Activation of mGluR5 and NMDA Receptor Pathways in the Rostral Ventrolateral Medulla as a Central Mechanism for Methamphetamine-Induced Pressor Effect in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Blood Pressure and Heart Rate
2.3. Intracerebroventricular Cannula Implantation
2.4. Microinjection into the RVLM
2.5. Microdialysis and HPLC for Detect Glutamate Concentration in the RVLM
2.6. Western Blotting for Detecting Fos protein (Fos) Expression and Phosphorylated GluN1
2.7. Determination of PKC Activity
2.8. Data Analysis
2.9. Chemical Agents
3. Results
3.1. i.p. MA-Induced a Pressor Effect in Conscious Free-Moving Rats
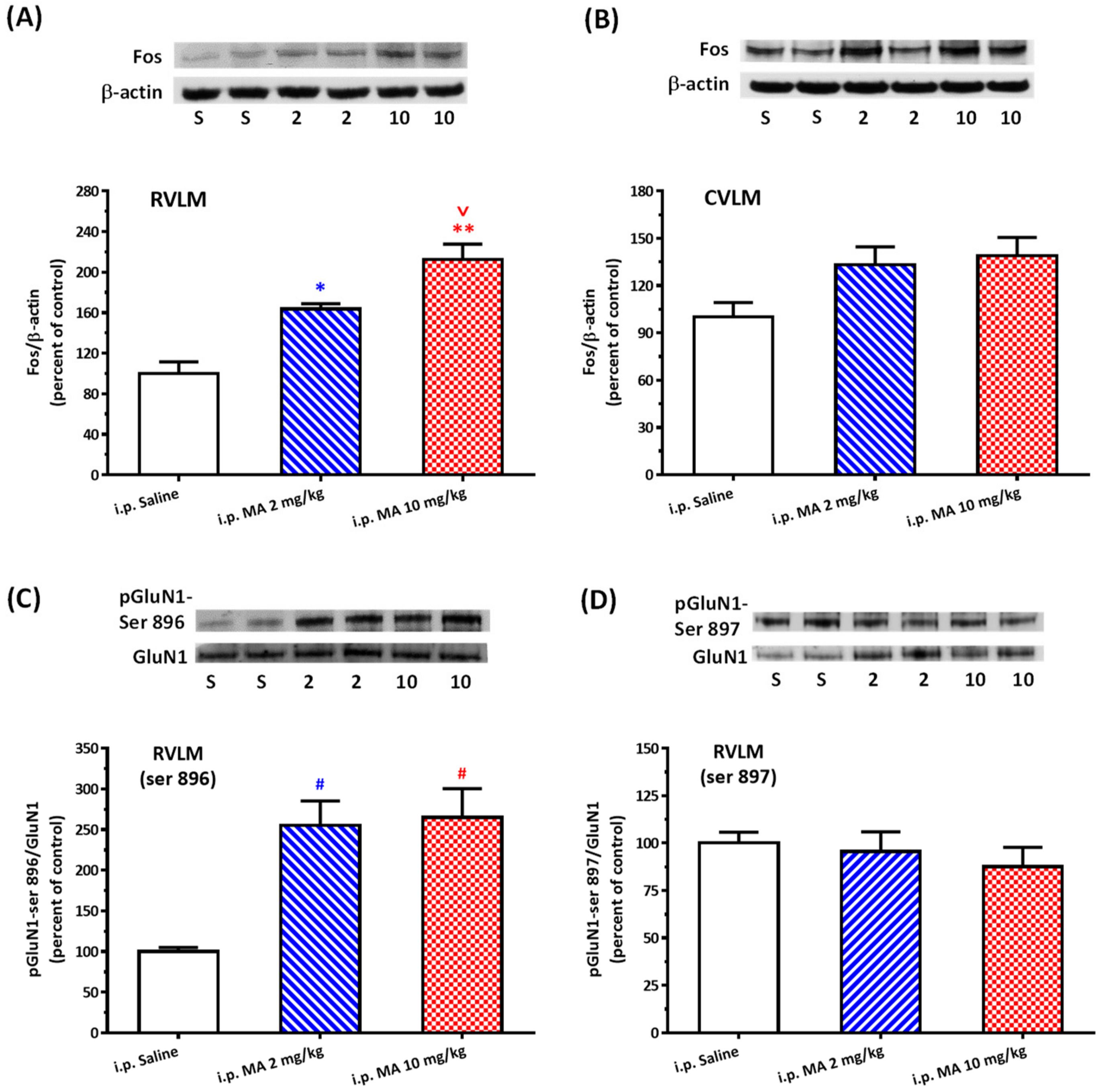
3.2. i.p. MA Increased FOS Expression and Phosphorylation of GluN1-ser 896 in the RVLM in Conscious Free-Moving Rats
3.3. i.c.v. MA-Induced a Pressor Effect in Urethane-Anesthetized Rats
3.4. i.c.v. MA Increased FOS Expression and Phosphorylation of GluN1-ser 896 in the RVLM in Urethane-Anesthetized Rats
3.5. MA Increased PKC Activity in the RVLM
3.6. The PKC Inhibitor Attenuated MA-Induced Increase in MAP and pGluN1-ser 896
3.7. i.c.v. Administration of MA increased the Glutamate Concentration in the RVLM
3.8. The mGluR5 Antagonist Attenuated MA-Induced Increase in MAP and pGluN1-ser 896
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, L.; Shen, Q.; Bai, X.; Di, X. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav. Neurol. 2015, 2015, 103969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, K.E.; Ray, L.A. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014, 143, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paratz, E.D.; Cunningham, N.J.; MacIsaac, A.I. The Cardiac Complications of Methamphetamines. Heart Lung Circ. 2016, 25, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef]
- Seiden, L.S.; Sabol, K.E.; Ricaurte, G.A. Amphetamine: Effects on catecholamine systems and behavior. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 639–677. [Google Scholar] [CrossRef]
- Schindler, C.W.; Zheng, J.W.; Tella, S.R.; Goldberg, S.R. Pharmacological mechanisms in the cardiovascular effects of methamphetamine in conscious squirrel monkeys. Pharmacol. Biochem. Behav. 1992, 42, 791–796. [Google Scholar] [CrossRef]
- Polesskaya, O.; Silva, J.; Sanfilippo, C.; Desrosiers, T.; Sun, A.; Shen, J.; Feng, C.; Polesskiy, A.; Deane, R.; Zlokovic, B.; et al. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011, 1373, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.L.; Gunderson, E.W.; Perez, A.; Kirkpatrick, M.G.; Thurmond, A.; Comer, S.D.; Foltin, R.W. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology 2008, 33, 1847–1855. [Google Scholar] [CrossRef]
- Riviere, G.J.; Gentry, W.B.; Owens, S.M. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J. Pharmacol. Exp. Ther. 2000, 292, 1042–1047. [Google Scholar]
- Li, F.C.; Yen, J.C.; Chan, S.H.; Chang, A.Y. Defunct brain stem cardiovascular regulation underlies cardiovascular collapse associated with methamphetamine intoxication. J. Biomed. Sci. 2012, 19, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Sved, A.F.; Ito, S.; Yajima, Y. Role of excitatory amino acid inputs to the rostral ventrolateral medulla in cardiovascular regulation. Clin. Exp. Pharmacol. Physiol. 2002, 29, 503–506. [Google Scholar] [CrossRef]
- Sun, M.K.; Reis, D.J. NMDA receptor-mediated sympathetic chemoreflex excitation of RVL-spinal vasomotor neurones in rats. J. Physiol. 1995, 482, 53–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Q.; Guo, M.L.; Jin, D.Z.; Xue, B.; Fibuch, E.E.; Mao, L.M. Roles of subunit phosphorylation in regulating glutamate receptor function. Eur. J. Pharmacol. 2014, 728, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.H.; Cheng, T.T.; Lo, H.; Lin, Y.C.; Lai, C.C. Spontaneously hypertensive rats exhibit higher sensitivity to ethanol-induced hypotensive effects: Role of NMDA receptors and nitric oxide in rostral ventrolateral medulla. Alcohol 2018, 73, 25–35. [Google Scholar] [CrossRef]
- Lai, C.C.; Yuan, Z.F.; Chu, L.Y.; Chuang, K.T.; Lin, H.H. Roles of cocaine- and amphetamine-regulated transcript peptide in the rostral ventrolateral medulla in cardiovascular regulation in rats. Brain Res. 2019, 1710, 117–124. [Google Scholar] [CrossRef]
- Lo, H.; Lin, H.H.; Chen, J.K.; Situmorang, J.H.; Lai, C.C. Involvement of NMDA Receptors, Nitric Oxide, and GABA in Rostral Ventrolateral Medulla in Acute Ethanol-Induced Cardiovascular Responses in Rats. Alcohol Clin. Exp. Res. 2018, 42, 1418–1430. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Lin, H.H.; Chang, S.J.; Shie, H.J.; Lai, C.C. Ethanol inhibition of NMDA-induced responses and acute tolerance to the inhibition in rat rostral ventrolateral medulla in vivo: Involvement of cAMP-dependent protein kinases. Neuropharmacology 2006, 51, 747–755. [Google Scholar] [CrossRef]
- Lai, C.C.; Lo, H.; Lin, H.G.; Lin, H.H. Potentiation of NMDA-Mediated Responses by Amyloid-beta Peptide 1-40 in Rat Sympathetic Preganglionic Neurons. J. Alzheimers Dis. 2019, 67, 1291–1303. [Google Scholar] [CrossRef]
- Chen, B.S.; Roche, K.W. Regulation of NMDA receptors by phosphorylation. Neuropharmacology 2007, 53, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benquet, P.; Gee, C.E.; Gerber, U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J. Neurosci. 2002, 22, 9679–9686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varner, K.J.; Daigle, K.; Weed, P.F.; Lewis, P.B.; Mahne, S.E.; Sankaranarayanan, A.; Winsauer, P.J. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology 2013, 225, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Owens, S.M.; Gentry, W.B. Intravenous (+)-methamphetamine causes complex dose-dependent physiologic changes in awake rats. Eur. J. Pharmacol. 2001, 426, 81–87. [Google Scholar] [CrossRef]
- Schindler, C.W.; Gilman, J.P.; Graczyk, Z.; Wang, G.; Gee, W.L. Reduced cardiovascular effects of methamphetamine following treatment with selegiline. Drug Alcohol Depend. 2003, 72, 133–139. [Google Scholar] [CrossRef]
- Hassan, S.F.; Wearne, T.A.; Cornish, J.L.; Goodchild, A.K. Effects of acute and chronic systemic methamphetamine on respiratory, cardiovascular and metabolic function, and cardiorespiratory reflexes. J. Physiol. 2016, 594, 763–780. [Google Scholar] [CrossRef] [Green Version]
- Melega, W.P.; Cho, A.K.; Harvey, D.; Lacan, G. Methamphetamine blood concentrations in human abusers: Application to pharmacokinetic modeling. Synapse 2007, 61, 216–220. [Google Scholar] [CrossRef]
- Northrop, N.A.; Yamamoto, B.K. Methamphetamine effects on blood-brain barrier structure and function. Front. Neurosci. 2015, 9, 69. [Google Scholar] [CrossRef]
- Gulaboski, R.; Cordeiro, M.N.; Milhazes, N.; Garrido, J.; Borges, F.; Jorge, M.; Pereira, C.M.; Bogeski, I.; Morales, A.H.; Naumoski, B.; et al. Evaluation of the lipophilic properties of opioids, amphetamine-like drugs, and metabolites through electrochemical studies at the interface between two immiscible solutions. Anal. Biochem. 2007, 361, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Loscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005, 76, 22–76. [Google Scholar] [CrossRef]
- Mengler, L.; Khmelinskii, A.; Diedenhofen, M.; Po, C.; Staring, M.; Lelieveldt, B.P.; Hoehn, M. Brain maturation of the adolescent rat cortex and striatum: Changes in volume and myelination. Neuroimage 2014, 84, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Mark, K.A.; Quinton, M.S.; Russek, S.J.; Yamamoto, B.K. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J. Neurosci. 2007, 27, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, R.; Luo, J.; He, L.; Liu, J.; Zhang, J. Increased Absolute Glutamate Concentrations and Glutamate-to-Creatine Ratios in Patients with Methamphetamine Use Disorders. Front. Psychiatry 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Jin, Y.; Liu, X.; Yang, L.; Ge, Z.; Wang, H.; Li, J.; Zheng, J. Methamphetamine modulates glutamatergic synaptic transmission in rat primary cultured hippocampal neurons. Brain Res. 2014, 1582, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dhami, G.K.; Ferguson, S.S. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol. Ther. 2006, 111, 260–271. [Google Scholar] [CrossRef]
- Ferraguti, F.; Crepaldi, L.; Nicoletti, F. Metabotropic glutamate 1 receptor: Current concepts and perspectives. Pharmacol. Rev. 2008, 60, 536–581. [Google Scholar] [CrossRef]
- Schoepp, D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001, 299, 12–20. [Google Scholar]
- Tsuchihashi, T.; Liu, Y.; Kagiyama, S.; Matsumura, K.; Abe, I.; Fujishima, M. Metabotropic glutamate receptor subtypes involved in cardiovascular regulation in the rostral ventrolateral medulla of rats. Brain Res. Bull. 2000, 52, 279–283. [Google Scholar] [CrossRef]
- Perry, C.J.; Zbukvic, I.; Kim, J.H.; Lawrence, A.J. Role of cues and contexts on drug-seeking behaviour. Br. J. Pharmacol. 2014, 171, 4636–4672. [Google Scholar] [CrossRef] [Green Version]
- Stocker, S.D.; Simmons, J.R.; Stornetta, R.L.; Toney, G.M.; Guyenet, P.G. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J. Comp. Neurol. 2006, 494, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koba, S.; Hanai, E.; Kumada, N.; Kataoka, N.; Nakamura, K.; Watanabe, T. Sympathoexcitation by hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla. J. Physiol. 2018, 596, 4581–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.J.; Dang, D.K.; Hwang, Y.G.; Tran, H.Q.; Sharma, N.; Jeong, J.H.; Jang, C.G.; Nah, S.Y.; Nabeshima, T.; Yoneda, Y.; et al. Significance of protein kinase C in the neuropsychotoxicity induced by methamphetamine-like psychostimulants. Neurochem. Int. 2019, 124, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Scheefhals, N.; MacGillavry, H.D. Functional organization of postsynaptic glutamate receptors. Mol. Cell. Neurosci. 2018, 91, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-C.; Fang, C.; Kuo, C.-Y.; Wu, Y.-W.; Lin, H.-H. Activation of mGluR5 and NMDA Receptor Pathways in the Rostral Ventrolateral Medulla as a Central Mechanism for Methamphetamine-Induced Pressor Effect in Rats. Biomolecules 2020, 10, 149. https://doi.org/10.3390/biom10010149
Lai C-C, Fang C, Kuo C-Y, Wu Y-W, Lin H-H. Activation of mGluR5 and NMDA Receptor Pathways in the Rostral Ventrolateral Medulla as a Central Mechanism for Methamphetamine-Induced Pressor Effect in Rats. Biomolecules. 2020; 10(1):149. https://doi.org/10.3390/biom10010149
Chicago/Turabian StyleLai, Chih-Chia, Chi Fang, Chung-Yi Kuo, Ya-Wen Wu, and Hsun-Hsun Lin. 2020. "Activation of mGluR5 and NMDA Receptor Pathways in the Rostral Ventrolateral Medulla as a Central Mechanism for Methamphetamine-Induced Pressor Effect in Rats" Biomolecules 10, no. 1: 149. https://doi.org/10.3390/biom10010149
APA StyleLai, C.-C., Fang, C., Kuo, C.-Y., Wu, Y.-W., & Lin, H.-H. (2020). Activation of mGluR5 and NMDA Receptor Pathways in the Rostral Ventrolateral Medulla as a Central Mechanism for Methamphetamine-Induced Pressor Effect in Rats. Biomolecules, 10(1), 149. https://doi.org/10.3390/biom10010149




