoordinate Carbon |
| ptAl | Planar Tetracoordinate Aluminum |
| ppAl | Planar Pentacoordinate Aluminum |
| WBI | Wiberg Bond Index |
| LP | Lone Pair |
References
- Leyva-Parra, L.; Inostroza, D.; Yañez, O.; Cruz, J.C.; Garza, J.; García, V.; Tiznado, W. Persistent Planar Tetracoordinate Carbon in Global Minima Structures of Silicon-Carbon Clusters. Atoms 2022, 10, 27. [Google Scholar] [CrossRef]
- Das, P.; Khatun, M.; Anoop, A.; Chattaraj, P.K. CSinGe4−n2+ (n = 1–3): Prospective Systems Containing Planar Tetracoordinate Carbon (PtC). Phys. Chem. Chem. Phys. 2022, 24, 16701–16711. [Google Scholar] [CrossRef]
- Yañez, O.; Vásquez-Espinal, A.; Báez-Grez, R.; Rabanal-León, W.A.; Osorio, E.; Ruiz, L.; Tiznado, W. Carbon Rings Decorated with Group 14 Elements: New Aromatic Clusters Containing Planar Tetracoordinate Carbon. New J. Chem. 2019, 43, 6781–6785. [Google Scholar] [CrossRef]
- Yang, L.M.; Ganz, E.; Chen, Z.; Wang, Z.X.; Schleyer, P.V.R. Four Decades of the Chemistry of Planar Hypercoordinate Compounds. Angew. Chem.-Int. Ed. 2015, 54, 9468–9501. [Google Scholar] [CrossRef]
- Merino, G.; Méndez-Rojas, M.A.; Beltrán, H.I.; Corminboeuf, C.; Heine, T.; Vela, A. Theoretical Analysis of the Smallest Carbon Cluster Containing a Planar Tetracoordinate Carbon. J. Am. Chem. Soc. 2004, 126, 16160–16169. [Google Scholar] [CrossRef]
- Pancharatna, P.D.; Méndez-Rojas, M.A.; Merino, G.; Vela, A.; Hoffmann, R. Planar Tetracoordinate Carbon in Extended Systems. J. Am. Chem. Soc. 2004, 126, 15309–15315. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Zhai, H.; Zhou, R.; Ni, G.; Xu, Z. Mass Spectrometric and First Principles Study of AlnC− Clusters. Solid State Commun. 2002, 122, 543–547. [Google Scholar] [CrossRef]
- Jensen, C.M.; Gross, K.J. Development of Catalytically Enhanced Sodium Aluminum Hydride as a Hydrogen-Storage Material. Appl. Phys. A Mater. Sci. Process. 2001, 72, 213–219. [Google Scholar] [CrossRef]
- Leskiw, B.D.; Castleman, A.W. The Interplay between the Electronic Structure and Reactivity of Aluminum Clusters: Model Systems as Building Blocks for Cluster Assembled Materials. Chem. Phys. Lett. 2000, 316, 31–36. [Google Scholar] [CrossRef]
- Suresh, C.H.; Frenking, G. Direct 1−3 Metal−Carbon Bonding and Planar Tetracoordinated Carbon in Group 6 Metallacyclobutadienes. Organometallics 2010, 29, 4766–4769. [Google Scholar] [CrossRef]
- Ghana, P.; Rump, J.; Schnakenburg, G.; Arz, M.I.; Filippou, A.C. Planar Tetracoordinated Silicon (PtSi): Room-Temperature Stable Compounds Containing Anti-van’t Hoff/Le Bel Silicon. J. Am. Chem. Soc. 2021, 143, 420–432. [Google Scholar] [CrossRef]
- Ebner, F.; Greb, L. Calix [4]Pyrrole Hydridosilicate: The Elusive Planar Tetracoordinate Silicon Imparts Striking Stability to Its Anionic Silicon Hydride. J. Am. Chem. Soc. 2018, 140, 17409–17412. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Yu, S.; Ding, Y.H.; Bowen, K.H. Identifying the Hydrogenated Planar Tetracoordinate Carbon: A Combined Experimental and Theoretical Study of CAl4H and CAl4H−. J. Phys. Chem. Lett. 2017, 8, 2263–2267. [Google Scholar] [CrossRef]
- Keese, R. Carbon Flatland: Planar Tetracoordinate Carbon and Fenestranes. Chem. Rev. 2006, 106, 4787–4808. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.-F.; Wang, L.-S.; Geske, G.D.; Boldyrev, A.I. Pentaatomic Tetracoordinate Planar Carbon, [CAl4]2−: A New Structural Unit and Its Salt Complexes. Angew. Chem. 2000, 39, 3630–3632. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.S.; Boldyrev, A.I.; Simons, J. Tetracoordinated Planar Carbon in the Al4C− Anion. A Combined Photoelectron Spectroscopy and Ab Initio Study. J. Am. Chem. Soc. 1999, 121, 6033–6038. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Simons, J. Tetracoordinated Planar Carbon in Pentaatomic Molecules. J. Am. Chem. Soc. 1998, 120, 7967–7972. [Google Scholar] [CrossRef]
- Erker, G. Planar-Tetracoordinate Carbon: Making Stable Anti-van’t Hoff/LeBel Compounds. Comments Inorg. Chem. 1992, 13, 111–131. [Google Scholar] [CrossRef]
- Cotton, F.A.; Millar, M. The Probable Existence of a Triple Bond between Two Vanadium Atoms. J. Am. Chem. Soc. 1977, 99, 7886–7891. [Google Scholar] [CrossRef]
- Röttger, D.; Erker, G. Compounds Containing Planar-Tetracoordinate Carbon. Angew. Chem. Int. Ed. Eng. 1997, 36, 812–827. [Google Scholar] [CrossRef]
- Van’t Hoff, J.H. A Suggestion Looking To the Extension Into Space of the Structural Formulas At Present Used in Chemistry. and a Note Upon the Relation Between the Optical Activity and the Chemical Constitution of Organic Compounds. Arch. Neerl. Des Sci. Exactes Nat. 1874, 9, 445–454. [Google Scholar]
- Le Bel, J.A. On the Relations Which Exist between the Atomic Formulas of Organic Compounds and the Rotatory Power of Their Solutions. Bull. Soc. Chim. Fr. 1874, 22, 337–347. [Google Scholar]
- Vassilev-Galindo, V.; Pan, S.; Donald, K.J.; Merino, G. Planar Pentacoordinate Carbons. Nat. Rev. Chem. 2018, 2, 0114. [Google Scholar] [CrossRef]
- Monkhorst, J.H. Activation Energy for Interconversion of enantiomers containing an asymmetric carbon atom without breaking bonds. Chem. Commun. 1968, 1111–1112. [Google Scholar] [CrossRef]
- Hoffmann, R.; Alder, R.W.; Wilcox, C.F. Planar Tetracoordinate Carbon. J. Am. Chem. Soc. 1970, 92, 4992–4993. [Google Scholar] [CrossRef]
- Collins, J.B.; Dill, J.D.; Jemmis, E.D.; Apeloig, Y.; Schleyer, P.V.R.; Collins, J.B.; Jemmis, E.D.; Schleyer, P.V.R.; Seeger, R.; Pople, J.A. Stabilization of Planar Tetracoordinate Carbon. J. Am. Chem. Soc. 1976, 98, 5419–5427. [Google Scholar] [CrossRef]
- Pei, Y.; An, W.; Ito, K.; Schleyer, P.V.R.; Xiao, C.Z. Planar Pentacoordinate Carbon in CAI5+: A Global Minimum. J. Am. Chem. Soc. 2008, 130, 10394–10400. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; Schleyer, P.V.R.; Chen, Z. Al2C Monolayer: The Planar Tetracoordinate Carbon Global Minimum. Nanoscale 2014, 6, 10784–10791. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, Y.; Chen, Z. Be2C Monolayer with Quasi-Planar Hexacoordinate Carbons: A Global Minimum Structure. Angew. Chem.-Int. Ed. 2014, 53, 7248–7252. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Yakobson, B.I.; Guo, W. Two-Dimensional Tetragonal TiC Monolayer Sheet and Nanoribbons. J. Am. Chem. Soc. 2012, 134, 19326–19329. [Google Scholar] [CrossRef]
- Wu, X.; Pei, Y.; Zeng, X.C. B2C Graphene, Nanotubes, and Nanoribbons. Nano Lett. 2009, 9, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Li, Y.; Chen, Z. Semi-Metallic Be5C2 Monolayer Global Minimum with Quasi-Planar Pentacoordinate Carbons and Negative Poisson’s Ratio. Nat. Commun. 2016, 7, 11488. [Google Scholar] [CrossRef]
- Wang, L.-S.; Boldyrev, A.I.; Li, X.; Simons, J. Experimental Observation of Pentaatomic Tetracoordinate Planar Carbon-Containing Molecules. J. Am. Chem. Soc. 2000, 122, 7681–7687. [Google Scholar] [CrossRef]
- Zhai, H.J.; Wang, L.S.; Alexandrova, A.N.; Boldyrev, A.I. Electronic Structure and Chemical Bonding of B5− and B5 by Photoelectron Spectroscopy and Ab Initio Calculations. J. Chem. Phys. 2002, 117, 7917–7924. [Google Scholar] [CrossRef]
- Wang, L.M.; Huang, W.; Averkiev, B.B.; Boldyrev, A.I.; Wang, L.S. CB7−: Experimental and Theoretical Evidence against Hypercoordinate Planar Carbon. Angew. Chem.-Int. Ed. 2007, 46, 4550–4553. [Google Scholar] [CrossRef]
- Averkiev, B.B.; Zubarev, D.Y.; Wang, L.M.; Huang, W.; Wang, L.S.; Boldyrev, A.I. Carbon Avoids Hypercoordination in CB6−, CB62−, and C2B5− Planar Carbon-Boron Clusters. J. Am. Chem. Soc. 2008, 130, 9248–9250. [Google Scholar] [CrossRef]
- Dong, F.; Heinbuch, S.; Xie, Y.; Rocca, J.J.; Bernstein, E.R. Experimental and Theoretical Study of Neutral AlmCn and AlmCnHx Clusters. Phys. Chem. Chem. Phys. 2010, 12, 2569–2581. [Google Scholar] [CrossRef]
- Sergeeva, A.P.; Piazza, Z.A.; Romanescu, C.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. B22− and B23−: All-Boron Analogues of Anthracene and Phenanthrene. J. Am. Chem. Soc. 2012, 134, 18065–18073. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Wang, P.; Xu, X.-L.; Xu, H.-G.; Zheng, W.-J. Photoelectron Spectroscopy and Theoretical Study of AlnC5−/0 (n = 1–5) Clusters: Structural Evolution, Relative Stability of Star-like Clusters, and Planar Tetracoordinate Carbon Structures. Phys. Chem. Chem. Phys. 2021, 23, 1967–1975. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Lu, H.-G.; Li, S.-D.; Wang, Z.-X. Simplest Neutral Singlet C2E4 (E = Al, Ga, In, and Tl) Global Minima with Double Planar Tetracoordinate Carbons: Equivalence of C2 Moieties in C2E4 to Carbon Centers in CAl42− and CAl5+. J. Phys. Chem. A 2009, 113, 3395–3402. [Google Scholar] [CrossRef]
- Wu, Y.B.; Jiang, J.L.; Lu, H.G.; Wang, Z.X.; Perez-Peralta, N.; Islas, R.; Contreras, M.; Merino, G.; Wu, J.I.C.; Von Ragué Schleyer, P. Starlike Aluminum-Carbon Aromatic Species. Chem. Eur. J. 2011, 17, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.B.; Ding, Y.H. C Al4 X (X=Si,Ge): Molecules with Simultaneous Planar Tetracoordinate Carbon, Aluminum, and Silicon/Germanium. J. Chem. Phys. 2007, 126, 184302. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.J.; Naumkin, F.Y. Communication: A Density Functional Investigation of Structure-Property Evolution in the Tetrakis Hexahedral C4Al14 Nanocluster. J. Chem. Phys. 2014, 141, 131102. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.I.; Simons, J.; Li, X.; Wang, L. Photoelectron Spectroscopy and Ab Initio Study. J. Am. Chem. Soc. 1999, 121, 10193–10197. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, W.; Xu, H.; Xu, X.; Zheng, W. Structural Evolution of Carbon-Doped Aluminum Clusters AlnC− (n = 6–15): Anion Photoelectron Spectroscopy and Theoretical Calculations. J. Phys. Chem. A 2022, 126, 5621–5631. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, F.Y. Flat-Structural Motives in Small Alumino−Carbon Clusters CnAlm (n = 2−3, m = 2−8). J. Phys. Chem. A 2008, 112, 4660–4668. [Google Scholar] [CrossRef]
- Das, P.; Chattaraj, P.K. CSiGaAl2−/0 and CGeGaAl2−/0 Having Planar Tetracoordinate Carbon Atoms in Their Global Minimum Energy Structures. J. Comput. Chem. 2022, 43, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Maatallah, M.; Guo, M.; Cherqaoui, D.; Jarid, A.; Liebman, J.F. Aluminium Clusters for Molecular Hydrogen Storage and the Corresponding Alanes as Fuel Alternatives: A Structural and Energetic Analysis. Int. J. Hydrogen Energy 2013, 38, 5758–5767. [Google Scholar] [CrossRef]
- Dai, J.; Wu, X.; Yang, J.; Zeng, X.C. AlxC Monolayer Sheets: Two-Dimensional Networks with Planar Tetracoordinate Carbon and Potential Applications as Donor Materials in Solar Cell. J. Phys. Chem. Lett. 2014, 5, 2058–2065. [Google Scholar] [CrossRef]
- Job, N.; Khatun, M.; Thirumoorthy, K.; CH, S.S.R.; Chandrasekaran, V.; Anoop, A.; Thimmakondu, V.S. CAl4Mg0/−: Global Minima with a Planar Tetracoordinate Carbon Atom. Atoms 2021, 9, 24. [Google Scholar] [CrossRef]
- Khatun, M.; Roy, S.; Giri, S.; CH, S.S.R.; Anoop, A.; Thimmakondu, V.S. BAl4Mg−/0/+: Global Minima with a Planar Tetracoordinate or Hypercoordinate Boron Atom. Atoms 2021, 9, 89. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- A/RES/71/313. Available online: https://web.archive.org/web/20201128194012/https://undocs.org/A/RES/71/313 (accessed on 7 October 2022).
- Tian, Y.H.; Hu, S.; Sheng, X.; Duan, Y.; Jakowski, J.; Sumpter, B.G.; Huang, J. Non-Transition-Metal Catalytic System for N2 Reduction to NH3: A Density Functional Theory Study of Al-Doped Graphene. J. Phys. Chem. Lett. 2018, 9, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, D.; Sun, W.; Han, Z.; Liu, C. A Novel Aluminum-Doped Carbon Nanotubes Sensor for Carbon Monoxide. J. Mol. Struct. THEOCHEM 2007, 806, 93–97. [Google Scholar] [CrossRef]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas Adsorption on Graphene Doped with B, N, Al, and S: A Theoretical Study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Compaan, K.R.; Agarwal, J.; Dye, B.E.; Yamaguchi, Y.; Schaefer, H.F. Toward Detection of AlCH2 and AlCH2+ in the Interstellar Medium. Astrophys. J. 2013, 778, 125–130. [Google Scholar] [CrossRef]
- Janczyk, A.; Ziurys, L.M. Laboratory Detection and Pure Rotational Spectrum of AlSH (X∼1A’). Astrophys. J. 2006, 639, L107–L110. [Google Scholar] [CrossRef][Green Version]
- Yousefi, M.; Bernath, P.F. Line Lists for AlF and AlCl in the X 1Σ+ Ground State. Astrophys. J. Suppl. Ser. 2018, 237, 8. [Google Scholar] [CrossRef]
- Hedderich, H.G.; Dulick, M.; Bernath, P.F. High Resolution Emission Spectroscopy of AlCl at 20 μ. J. Chem. Phys. 1993, 99, 8363–8370. [Google Scholar] [CrossRef]
- Hoeft, J.; Lovas, F.J.; Tiemann, E.; Törring, T. Microwave Absorption Spectra of AlF, GaF, InF, and TIF. Z. Naturforsch. A 1970, 25, 1029–1035. [Google Scholar] [CrossRef]
- Tenenbaum, E.D.; Ziurys, L.M. Exotic Metal Molecules in Oxygen-Rich Envelopes: Detection of AlOH (X 1Σ+) in VY Canis Majoris. Astrophys. J. Lett. 2010, 712, 93–97. [Google Scholar] [CrossRef]
- Walker, K.A.; Gerry, M.C. Laboratory Microwave Spectroscopy of Aluminium Cyanide. Chem. Phys. Lett. 1999, 301, 200–204. [Google Scholar] [CrossRef]
- Ma, B.; Yamaguchi, Y.; Schaefer, H.F. Spectroscopic Constants and Potential Energy Surfaces for the Possible Interstellar Molecules A1NC and A1CN. Mol. Phys. 1995, 86, 1331–1337. [Google Scholar] [CrossRef]
- Ziurys, L.M.; Savage, C.; Highberger, J.L.; Apponi, A.J.; Guélin, M.; Cernicharo, J. More Metal Cyanide Species: Detection of AlNC (X 1Σ+) toward IRC +10216. Astrophys. J. 2002, 564, L45–L48. [Google Scholar] [CrossRef]
- Tenenbaum, E.D.; Ziurys, L.M. Millimeter Detection of Alo (X 2Σ+): Metal Oxide Chemistry in the Envelope of VY Canis Majoris. Astrophys. J. 2009, 694, 59–63. [Google Scholar] [CrossRef]
- Kamiński, T.; Müller, H.S.P.; Schmidt, M.R.; Cherchneff, I.; Wong, K.T.; Brünken, S.; Menten, K.M.; Winters, J.M.; Gottlieb, C.A.; Patel, N.A. An Observational Study of Dust Nucleation in Mira (o Ceti). Astron. Astrophys. 2017, 599, A59. [Google Scholar] [CrossRef]
- Gobrecht, D.; Plane, J.M.C.; Bromley, S.T.; Decin, L.; Cristallo, S.; Sekaran, S. Bottom-up Dust Nucleation Theory in Oxygen-Rich Evolved Stars I. Aluminium Oxide Clusters. Astron. Astrophys. 2021, 658, A167. [Google Scholar] [CrossRef]
- Kramers, J.D.; Belyanin, G.A.; Przybyłowicz, W.J.; Winkler, H.; Andreoli, M.A.G. The Chemistry of the Extraterrestrial Carbonaceous Stone “Hypatia”: A Perspective on Dust Heterogeneity in Interstellar Space. Icarus 2022, 382, 115043. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-Augmented Basis Sets for Anion Calculations. III. The 3-21+G Basis Set for First-Row Elements, Li-F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. ”Developing Paradigms of Chemical Bonding: Adaptive Natural Density Partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, D.Y.; Boldyrev, A.I. Revealing Intuitively Assessable Chemical Bonding Patterns in Organic Aromatic Molecules via Adaptive Natural Density Partitioning. J. Org. Chem. 2008, 73, 9251–9258. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Weinhold, F. Natural Resonance Theory: I. General Formalism. J. Comput. Chem. 1998, 19, 593–609. [Google Scholar] [CrossRef]
- Von Ragué Schleyer, P.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Schlegel, H.B.; Millam, J.M.; Iyengar, S.S.; Voth, G.A.; Daniels, A.D.; Scuseria, G.E.; Frisch, M.J. Ab Initio Molecular Dynamics: Propagating the Density Matrix with Gaussian Orbitals. J. Chem. Phys. 2001, 114, 9758–9763. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).