Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
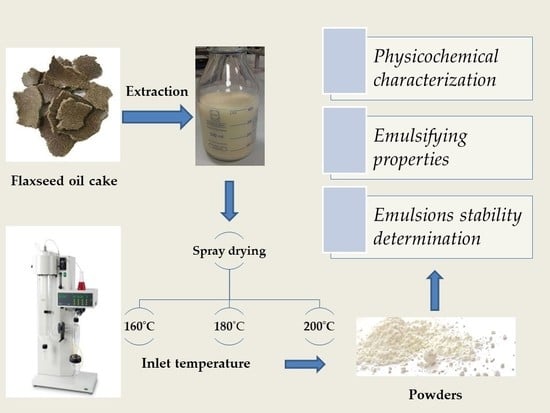
2.2. Preparation of Flaxseed Oil Cake Extract (FOCE)
2.3. Preparation of Spray-Dried FOCE Powders
2.4. Determination of Total Solids Content, Solubility, and Water Activity of Spray-Dried FOCE Powders
2.5. Determination of Water-Holding and Oil-Binding Capacities
2.6. SEM Observations
2.7. Determination of Sulfhydryl Groups (–SH) and Disulfide Bonds (–S–S–) Contents
2.8. FTIR Analysis of Powders
2.9. Determination of Powders Antioxidant Activity
2.10. Emulsifying Properties of Powders and Emulsions Characterization
2.11. Determination of Emulsions Particles Size Distribution
2.12. Emulsions Optical Microscopic Examination
2.13. Powders and Emulsions Color Measurements
2.14. Statistical Analysis
3. Results and Discussion
3.1. The Proximate Composition of FOCE
3.2. The Changes of Water-Holding Capacity, Oil-Binding Capacity, Solubility, Dry Matter Content, Water Activity, Free Sulphydryl Groups, and Disulfide Bonds Contents
3.3. The Changes of Powders Antioxidant Activity
3.4. The Changes in Powders Chemical Composition
3.5. The Change of Powders Color
3.6. Powders Surface Morphology
3.7. Emulsifying Activity of Powders and Emulsions Stability
3.8. Emulsions Color
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-Like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Papadaki, A.; Kopsahelis, N.; Mallouchos, A.; Mandala, I.; Koutinas, A.A. Bioprocess development for the production of novel oleogels from soybean and microbial oils. Food Res. Int. 2019, 126, 108684. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł. Waste from the harvesting of button mushroom (Agaricus bisporus) as a source of natural melanin. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2018, 343, 23–42. [Google Scholar] [CrossRef]
- Turon, X.; Venus, J.; Arshadi, M.; Koutinas, M.; Lin, C.S.K.; Koutinas, A. Food waste and byproduct valorization through bio-processing: Opportunities and challenges. BioResources 2014, 9, 5774–5777. [Google Scholar] [CrossRef]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2019, 16, 1–19. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Day, L. Proteins from land plants - Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- United Nations. Department of Economic and Social Affairs Population Dynamics. Available online: https://population.un.org/wpp/ (accessed on 14 January 2020).
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of canola and flaxseed protein isolates produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2991–2998. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Wang, L.J.; Özkan, N. Effect of concentrated flaxseed protein on the stability and rheological properties of soybean oil-in-water emulsions. J. Food Eng. 2010, 96, 555–561. [Google Scholar] [CrossRef]
- Dickinson, E. Milk protein adsorbed layers and the relationship to emulsion stability and rheology. Stud. Surf. Sci. Catal. 2001, 132, 973–978. [Google Scholar]
- Joshi, H.C.; Pandey, I.P.; Kumar, A.; Gar, N. A study of various factors determining the stability of molecules. Adv. Pure Appl. Chem. 2012, 1, 7–11. [Google Scholar]
- Gong, K.-J.; Shi, A.-M.; Liu, H.-Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Suresh Kumar, P.; Choudhary, V.K.; Devi, P.; Kanwat, M.; Sangeetha, A. Influence of Processing Variables and Storage on the Fruit Drink Developed from Taktir; Wild Fruit of Himalaya. Proc. Natl. Acad. Sci. India Sect. B: Biol. Sci. 2015, 85, 767–775. [Google Scholar] [CrossRef]
- Dzuvor, C.; Taylor, J.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of Functional Ingredients from Flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef]
- Krause, J.P.; Schultz, M.; Dudek, S. Effect of extraction conditions on composition, surface activity and rheological properties of protein isolates from flaxseed (Linum usitatissimum L). J. Sci. Food Agric. 2002, 82, 970–976. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and flaxseed cake as a source of compounds for food industry. J. Soil Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Anjum, F.M.; Butt, M.S.; Siddiq, M.; Sheikh, M.A. Reduction of cyanogenic compounds in flaxseed (Linum usitatissimum L.) meal using thermal treatment. Int. J. Food Prop. 2013, 16, 1809–1818. [Google Scholar] [CrossRef] [Green Version]
- Roozegar, M.H.; Shahedi, M.; Keramet, J.; Hamdami, N.; Roshanak, S. Effect of coated and uncoated ground flaxseed addition on rheological, physical and sensory properties of Taftoon bread. J. Food Sci. Technol. 2015, 52, 5102–5110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Goli, S.A.H.; Sedaghat Doost, A.; Dewettinck, K.; Van der Meeren, P. Bioparticles of flaxseed protein and mucilage enhance the physical and oxidative stability of flaxseed oil emulsions as a potential natural alternative for synthetic surfactants. Colloids Surf. B Biointerfaces 2019, 184, 110489. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Optimization of a spray drying process for flaxseed gum. Int. J. Food Sci. Technol. 2001, 36, 135–143. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G.; Cui, W. Optimization of protein extraction from flaxseed meal. Food Res. Int. 1994, 27, 355–361. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flaxseed proteins—A review. Food Chem. 1993, 48, 109–114. [Google Scholar] [CrossRef]
- Feng, D.; Shen, Y.; Chavez, E.R. Effectiveness of different processing methods in reducing hydrogen cyanide content of flaxseed. J. Sci. Food Agric. 2003, 83, 836–841. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess. Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Chung, M.W.Y.; Lei, B.; Li-Chan, E.C.Y. Isolation and structural characterization of the major protein fraction from NorMan flaxseed (Linum usitatissimum L.). Food Chem. 2005, 90, 271–279. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Functional Properties of Acylated Flax Protein Isolates. J. Agric. Food Chem. 1997, 45, 2431–2441. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.-J.; Li, S.-J.; Adhikari, B. Effects of drying methods on the functional properties of flaxseed gum powders. Carbohydr. Polym. 2010, 81, 128–133. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The effect of native and denaturated flaxseed meal extract on physiochemical properties of low fat mayonnaises. Food Meas. 2020, 1–11. [Google Scholar] [CrossRef]
- Martínez-Flores, H.E.; Barrera, E.S.; Garnica-Romo, M.G.; Penagos, C.J.C.; Saavedra, J.P.; Macazaga-Alvarez, R. Functional characteristics of protein flaxseed concentrate obtained applying a response surface methodology. J. Food Sci. 2006, 71, 495–498. [Google Scholar] [CrossRef]
- Claussen, I.C.; Strømmen, I.; Egelandsdal, B.; Strætkvern, K.O. Effects of drying methods on functionality of a native potato protein concentrate. Dry. Technol. 2007, 25, 1091–1098. [Google Scholar] [CrossRef]
- Chegini, G.; HamidiSepehr, A.; Dizaji, M.F.; Mirnezami, S.V. Study of physical and chemical properties of spray drying whey powder. Int. J. Recycl. Org. Waste Agric. 2014. [Google Scholar] [CrossRef] [Green Version]
- Koca, N.; Erbay, Z.; Kaymak-Ertekin, F. Effects of spray-drying conditions on the chemical, physical, and sensory properties of cheese powder. J. Dairy Sci. 2015, 98, 2934–2943. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Rogers, S.; Selomulya, C.; Chen, X.D. Functionality of milk protein concentrate: Effect of spray drying temperature. Biochem. Eng. J. 2012, 62, 101–105. [Google Scholar] [CrossRef]
- Goa, J. A Micro Biuret Method for Protein Determination Determination of Total Protein in Cerebrospinal Fluid. Scand. J. Clin. Lab. Investig. 1953, 5, 218–222. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Tong, T.; Liu, Y.-J.; Kang, J.; Zhang, C.-M.; Kang, S.-G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Jędra, F.; Mizielińska, M. New Poly (lactic acid) Active Packaging Composite Films Incorporated with Fungal Melanin. Polymers 2018, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Cameron, D.R.; Weber, M.E.; Idziak, E.S.; Neufeld, R.J.; Cooper, D.G. Determination of interfacial areas in emulsions using turbidimetric and droplet size data: Correction of the formula for emulsifying activity index. J. Agric. Food Chem. 1991, 39, 655–659. [Google Scholar] [CrossRef]
- Joscelyne, S.M.; Trägårdh, G. Food emulsions using membrane emulsification: Conditions for producing small droplets. J. Food Eng. 1999, 39, 59–64. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Casagrande, R.; Moura-de-Carvalho Vicentini, F.T.; Verri, W.A.; Fonseca, M.J.V. Evaluation of the antioxidant activity of soybean extract by different in vitro methods and investigation of this activity after its incorporation in topical formulations. Eur. J. Pharm. Biopharm. 2006, 64, 99–106. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Georgetti, S.R.; Salvador, M.J.; José, M.; Fonseca, V.; Oliveira, W.P. Antioxidant activity and physical-chemical properties of spray and spouted bed dried extracts of Bauhinia forficata. Braz. J. Pharm. Sci. 2009, 45, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Pag, A.I.; Radu, D.G.; Drǎgǎnescu, D.; Popa, M.I.; Sîrghie, C. Flaxseed cake—A sustainable source of antioxidant and antibacterial extracts. Cellul. Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Gerstenmeyer, E.; Reimer, S.; Berghofer, E.; Schwartz, H.; Sontag, G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K. Nanocomposite Furcellaran Films—The Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers 2019, 11, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Complexation between flaxseed protein isolate and phenolic compounds: Effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. 2019, 94, 20–29. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine “Carabao” var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between secondary structure and surface hydrophobicity of soybean protein isolate subjected to heat treatment. J. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Drozłowska, E.; Weronis, M.; Bartkowiak, A. The influence of thermal hydrolysis process on emulsifying properties of potato protein isolate. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Laca, A.; Sáenz, M.C.; Paredes, B.; Díaz, M. Rheological properties, stability and sensory evaluation of low-cholesterol mayonnaises prepared using egg yolk granules as emulsifying agent. J. Food Eng. 2010, 97, 243–252. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Corredig, M. Heat-Induced Changes Occurring in Oil/Water Emulsions Stabilized by Soy Glycinin and β-Conglycinin. J. Agric. Food Chem. 2010, 58, 9171–9180. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Green tea polyphenols-β-lactoglobulin nanocomplexes: Interfacial behavior, emulsification and oxidation stability of fish oil. Food Hydrocoll. 2014, 35, 505–511. [Google Scholar] [CrossRef]
- Sathivel, S.; Bechtel, P.J.; Babbitt, J.K.; Prinyawiwatkul, W.; Patterson, M. Functional, Nutritional, and Rheological Properties of Protein Powders from Arrowtooth Flounder and their Application in Mayonnaise. J. Food Sci. 2005, 70, E57–E63. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Agric. Biol. Chem. 1972, 36, 719–727. [Google Scholar] [CrossRef]




| Dry matter | 3 ± 0.2% |
| Proteins | 14 ± 0.2 mg/mL |
| Saccharides | 6.5 ± 0.2 mg/mL |
| Other Extractable Compounds | 9.5 ± 0.02 mg/mL |
| Powder Sample | OBC (%) | WHC (%) | Solubility (%) | Dry Matter (%) | aw | –SH (µmol/g) | –S–S– (µmol/g) |
|---|---|---|---|---|---|---|---|
| A | 217.51 ± 0.40 c | 375.12 ± 0.30 c | 74.29 ± 0.24 a | 91.95 ± 5.06 a | 0.52 ± 0.01 a | 76.93 ± 0.05 a | 33.46 ± 0.02 c |
| B | 275.47 ± 0.25 b | 401.93 ± 0.31 b | 65.24 ± 0.57 b | 94.65 ± 0.86 a | 0.48 ± 0.00 b | 75.12 ± 0.01 b | 77.81 ± 0.14 b |
| C | 296.09 ± 0.12 a | 454.92 ± 0.27 a | 64.82 ± 0.39 b | 98.41 ± 2.75 a | 0.36 ± 0.00 c | 71.52 ± 0.00 c | 88.03 ± 0.08 a |
| Powder Sample | DPPH (%) | ABTS (%) |
|---|---|---|
| A | 79.20 ± 0.16 a | 95.33 ± 1.01 a |
| B | 76.07 ± 0.78 b | 70.72 ± 1.30 b |
| C | 69.23 ± 0.78 c | 60.33 ± 0.30 c |
| Powder Sample | L* | a* | b* |
|---|---|---|---|
| A | 72.71 ± 0.01 c | −1.06 ± 0.01 b | 17.74 ± 0.01 a |
| B | 72.74 ± 0.01 a | −1.43 ± 0.01 a | 21.14 ± 0.02 c |
| C | 71.69 ± 0.01 b | −0.93 ± 0.01 | 19.91 ± 0.02 b |
| Emulsion | D4.3 (µm) | SPAN (-) |
|---|---|---|
| A—0.5% | 37.42 ± 0.20 a | 8.02 |
| B—0.5% | 22.52 ± 0.21 b | 6.27 |
| C—0.5% | 20.91 ± 0.05 d | 5.22 |
| A—1% | 21.56 ± 0.05 c | 7.20 |
| B—1% | 18.76 ± 0.10 e | 5.53 |
| C—1% | 8.12 ± 0.10 f | 4.39 |
| A—3% | 7.89 ± 0.11 g | 6.76 |
| B—3% | 7.86 ± 0.23 h | 4.40 |
| C—3% | 7.51 ± 0.02 i | 3.71 |
| Emulsion | L* | a* | b* |
|---|---|---|---|
| A—0.5% | 86.36 ± 0.05 f | −1.94 ± 0.01 i | 12.29 ± 0.05 h |
| A—1% | 86.42 ± 0.02 f | −1.35 ± 0.01 e | 15.99 ± 0.02 e |
| A—3% | 83.41 ± 0.00 g | −0.59 ± 0.00 a | 18.80 ± 0.01 a |
| B—0.5% | 88.57 ± 0.02 a | −1.61 ± 0.01 f | 17.31 ± 0.02 b |
| B—1% | 87.18 ± 0.04 e | −1.64 ± 0.00 g | 16.94 ± 0.04 c |
| B—3% | 88.49 ± 0.02 b | −1.21 ± 0.00 d | 16.81 ± 0.01 d |
| C—0.5% | 88.57 ± 0.02 a | −1.80 ± 0.00 h | 12.93 ± 0.00 g |
| C—1% | 87.33 ± 0.02 d | −1.12 ± 0.01 c | 15.78 ± 0.01 f |
| C—3% | 87.64 ± 0.02 c | −0.78 ± 0.01 b | 15.98 ± 0.01 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules 2020, 10, 153. https://doi.org/10.3390/biom10010153
Drozłowska E, Łopusiewicz Ł, Mężyńska M, Bartkowiak A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules. 2020; 10(1):153. https://doi.org/10.3390/biom10010153
Chicago/Turabian StyleDrozłowska, Emilia, Łukasz Łopusiewicz, Monika Mężyńska, and Artur Bartkowiak. 2020. "Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers" Biomolecules 10, no. 1: 153. https://doi.org/10.3390/biom10010153
APA StyleDrozłowska, E., Łopusiewicz, Ł., Mężyńska, M., & Bartkowiak, A. (2020). Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules, 10(1), 153. https://doi.org/10.3390/biom10010153