Emission of Fast Hydrogen Atoms in a Low Density Gas Discharge—The Most “Natural” Mirror Laboratory
Abstract
:1. Introduction
2. Fast Atoms at the Plasma–Solid Interface
2.1. Source of Fast Atoms at the Mirror
2.2. Source of Emission of Fast Atoms
3. Experimental Setup at the Linear Plasma Device PSI-2
4. Emission of Fast Reflected Atoms and Measurements of Reflectance
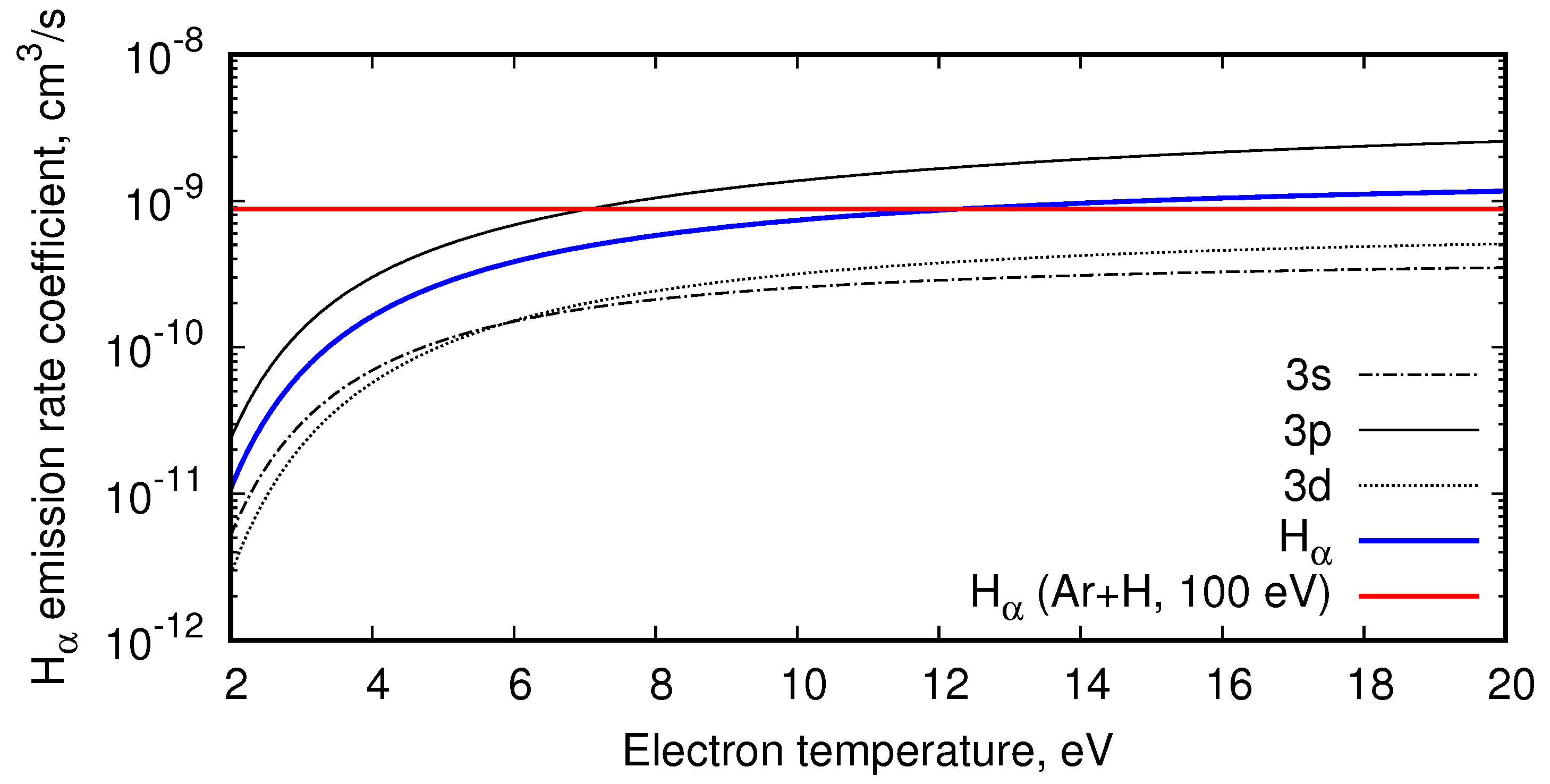
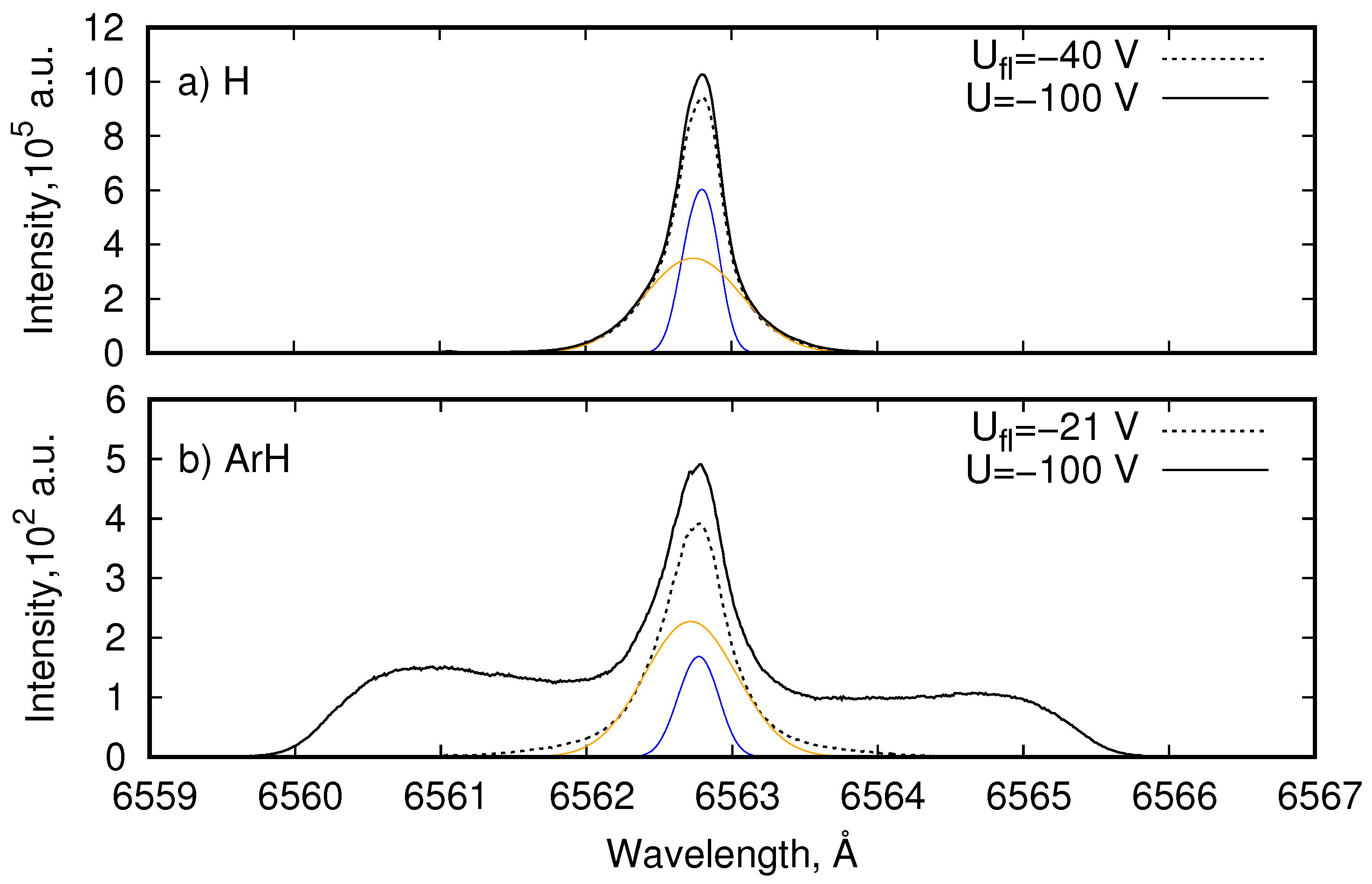
4.1. Balmer- Line Emission in H and ArH Plasmas
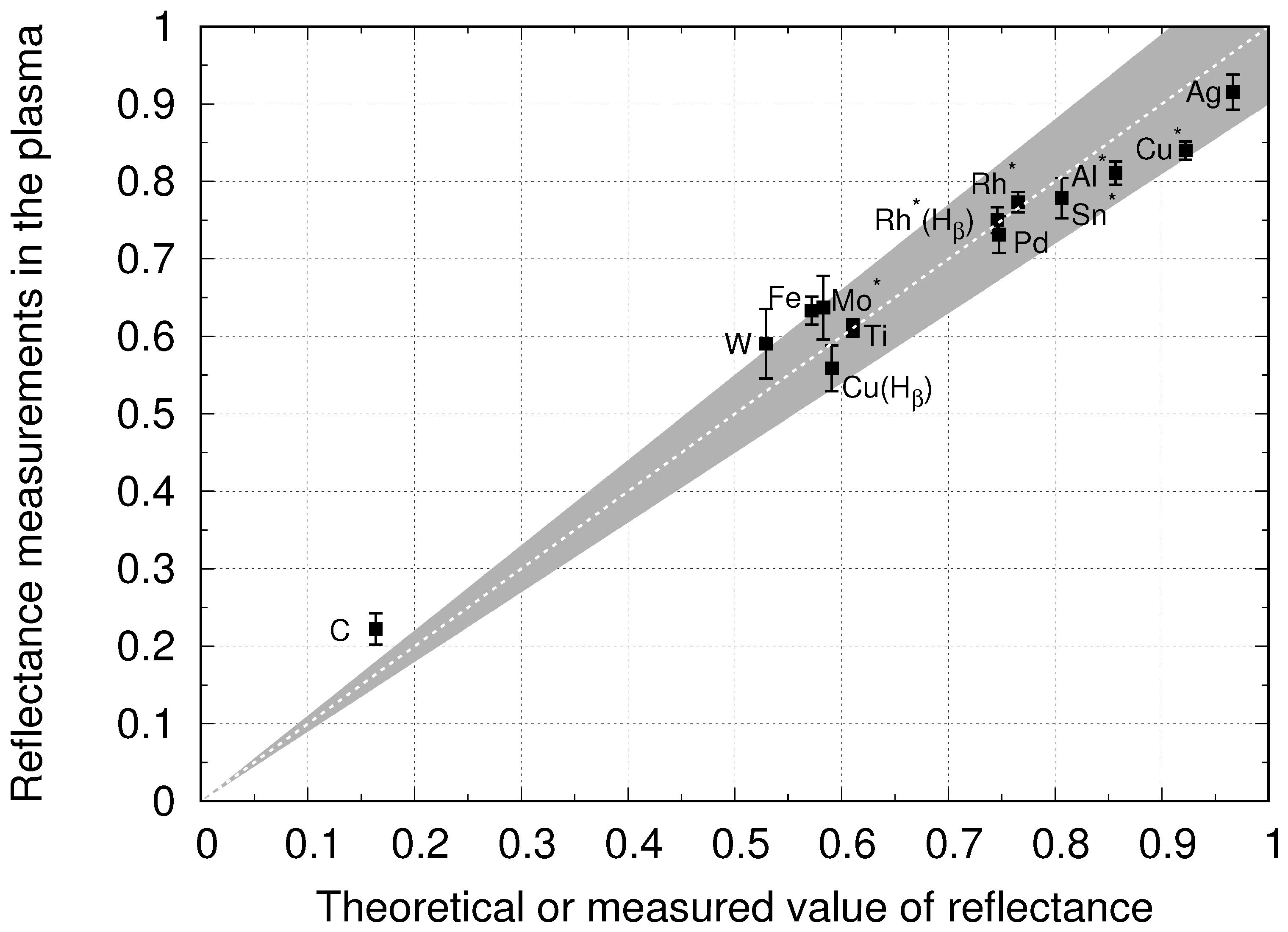
4.2. Reflectance Measurements at PSI-2
4.3. Polarization of Balmer Lines by Reflection on Metallic Surfaces
4.4. Comparison of Emission in ArH, KrH, and NeH Plasma
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchuk, O.; Brandt, C.; Pospieszczyk, A. Verfahren zur Bestimmung der Oberflächeneigenschaften von Targets. German Patent DE102016002270B3, 10 August 2017. [Google Scholar]
- Dickheuer, S.; Marchuk, O.; Brandt, C.; Pospieszczyk, A.; Goriaev, A.; Ialovega, M.; Göths, B.; Krasikov, Y.; Krimmer, A.; Mertens, P.; et al. In-situ measurements of the spectral reflectance of metallic mirrors at the Hα line in a low density Ar-H plasma. Rev. Sci. Instrum. 2018, 89, 063112. [Google Scholar] [CrossRef] [PubMed]
- NRL Plasma Formulary; NRL/PU/6790-18-640; Plasma Physics Division, Naval Research Laboratory: Washington, DC, USA, 2018.
- Stangeby, P.C. The Plasma Boundary of Magnetic Fusion Devices; IoP Publishing Ltd.: Bristol, UK; Philadelphia, PA, USA, 2000; p. 73. [Google Scholar]
- Videnović, I.R.; Konjević, N.; Kuraica, M.M. Spectroscopic investigations of a cathode fall region of the Grimm-type glow discharge. Spectrochim. Acta Part B 1996, 51, 1707–1731. [Google Scholar] [CrossRef]
- Verbeek, H. Backscattering of light ions from metal surfaces. In Symposium on Radiation Effects on Solid Surfaces; Kaminsky, M., Ed.; American Chemical Society: Washington, DC, USA, 1976; pp. 245–261. [Google Scholar]
- Smith, D.P. Analysis of surface composition with low-energy backscattered ions. Surf. Sci. 1976, 25, 171–191. [Google Scholar] [CrossRef]
- Aratari, R.; Eckstein, W. Backscattering of atomic and molecular hydrogen from nickel and carbon. Nucl. Instrum. Methods Phys. Res. B 1989, 42, 11–18. [Google Scholar] [CrossRef]
- Van Zyl, B.; Neumann, H.; Rothwell, H.L.; Amme, R.C. Balmer-α and Balmer-β emission cross-sections for H+Ar collisions. Phys. Rev. A 1980, 21, 716. [Google Scholar] [CrossRef]
- Van Zyl, B.; Gealy, M.W.; Neumann, H. Balmer-α and Balmer-β emission cross-sections for low-energy H collisions with He and H2. Phys. Rev. A 1983, 28, 176. [Google Scholar] [CrossRef]
- Van Zyl, B.; Gealy, M.W.; Neumann, H. Excitation of low-energy H atoms in H + Ne collisions. Phys. Rev. A 1985, 31, 2922. [Google Scholar] [CrossRef]
- Van Zyl, B.; Neumann, H.; Gealy, M.W. Balmer-line emission from low-energy H impact on Kr and Xe. Phys. Rev. A 1986, 33, 2093. [Google Scholar] [CrossRef]
- Van Zyl, B.; Gealy, M.W. Lyman-α emission from low-energy H impact on rare-gas atoms. Phys. Rev. A 1987, 35, 3741. [Google Scholar] [CrossRef]
- Eckstein, W. Reflection (Backscattering). Garching: Max-Planck-Institut für Plasmaphysik, 2009. Available online: https://pure.mpg.de/rest/items/item_2141005_1/component/file_2141004/content (accessed on 11 November 2014).
- Bray, I.; Ralchenko, Y. CCC Data Base. 1997. Available online: http://atom.curtin.edu.au/CCC-WWW/ (accessed on 11 November 2014).
- Herschkowitz, N. Sheaths: More complicated than you think. Phys. Plasma 2005, 12, 055502. [Google Scholar] [CrossRef]
- Kreter, A.; Brandt, C.; Huber, A.; Kraus, S.; Möller, S.; Reinhart, M.; Schweer, B.; Sergienko, G.; Unterberg, B. Linear plasma device PSI-2 for plasma-material interaction studies. Fusion Sci. Technol. 2015, 68, 8–14. [Google Scholar] [CrossRef]
- Dickheuer, S.; Marchuk, O.; Krasikov, Y.; Mertens, P.; Brandt, C.; Ertmer, S.; Börner, P.; Reiter, D.; Göths, B.; von Bovert, K.; et al. Polarization by light reflection at metallic surfaces observed in the shape of the Balmer-α line of low density plasmas. Phys. Plasma 2019, 26, 073513. [Google Scholar] [CrossRef]
- Dickheuer, S.; Marchuk, O.; Tsankov, T.V.; Luggenhølscher, D.; Czarnetzki, U.; Gromelski, W.; Ertmer, S.; Kreter, A. Measurement of the Magnetic Field in a Linear Magnetized Plasma by Tunable Diode Laser Absorption Spectroscopy. Atoms 2019, 7, 48. [Google Scholar] [CrossRef]
- Brandt, C.; Marchuk, O.; Pospieszczyk, A.; Dickheuer, S. Emission of fast non-Maxwellian hydrogen atoms in low-density laboratory plasma. AIP Conf. Proc. 2017, 1811, 130001. [Google Scholar]
- Marchuk, O.; Brandt, C.; Pospieszczyk, A.; Reinhart, M.; Brezinsek, S.; Unterberg, B.; Dickheuer, S. Emission of fast hydrogen atoms at a plasma-solid interface in a low density plasma containing noble gases. J. Phys. B At. Mol. Opt. Phys. 2018, 51, 025702. [Google Scholar] [CrossRef]
- Waldmann, O.; Meyer, H.; Fussmann, G. Anomalous diffusion in a linear plasma generator. Contrib. Plasma Phys. 2007, 47, 691–702. [Google Scholar] [CrossRef]
- Severn, G.; Yip, C.-S.; Hershkowitz, N.; Baalrud, S.D. Experimental studies of ion flow near the sheath edge in multiple ion species plasma including argon, xenon and neon. Plasma Sources Sci. Technol. 2017, 26, 055021. [Google Scholar] [CrossRef]
- Mertens, P. The Core-Plasma CXRS Diagnostic for ITER: An Introduction to the Current Design. J. Fusion Energy 2019, 38, 264–282. [Google Scholar] [CrossRef]
- Polyanskiy, M.N. Refractive Index Database. Available online: https://refractiveindex.info (accessed on 26 June 2019).
- Werner, W.S.M.; Glantschnig, K.; Ambrosch-Draxl, C. Optical constants and inelastic electron-scattering data for 17 elemental metals. J. Phys. Chem. Ref. Data 2009, 38, 1013–1092. [Google Scholar] [CrossRef]
- Merten, P.; Boman, R.; Dickheuer, S.; Krasikov, Y.; Krimmer, A.; Leichtle, D.; Liegeois, K.; Linsmeier, C.; Litnovsky, A.; Marchuk, O.; et al. On the use of rhodium mirrors for optical diagnostics in ITER. Fusion Eng. Des. 2019. [Google Scholar] [CrossRef]
- Minissale, M.; Pardanaud, C.; Bisson, R.; Gallais, L. The temperature dependence of optical properties of tungsten in the visible and near-infrared domains: An experimental and theoretical study. J. Phys. D Appl. Phys. 2017, 50, 455601. [Google Scholar] [CrossRef]
- Fujimoto, T.; Iwamae, A. Plasma Polarization Spectroscopy; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Rakic, A.D.; Djurišic, A.B.; Elazar, J.M.; Majewski, M.L. Optical properties of metallic films for vertical-cavity optoelectronic devices. Appl. Opt. 1998, 37, 5271–5283. [Google Scholar] [CrossRef]
- Phelps, A.V. Energetic ion, atom, and molecule reactions and excitation in low-current H2 discharges: Model. Phys. Rev. E 2009, 79, 066401. [Google Scholar] [CrossRef]
- Phelps, A.V. Collisional kinetics of non-uniform electric field, low-pressure, direct-current discharges in H2. Plasma Sources Sci. Technol. 2011, 20, 043001. [Google Scholar] [CrossRef]
- Lavrov, B.P.; Melnikov, A.S. Observation of charge-exchange effects from the Doppler profiles of Balmer lines in H2+Ne, Ar and Kr hollow-cathode discharges. Optika i Spektroskopiya 1993, 75, 1152–1163. [Google Scholar]
- Clyne, M.A.A.; Heaven, M.C.; Bayes, K.D.; Monkhouse, P. Energy transfer in collisions of excited Ar(3P0,2) metastable atoms with H(2S) atoms. II. Lyman-α emission profile. Chem. Phys. 1980, 47, 179–188. [Google Scholar] [CrossRef]
- Brooks, N.H.; Isler, R.C.; Whyte, D.G.; Fenstermacher, M.E.; Groebner, R.J.; Stangeby, P.C.; Heidbrink, W.W.; Jackson, G.L.; Mahdavi, M.A.; West, W.P.; et al. Measured signatures of low energy, physical sputtering in the line shape of neutral carbon emission. J. Nucl. Mater. 2005, 337–339, 227–231. [Google Scholar] [CrossRef]
- Šišović, N.M.; Majstorović, G.L.; Konjević, N. Excessive Doppler broadening of the Hα line in a hollow cathode glow discharge. Eur. Phys. J. D 2007, 41, 143–150. [Google Scholar] [CrossRef]
- Babkina, T.; Gans, T.; Czarnetzki, U. Energy analysis of hyperthermal hydrogen atoms generated through surface neutralisation of ions. Europhys. Lett. 2005, 72, 235. [Google Scholar] [CrossRef]
- Banerjee, S.; Vasu, P.; von Hellermann, M.; Jaspers, R.J.E. Wall reflection modeling for charge exchange recombination spectroscopy (CXRS) measurements on Textor and ITER. Plasma Phys. Contr. Fusion 2010, 52, 125006. [Google Scholar] [CrossRef]
- Schunke, B.; Huysmans, G.; Thomas, P. Evidence of the influence of reflections on the Zeff profile measurements and their mitigation. In Proceedings of the 31st EPS Conference on Plasma Physics, London, UK, 28 June–2 July 2004. [Google Scholar]
- Hjartarson, A.T.; Thorsteinsson, E.G.; Gudmundsson, J.T. Low pressure hydrogen discharges diluted with argon explored using a global model. Plasma Sources Sci. Technol. 2010, 19, 065008. [Google Scholar] [CrossRef]
- Dickheuer, S.; Marchuk, O.; Ertmer, S.; Goriaev, A.; Ialovega, M.; Göths, B.; Krasikov, Y.; Mertens, P.; Kreter, A. In-situ measurement of the spectral reflectance of mirror-like metallic surfaces during plasma exposition. Nucl. Mater. Energy 2018, 17, 302–306. [Google Scholar] [CrossRef]
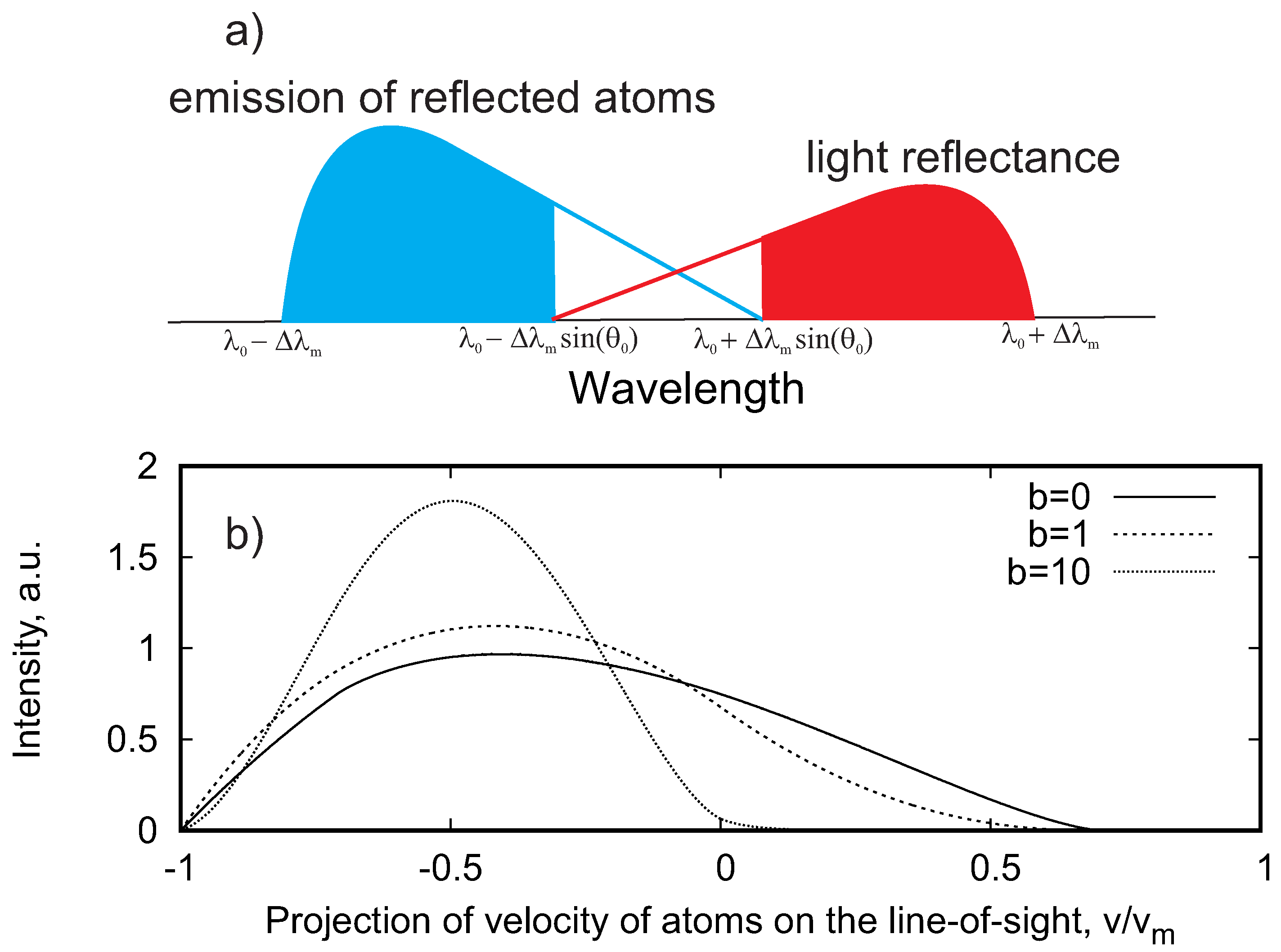
| 1 | The emission of reflected atoms appeared also at the red-shifted wavelength and vice versa for all angles with 90 > > 0; however, we refer to the blue-shifted emission for the direct signal and to the red-shifted one for the light reflectance |








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchuk, O.; Dickheuer, S.; Ertmer, S.; Krasikov, Y.; Mertens, P.; Brandt, C.; Brezinsek, S.; Goriaev, A.; Ialovega, M.; Göths, B.; et al. Emission of Fast Hydrogen Atoms in a Low Density Gas Discharge—The Most “Natural” Mirror Laboratory. Atoms 2019, 7, 81. https://doi.org/10.3390/atoms7030081
Marchuk O, Dickheuer S, Ertmer S, Krasikov Y, Mertens P, Brandt C, Brezinsek S, Goriaev A, Ialovega M, Göths B, et al. Emission of Fast Hydrogen Atoms in a Low Density Gas Discharge—The Most “Natural” Mirror Laboratory. Atoms. 2019; 7(3):81. https://doi.org/10.3390/atoms7030081
Chicago/Turabian StyleMarchuk, Oleksandr, Sven Dickheuer, Stephan Ertmer, Yuri Krasikov, Philippe Mertens, Christian Brandt, Sebastijan Brezinsek, Andrei Goriaev, Mykola Ialovega, Beatrix Göths, and et al. 2019. "Emission of Fast Hydrogen Atoms in a Low Density Gas Discharge—The Most “Natural” Mirror Laboratory" Atoms 7, no. 3: 81. https://doi.org/10.3390/atoms7030081
APA StyleMarchuk, O., Dickheuer, S., Ertmer, S., Krasikov, Y., Mertens, P., Brandt, C., Brezinsek, S., Goriaev, A., Ialovega, M., Göths, B., Kreter, A., & Linsmeier, C. (2019). Emission of Fast Hydrogen Atoms in a Low Density Gas Discharge—The Most “Natural” Mirror Laboratory. Atoms, 7(3), 81. https://doi.org/10.3390/atoms7030081






