The Ovarian Transcriptome of Reproductively Aged Multiparous Mice: Candidate Genes for Ovarian Cancer Protection †
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Study Scheme, and Sample Collection
2.2. Count of Ovarian Follicles
2.3. Microarray Profiling of Mouse Ovaries
2.4. Analysis of Transcriptomic Data
2.5. Determination of Circulating Chemokines
2.6. Quantitative RT-PCR Assays
2.7. Statistics
3. Results
3.1. Study Scheme and Validation of Platforms
3.2. Remnant Follicles in Aged Mouse Ovaries of Divergent Parity History
3.3. The Differential Mouse Ovarian Transcriptome: Age and Parity Comparisons
3.4. Gene Ontology Profile of the Aged Multiparous Mouse Ovary
3.4.1. Follicle and Oocyte Homeostasis
3.4.2. Cell Immunity and Inflammation
3.4.3. Transcriptional Regulation
3.4.4. Cell Death
3.5. Correlation of Follicle Counts with the Ovarian Transcriptome
3.6. Parity-Dependent Serum Cytokine Levels and Correlation with the Ovarian Transcriptome
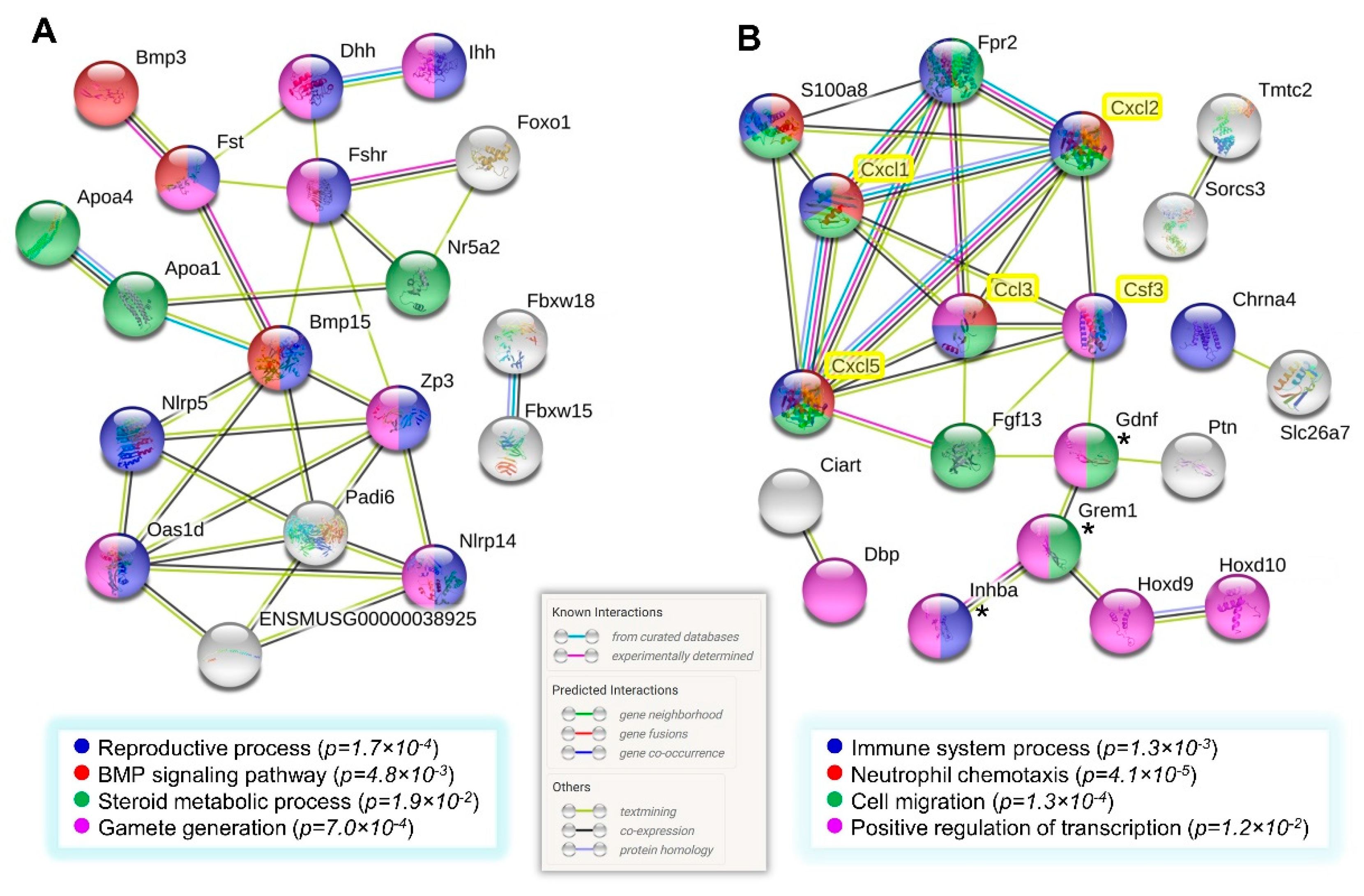
3.7. Network Analysis of Gene Clusters of Higher Expression in the Multiparous Ovary
4. Discussion
4.1. Evidence of Remnant Follicles in the Aged Multiparous Ovary
4.2. Former Parity Might Sustain Residual Steroidogenesis in the Aged Ovaries
4.3. Transcriptional Control in the Aged Multiparous Ovary
4.4. Parity History Improves the Ovarian and Systemic Immune-Chemotactic Activity of Aged Mice
4.5. Apoptotic Activity in the Aged Multiparous Mouse Ovary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Van der Hyden, B.C. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res. 2005, 322, 117–124. [Google Scholar] [CrossRef]
- Smith, E.R.; Xu, X.X. Ovarian ageing, follicle depletion, and cancer: A hypothesis for the aetiology of epithelial ovarian cancer involving follicle depletion. Lancet Oncol. 2008, 9, 1108–1111. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, K.Q.; Smith, E.R.; Yeasky, T.M.; Moore, R.; Ganjei-Azar, P.; Klein-Szanto, A.J.; Godwin, A.K.; Hamilton, T.C.; Xu, X.X. Follicle Depletion Provides a Permissive Environment for Ovarian Carcinogenesis. Mol. Cell. Biol. 2016, 36, 2418–2430. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.M.; Wimberger, P. Cells of origin of ovarian cancer: Ovarian surface epithelium or fallopian tube? Arch. Gynecol. Obstet. 2017, 296, 1055–1062. [Google Scholar] [CrossRef]
- Cai, K.Q.; Wang, Y.; Smith, E.R.; Smedberg, J.L.; Yang, D.H.; Yang, W.L.; Xu, X.X. Global deletion of Trp53 reverts ovarian tumor phenotype of the germ cell-deficient white spotting variant (Wv) mice. Neoplasia 2015, 17, 89–100. [Google Scholar] [CrossRef]
- Smith, E.R.; Wang, Y.; Xu, X.X. Development of a mouse model of menopausal ovarian cancer. Front. Oncol. 2014, 4, 36. [Google Scholar] [CrossRef]
- Scully, R.E. Pathology of ovarian cancer precursors. J. Cell. Biochem. 1995, 59, 208–218. [Google Scholar] [CrossRef]
- Banet, N.; Kurman, R.J. Two types of ovarian cortical inclusion cysts: Proposed origin and possible role in ovarian serous carcinogenesis. Int. J. Gynecol. Pathol. 2015, 34, 3–8. [Google Scholar] [CrossRef]
- Hunn, J.; Rodriguez, G.C. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef]
- Fougère, B.; Boulanger, E.; Nourhashémi, F.; Guyonnet, S.; Cesari, M. Chronic Inflammation: Accelerator of Biological Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Faddy, M.J.; Gosden, R.G. A model conforming the decline in follicle numbers to the age of menopause in women. Hum. Reprod. 1996, 11, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Moini, A.; Hedayatshodeh, M.; Hosseini, R.; Rastad, H. Association between parity and ovarian reserve in reproductive age women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Zhou, C.; Zhong, Y.; Kuan, P.F.; Fan, C.; Sampey, B.P.; Difurio, M.; Bae-Jump, V.L. Obesity increases tumor aggressiveness in a genetically engineered mouse model of serous ovarian cancer. Gynecol. Oncol. 2014, 133, 90–97. [Google Scholar] [CrossRef]
- Jia, D.; Nagaoka, Y.; Katsumata, M.; Orsulic, S. Inflammation is a key contributor to ovarian cancer cell seeding. Sci. Rep. 2018, 8, 12394. [Google Scholar] [CrossRef]
- Li, C.; Course, M.M.; McNeish, I.A.; Drescher, C.W.; Valdmanis, P.N.; Lieber, A. Prophylactic in vivo hematopoietic stem cell gene therapy with an immune checkpoint inhibitor reverses tumor growth in syngeneic mouse tumor models. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Urzua, U.; Chacon, C.; Lizama, L.; Sarmiento, S.; Villalobos, P.; Kroxato, B.; Marcelain, K.; Gonzalez, M.J. Parity History Determines a Systemic Inflammatory Response to Spread of Ovarian Cancer in Naturally Aged Mice. Aging Dis. 2017, 8, 546–557. [Google Scholar] [CrossRef][Green Version]
- Griffin, J.; Emery, B.R.; Huang, I.; Peterson, C.M.; Carrell, D.T. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J. Exp. Clin. Assist. Reprod. 2006, 3, 2. [Google Scholar] [CrossRef]
- Sharov, A.A.; Falco, G.; Piao, Y.; Poosala, S.; Becker, K.G.; Zonderman, A.B.; Longo, D.L.; Schlessinger, D.; Ko, M.S. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol. 2008, 6, 24. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Urzua, U.; Chacon, C.; Espinoza, R.; Martínez, S.; Hernandez, N. Parity-Dependent Hemosiderin and Lipofuscin Accumulation in the Reproductively Aged Mouse Ovary. Anal. Cell. Pathol. 2018, 2018, 1289103. [Google Scholar] [CrossRef] [PubMed]
- Malaquin, N.; Martinez, A.; Rodier, F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016, 82, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.A.; Li, X.; Umans, L.; Zwijsen, A.; Huylebroeck, D.; Gutierrez, C.; Wang, D.; Martin, J.F.; Jamin, S.P.; Behringer, R.R.; et al. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol. Cell. Biol. 2008, 28, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, J.W.; Wang, J.; Ma, L.; Martin, J.F.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. Bmp2 is critical for the murine uterine decidual response. Mol. Cell. Biol. 2007, 27, 5468–5478. [Google Scholar] [CrossRef] [PubMed]
- Finco, I.; LaPensee, C.R.; Krill, K.T.; Hammer, G.D. Hedgehog signaling and steroidogenesis. Annu. Rev. Physiol. 2015, 77, 105–129. [Google Scholar] [CrossRef]
- Wu, R.; Van der Hoek, K.H.; Ryan, N.K.; Norman, R.J.; Robker, R.L. Macrophage contributions to ovarian function. Hum. Reprod. Update 2004, 10, 119–133. [Google Scholar] [CrossRef]
- Turner, E.C.; Hughes, J.; Wilson, H.; Clay, M.; Mylonas, K.J.; Kipari, T.; Duncan, W.C.; Fraser, H.M. Conditional ablation of macrophages disrupts ovarian vasculature. Reproduction 2011, 141, 821–831. [Google Scholar] [CrossRef]
- Pepe, G.; Locati, M.; Della Torre, S.; Mornata, F.; Cignarella, A.; Maggi, A.; Vegeto, E. The estrogen-macrophage interplay in the homeostasis of the female reproductive tract. Hum. Reprod. Update 2018, 24, 652–672. [Google Scholar] [CrossRef]
- Hijikata, A.; Kitamura, H.; Kimura, Y.; Yokoyama, R.; Aiba, Y.; Bao, Y.; Fujita, S.; Hase, K.; Hori, S.; Ishii, Y.; et al. Construction of an open-access database that integrates cross-reference information from the transcriptome and proteome of immune cells. Bioinformatics 2007, 23, 2934–2941. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Lee, E.S.; Son, D.S. Potential Roles of Innate Immune Chemokine and Cytokine Network on Lipopolysaccharide-Based Therapeutic Approach in Ovarian Cancer. Immune Netw. 2019, 19, e22. [Google Scholar] [CrossRef]
- Gougeon, A.; Ecochard, R.; Thalabard, J.C. Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol. Reprod. 1994, 50, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Pangas, S.A. Regulation of the ovarian reserve by members of the transforming growth factor beta family. Mol. Reprod. Dev. 2012, 79, 666–679. [Google Scholar] [CrossRef]
- Knight, P.G.; Satchell, L.; Glister, C. Intra-ovarian roles of activins and inhibins. Mol. Cell. Endocrinol. 2012, 359, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, A.; Laszczyńska, M.; Brodowski, J.; Masiuk, M.; Starczewski, A. Analysis of pituitary gonadotropin concentration in blood serum and immunolocalization and immunoexpression of follicle stimulating hormone and luteinising hormone receptors in ovaries of postmenopausal women. Histol. Histopathol. 2012, 27, 241–248. [Google Scholar]
- Brodowska, A.; Brodowski, J.; Laszczyńska, M.; Słuczanowska-Głąbowska, S.; Rumianowski, B.; Rotter, I.; Starczewski, A.; Ratajczak, M.Z. Immunoexpression of aromatase cytochrome P450 and 17β-hydroxysteroid dehydrogenase in women’s ovaries after menopause. J. Ovarian Res. 2014, 7, 52. [Google Scholar] [CrossRef]
- Jabara, S.; Christenson, L.K.; Wang, C.Y.; McAllister, J.M.; Javitt, N.B.; Dunaif, A.; Strauss, J.F., 3rd. Stromal cells of the human postmenopausal ovary display a distinctive biochemical and molecular phenotype. J. Clin. Endocrinol. Metab. 2003, 88, 484–492. [Google Scholar] [CrossRef]
- Reame, N.E.; Lukacs, J.L.; Olton, P.; Ansbacher, R.; Padmanabhan, V. Differential effects of aging on activin A and its binding protein, follistatin, across the menopause transition. Fertil. Steril. 2007, 88, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Niu, W.; Wang, Y.; Teng, Z.; Wen, J.; Xia, G.; Wang, C. Follistatin288 Regulates Germ Cell Cyst Breakdown and Primordial Follicle Assembly in the Mouse Ovary. PLoS ONE 2015, 10, e0129643. [Google Scholar] [CrossRef] [PubMed]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Genet. 2018, 35, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- von Schönfeldt, V.; Chandolia, R.; Ochsenkühn, R.; Nieschlag, E.; Kiesel, L.; Sonntag, B. FSH prevents depletion of the resting follicle pool by promoting follicular number and morphology in fresh and cryopreserved primate ovarian tissues following xenografting. Reprod. Biol. Endocrinol. 2012, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Wu, H.; Pei, X.; Chang, Q.; Ma, W.; Ma, H.; Hei, C.; Zheng, X.; Cai, Y.; et al. The Increased Expression of Connexin and VEGF in Mouse Ovarian Tissue Vitrification by Follicle Stimulating Hormone. BioMed Res. Int. 2015, 2015, 397264. [Google Scholar] [CrossRef]
- Adashi, E.Y. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil. Steril. 1994, 62, 20–27. [Google Scholar] [CrossRef]
- Brodowski, J.; Brodowska, A.; Laszczyńska, M.; Chlubek, D.; Starczewski, A. Hormone concentrations in the homogenates of ovarian tissue and blood serum in postmenopausal women not using hormone therapy. Gynecol. Endocrinol. 2012, 28, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Fogle, R.H.; Stanczyk, F.Z.; Zhang, X.; Paulson, R.J. Ovarian androgen production in postmenopausal women. J. Clin. Endocrinol. Metab. 2007, 92, 3040–3043. [Google Scholar] [CrossRef] [PubMed]
- Hassa, H.; Aydin, Y.; Ozatik, O.; Erol, K.; Ozatik, Y. Effects of dehydroepiandrosterone (DHEA) on follicular dynamics in a diminished ovarian reserve in vivo model. Syst. Biol. Reprod. Med. 2015, 61, 117–121. [Google Scholar] [CrossRef]
- Martins, R.; Lithgow, G.J.; Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef]
- Herndon, M.K.; Law, N.C.; Donaubauer, E.M.; Kyriss, B.; Hunzicker-Dunn, M. Forkhead box O member FOXO1 regulates the majority of follicle-stimulating hormone responsive genes in ovarian granulosa cells. Mol. Cell. Endocrinol. 2016, 434, 116–126. [Google Scholar] [CrossRef]
- Shi, F.; LaPolt, P.S. Relationship between FoxO1 protein levels and follicular development, atresia, and luteinization in the rat ovary. J. Endocrinol. 2003, 179, 195–203. [Google Scholar] [CrossRef]
- Shen, M.; Liu, Z.; Li, B.; Teng, Y.; Zhang, J.; Tang, Y.; Sun, S.C.; Liu, H. Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis. 2014, 5, e1475. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rudd, M.D.; Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Fan, H.Y.; Zeleznik, A.J.; Richards, J.S. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol. Endocrinol. 2009, 23, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, K.; Gossen, J.; Schoonjans, K.; Murphy, B.D. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology 2014, 155, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Nechamen, C.A.; Thomas, R.M.; Dias, J.A. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol. Cell. Endocrinol. 2007, 260–262, 93–99. [Google Scholar] [CrossRef]
- Sun, X.F.; Sun, X.H.; Cheng, S.F.; Wang, J.J.; Feng, Y.N.; Zhao, Y.; Yin, S.; Hou, Z.M.; Shen, W.; Zhang, X.F. Interaction of the transforming growth factor-β and Notch signaling pathways in the regulation of granulosa cell proliferation. Reprod. Fertil. Dev. 2016, 28, 1873–1881. [Google Scholar] [CrossRef]
- Vanorny, D.A.; Prasasya, R.D.; Chalpe, A.J.; Kilen, S.M.; Mayo, K.E. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol. Endocrinol. 2014, 28, 499–511. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Madonna, R.; Melino, G.; Caruso, C. The emerging role of Notch pathway in ageing: Focus on the related mechanisms in age-related diseases. Ageing Res. Rev. 2016, 29, 50–65. [Google Scholar] [CrossRef]
- Carlock, C.; Wu, J.; Zhou, C.; Ross, A.; Adams, H.; Lou, Y. Ovarian phagocyte subsets and their distinct tissue distribution patterns. Reproduction 2013, 146, 491–500. [Google Scholar] [CrossRef]
- Rajarathnam, K.; Schnoor, M.; Richardson, R.M.; Rajagopal, S. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019, 54, 69–80. [Google Scholar] [CrossRef]
- Hol, J.; Wilhelmsen, L.; Haraldsen, G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J. Leukoc. Biol. 2010, 87, 501–508. [Google Scholar] [CrossRef]
- Luo, W.; Salih, S.M.; Bormann, C.L.; Wiltbank, M.C. Induction of chemokines and prostaglandin synthesis pathways in luteinized human granulosa cells: Potential role of luteotropin withdrawal and prostaglandin F2α in regression of the human corpus luteum. Reprod. Biol. 2015, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.W.; Kyme, P.A.; Arruda, A.; Ramanujan, V.K.; Tawackoli, W.; Liu, G.Y. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PLoS ONE 2012, 7, e41454. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, A.L.; Kovacs, E.J. G-CSF enhances resolution of Staphylococcus aureus wound infection in an age-dependent manner. Shock 2013, 40, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, A.L.; Rendon, J.L.; Ramirez, L.; Choudhry, M.A.; Kovacs, E.J. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J. Immunol. 2013, 190, 1746–1757. [Google Scholar] [CrossRef]
- Ryckman, C.; Vandal, K.; Rouleau, P.; Talbot, M.; Tessier, P.A. Proinflammatory activities of S100: Proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003, 170, 3233–3242. [Google Scholar] [CrossRef]
- Okada, K.; Arai, S.; Itoh, H.; Adachi, S.; Hayashida, M.; Nakase, H.; Ikemoto, M. CD68 on rat macrophages binds tightly to S100A8 and S100A9 and helps to regulate the cells’ immune functions. J. Leukoc. Biol. 2016, 100, 1093–1104. [Google Scholar] [CrossRef]
- Swindell, W.R.; Johnston, A.; Xing, X.; Little, A.; Robichaud, P.; Voorhees, J.J.; Fisher, G.; Gudjonsson, J.E. Robust shifts in S100a9 expression with aging: A novel mechanism for chronic inflammation. Sci. Rep. 2013, 3, 1215. [Google Scholar] [CrossRef]
- Yui, S.; Nakatani, Y.; Mikami, M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol. Pharm. Bull. 2003, 26, 753–760. [Google Scholar] [CrossRef]
- Moles, A.; Murphy, L.; Wilson, C.L.; Chakraborty, J.B.; Fox, C.; Park, E.J.; Mann, J.; Oakley, F.; Howarth, R.; Brain, J.; et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J. Hepatol. 2014, 60, 782–791. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef]
- De Buck, M.; Gouwy, M.; Berghmans, N.; Opdenakker, G.; Proost, P.; Struyf, S.; Van Damme, J. COOH-terminal SAA1 peptides fail to induce chemokines but synergize with CXCL8 and CCL3 to recruit leukocytes via FPR2. Blood 2018, 131, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Filep, J.G.; Sekheri, M.; El Kebir, D. Targeting formyl peptide receptors to facilitate the resolution of inflammation. Eur. J. Pharmacol. 2018, 833, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Grandjenette, C.; Dicato, M.; Diederich, M. Roles of Apoptosis and Cellular Senescence in Cancer and Aging. Curr. Drug Targets 2016, 17, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, A.; Laszczyńska, M.; Starczewski, A. Apoptosis in ovarian cells in postmenopausal women. Folia Histochem. Cytobiol. 2007, 45, 99–105. [Google Scholar] [PubMed]
- Thieblemont, N.; Witko-Sarsat, V.; Ariel, A. Regulation of macrophage activation by proteins expressed on apoptotic neutrophils: Subversion towards autoimmunity by proteinase 3. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12990. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin: Formation, distribution, and metabolic consequences. Ann. N. Y. Acad. Sci. 2007, 1119, 97–111. [Google Scholar] [CrossRef] [PubMed]




| Gene (alias) | Context (PMIDs) a | Ovarian Expression (This Work) |
|---|---|---|
| FST (follistatin) | Residual steroidogenesis; stroma (12519894) | Yes b; ↑ multiparous |
| GREM1 (gremlin−1) | Residual steroidogenesis; stroma (12519894) | Yes; ↑ multiparous |
| LHCGR (LH receptor) | Detected in OSE, cysts and stroma (22207559, 16253961) | No c; ↑ aged |
| FSHR (FSH receptor) | Detected in OSE, cysts and stroma (22207559) | Yes; ↑ multiparous |
| HSD3B (3β-hydroxysteroid dehydrogenase) | Detected in the cortical stroma (2643063) | NDE d |
| PR (progesterone receptor) | Detected in OSE, cysts and stroma (19056530, 20552551). | NDE |
| ESR1 (estrogen receptor alpha) | Detected in OSE, cysts and stroma (18165170, 20552551). | NDE |
| CYP19A1 (aromatase) | Detected in OSE, cysts, stroma and vascular endothelium (24855493) | NDE |
| HSD17B1 (17β-hydroxysteroid dehydrogenase) | Detected in OSE, cysts, stroma and vascular endothelium (24855493) | Yes, ↑ multiparous |
| AR (androgen receptor) | Detected in the OSE, cysts and stroma (20552551) | NDE |
| APCS (serum amyloid P component) | Differential pre-/post-menopausal proteome (25037597) | No; ↑ young (*) |
| HSPB1 (Hsp27) | Differential pre-/post-menopausal proteome (25037597) | NDE |
| GLO1 (glyoxalase-I) | Differential pre-/post-menopausal proteome (25037597) | NDE |
| UCHL1 (ubiquitin C-terminal hydrolase L1) | Differential pre-/post-menopausal proteome (25037597) | No; ↑ young |
| Functional Theme | GO Terms Associated | Genes |
|---|---|---|
| Follicle and oocyte homeostasis | Ovarian follicle development Germ cell development TGF-beta receptor binding BMP signaling Steroid metabolic process | Bmp15, Fshr, Inhba, Oas1d, Dhh, Ihh, Zp3, Gdnf, Bmp3, Fst, Grem1, Hsd17b1, Apoa1, Apoa4, and Nr5a2 |
| Cell immunity and inflammation | Regulation of inflammatory response Positive regulation of immune system process Chemotaxis Cell migration | Apoa1, S100a8, Zp3, Fpr2, Ihh, Inhba, Masp1, Rftn2, Sema5a, Fgf13, Gdnf, Grem1, and Efna5 |
| Transcription | Positive regulation of transcription, DNA-templated Transcription factor activity, sequence-specific DNA binding | Bmp15, Bmp3, Cdk5rap2, Dbp, Foxo1, Gdnf, Grem1, Hey2, Hoxd10, Hoxd9, Ihh, Inhba, Nr5a2, and Zp3 |
| Cell death | Cell death Positive regulation of apoptotic process | Nlrp5, S100a8, Serpina3g, Foxo1, Inhba, and Ptn |
| Gene | Fold Change b | Role in Ovarian Biology | PMID |
|---|---|---|---|
| Nlrp14 | 6.6 | Innate immunity of oocytes | 28423339 |
| Slc38a5 | 5.2 | Paralog Slc38a3 is expressed in adult mouse granulosa cells | 23083410 |
| Padi6 | 5.1 | Part of cytoplasmic lattice and cytoskeletal sheets in oocytes | 17587491, 27929740 |
| Nlrp5 | 4.6 | ER, calcium and mitochondrial homeostasis of oocytes | 24374158, 22357545 |
| Cpsf4l | 2.9 | Expressed in adult mouse granulosa cells | 23083410 |
| E330034G19Rik | 2.8 | Oocyte specific in mouse; meiotic maturation | 17567914 |
| Hey2 | 2.7 | Notch target involved in oocyte-follicle growth balance | 24552588 |
| Fbxw15 | 2.4 | Oocyte specific. Follicle assembly and early follicle growth | 18094359 |
| Serpine2 | 2.2 | Cumulus cell marker of oocyte maturation | 23082142 |
| Efna5 | 2.0 | Regulates proliferation and apoptosis of granulosa cells | 29619874 |
| Kcne2 | 1.9 | Expressed in adult mouse granulosa cells | 23083410 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urzúa, U.; Chacón, C.; Norambuena, M.; Lizama, L.; Sarmiento, S.; Asaki, E.; Powell, J.I.; Ampuero, S. The Ovarian Transcriptome of Reproductively Aged Multiparous Mice: Candidate Genes for Ovarian Cancer Protection. Biomolecules 2020, 10, 113. https://doi.org/10.3390/biom10010113
Urzúa U, Chacón C, Norambuena M, Lizama L, Sarmiento S, Asaki E, Powell JI, Ampuero S. The Ovarian Transcriptome of Reproductively Aged Multiparous Mice: Candidate Genes for Ovarian Cancer Protection. Biomolecules. 2020; 10(1):113. https://doi.org/10.3390/biom10010113
Chicago/Turabian StyleUrzúa, Ulises, Carlos Chacón, Maximiliano Norambuena, Luis Lizama, Sebastián Sarmiento, Esther Asaki, John I Powell, and Sandra Ampuero. 2020. "The Ovarian Transcriptome of Reproductively Aged Multiparous Mice: Candidate Genes for Ovarian Cancer Protection" Biomolecules 10, no. 1: 113. https://doi.org/10.3390/biom10010113
APA StyleUrzúa, U., Chacón, C., Norambuena, M., Lizama, L., Sarmiento, S., Asaki, E., Powell, J. I., & Ampuero, S. (2020). The Ovarian Transcriptome of Reproductively Aged Multiparous Mice: Candidate Genes for Ovarian Cancer Protection. Biomolecules, 10(1), 113. https://doi.org/10.3390/biom10010113





