Combining Physiological and Metabolomic Analysis to Unravel the Regulations of Coronatine Alleviating Water Stress in Tobacco (Nicotiana tabacum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and PEG Treatment
2.2. Measurement of Fresh Weight (FW) and Dry Weight (DW)
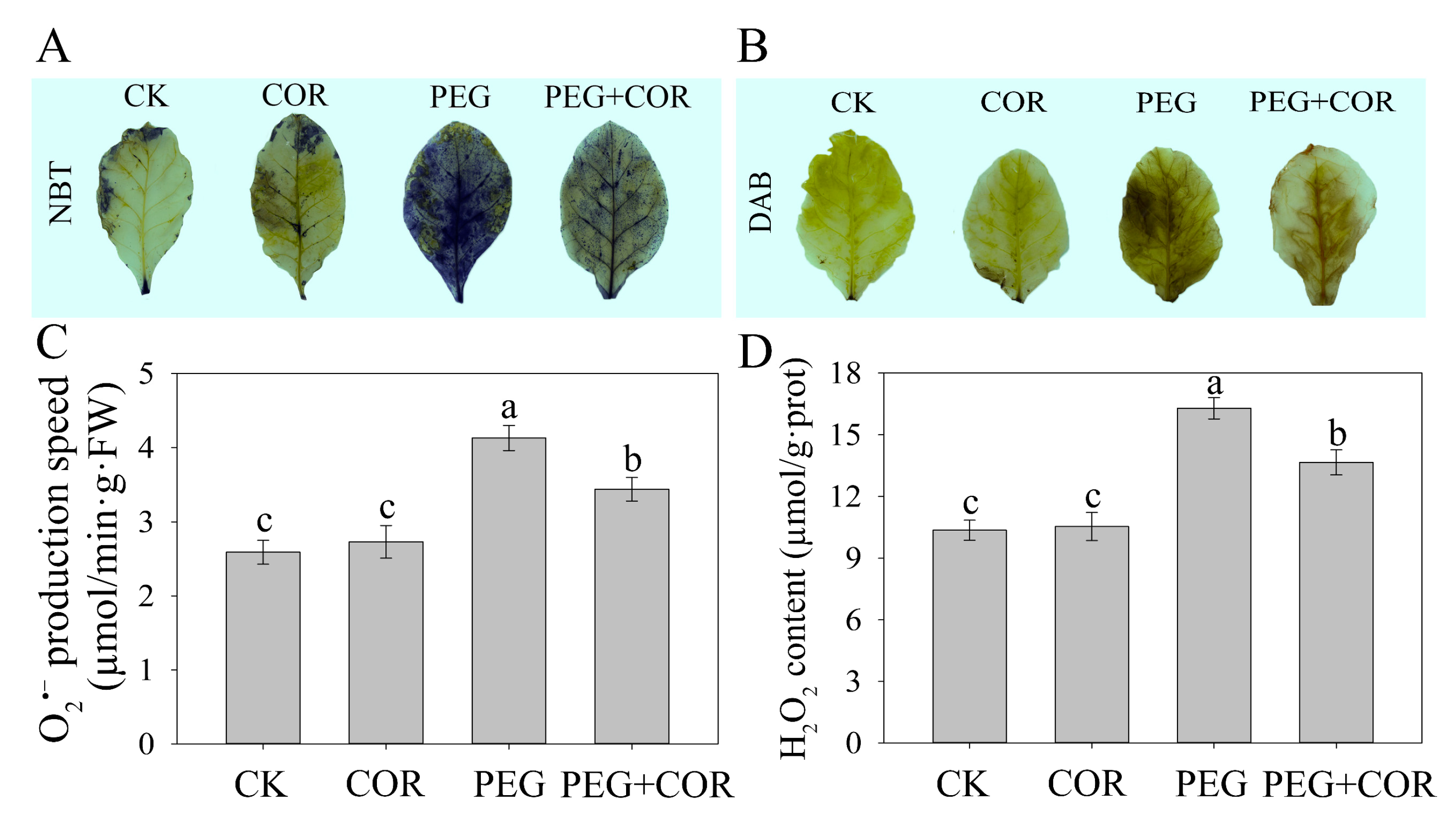
2.3. Determination of Superoxide Anion (O2•−) and Hydrogen Peroxide (H2O2) Concentrations
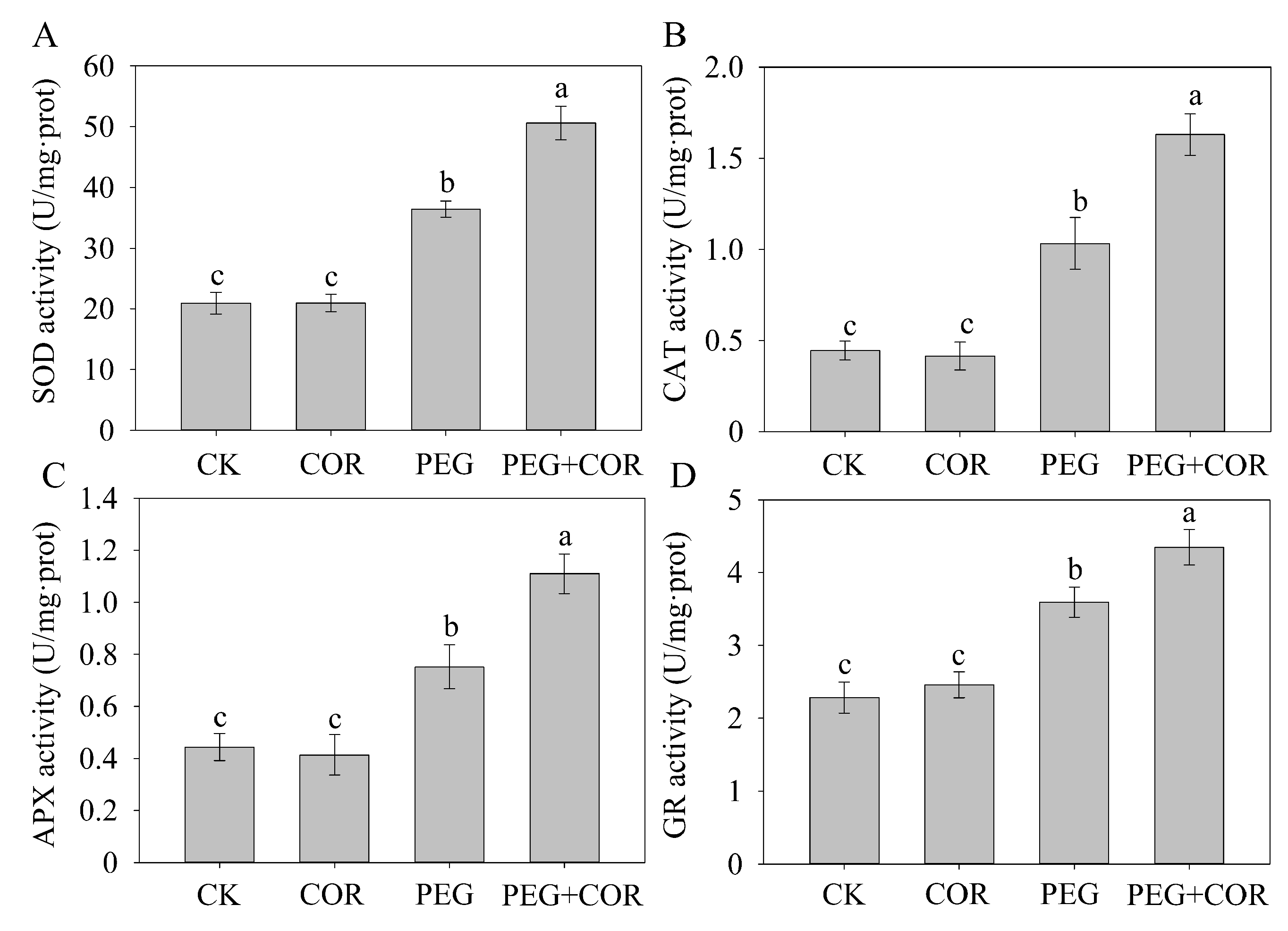
2.4. Determination of Antioxidant Enzyme Activities
2.5. Sample Preparation for LC-MS
2.6. UHPLC-Q-TOF/MS Conditions
2.7. Data Analysis
3. Results
3.1. Morphological Changes of Seedlings
3.2. Hydrogen Peroxide (H2O2) and Superoxide (O2•−) Concentrations
3.3. Activities of Antioxidant Enzymes
3.4. Metabolite Profiling
3.5. KEGG Metabolic Pathway Analysis of Significantly Changed Metabolites
4. Discussion
4.1. Exogenous COR Improves ROS Scavenging in Plants by Stimulating Antioxidant Enzymes
4.2. Exogenous COR Promotes the Accumulation of Carbohydrates Under PEG Conditions
4.3. Exogenous COR Enhances the Accumulation of Organic Acids Under PEG Stress
4.4. Increases in Some Nitrogen-Containing Metabolites Induced by COR Contribute to Osmotic Resistance
4.5. Increased Levels of Amino Acids in PEG-Stressed Tobacco Plants May Be an Indicator of Tissue Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hopkins, A.; Del Prado, A. Implications of climate change for grassland in Europe: Impacts, adaptations and mitigation options: A review. Grass Forage Sci. 2007, 62, 118–126. [Google Scholar] [CrossRef]
- Blum, A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Yu, C.Q.; Huang, X.; Chen, H.; Huang, G.R.; Ni, S.Q.; Wright, J.S.; Hall, J.; Ciais, P.; Zhang, J.; Xiao, Y.C.; et al. Assessing the Impacts of Extreme Agricultural Droughts in China Under Climate and Socioeconomic Changes. Earth Future 2018, 6, 689–703. [Google Scholar] [CrossRef]
- Kang, Z.Y.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Bender, C.; Palmer, D.; PenalozaVazquez, A.; Rangaswamy, V.; Ullrich, M. Biosynthesis of coronatine, a thermoregulated phytotoxin produced by the phytopathogen Pseudomonas syringae. Arch. Microbiol. 1996, 166, 71–75. [Google Scholar] [CrossRef]
- Tamogami, S.; Kodama, O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 2000, 54, 689–694. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ayoubi, P.; Weng, H.; Palmer, D.A.; Mitchell, R.E.; Jones, W.; Bender, C.L. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005, 42, 201–217. [Google Scholar] [CrossRef]
- Kenyon, J.S.; Turner, J.G. The stimulation of ethylene synthesis in nicotiana-tabacum leaves by the phytotoxin coronatine. Plant Physiol. 1992, 100, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, F.; Li, B.; Eneji, A.E.; Li, J.; Duan, L.; Wang, B.; Li, Z.; Tian, X. Coronatine-induced lateral-root formation in cotton (Gossypium hirsutum) seedlings under potassium-sufficient and -deficient conditions in relation to auxin. J. Plant Nutr. Soil Sci. 2009, 172, 435–444. [Google Scholar] [CrossRef]
- Feys, B.J.F.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.X.; Duan, L.S.; Tian, X.L.; Wang, B.M.; Eneji, A.E.; Li, Z.H. Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J. Plant Physiol. 2008, 165, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, Y.; Zhang, J.; Xie, Y.; Zhang, M.; Duan, L.; Li, Z. Coronatine enhances drought tolerance via improving antioxidative capacity to maintaining higher photosynthetic performance in soybean. Plant Sci. 2013, 210, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, X.; Li, Z.; Duan, L.; Zhang, M. Physiological Evaluation of Drought Stress Tolerance and Recovery in Cauliflower (Brassica oleracea L.) Seedlings Treated with Methyl Jasmonate and Coronatine. J. Plant Growth Regul. 2012, 31, 113–123. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Li, J.; Li, Z.; Tian, X.; Duan, L. Phytotoxin coronatine enhances heat tolerance via maintaining photosynthetic performance in wheat based on Electrophoresis and TOF-MS analysis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.J.; Wang, Q.; Eneji, A.E.; Li, Z.H.; Duan, L.S. Coronatine Enhances Chilling Tolerance in Cucumber (Cucumis sativus L.) Seedlings by Improving the Antioxidative Defence System. J. Agron. Crop Sci. 2009, 195, 377–383. [Google Scholar] [CrossRef]
- Gao, W.; Yu, C.X.; Ai, L.; Zhou, Y.Y.; Duan, L.S. Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica). Int. J. Mol. Sci. 2019, 20, 17. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.W.; Li, K.; Alseekh, S.; Omranian, N.; Zhao, L.J.; Zhou, Y.; Xiao, Y.J.; Jin, M.; Yang, N.; Liu, H.J.; et al. Genetic Determinants of the Network of Primary Metabolism and Their Relationships to Plant Performance in a Maize Recombinant Inbred Line Population. Plant Cell 2015, 27, 1839–1856. [Google Scholar] [CrossRef] [Green Version]
- Go, E.P. Database Resources in Metabolomics: An Overview. J. Neuroimmune Pharm. 2010, 5, 18–30. [Google Scholar] [CrossRef]
- Simo, C.; Ibanez, C.; Valdes, A.; Cifuentes, A.; Garcia-Canas, V. Metabolomics of Genetically Modified Crops. Int. J. Mol. Sci. 2014, 15, 18941–18966. [Google Scholar] [CrossRef] [Green Version]
- Scalabrin, E.; Radaelli, M.; Rizzato, G.; Bogani, P.; Buiatti, M.; Gambaro, A.; Capodaglio, G. Metabolomic analysis of wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses: Unraveling metabolic responses. Anal. Bioanal. Chem. 2015, 407, 6357–6368. [Google Scholar] [CrossRef] [PubMed]
- Mibei, E.K.; Owino, W.O.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N. Metabolomic analyses to evaluate the effect of drought stress on selected African Eggplant accessions. J. Sci. Food Agric. 2018, 98, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Jiang, F.L.; Wu, Z. The apoplastic oxidative burst as a key factor of hyperhydricity in garlic plantlet in vitro. Plant Cell Tiss. Organ Cult. 2015, 120, 571–584. [Google Scholar] [CrossRef]
- Scarpeci, T.E.; Zanor, M.I.; Carrillo, N.; Mueller-Roeber, B.; Valle, E.M. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: A focus on rapidly induced genes. Plant Mol. Biol. 2008, 66, 361–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudi, A.; Cheng, Z.Y.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The Apoplastic Oxidative Burst Peroxidase in Arabidopsis Is a Major Component of Pattern-Triggered Immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, Y.C.; Ma, Y.Y.; Zhang, T.T.; Bi, Y.; Wang, Y.; Prusky, D. Exogenous polyamines enhance resistance to Alternaria alternata by modulating redox homeostasis in apricot fruit. Food Chem. 2019, 301, 8. [Google Scholar] [CrossRef]
- Adusumilli, R.; Mallick, P. Data Conversion with ProteoWizard msConvert. In Proteomics: Methods and Protocols; Comai, L., Katz, J.E., Mallick, P., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 1550, pp. 339–368. [Google Scholar]
- Patti, G.J.; Tautenhahn, R.; Siuzdak, G. Meta-analysis of untargeted metabolomic data from multiple profiling experiments. Nat. Protoc. 2012, 7, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using Nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Fan, W.Q.; Ge, G.T.; Liu, Y.H.; Wang, W.; Liu, L.Y.; Jia, Y.S. Proteomics integrated with metabolomics: Analysis of the internal causes of nutrient changes in alfalfa at different growth stages. BMC Plant Biol. 2018, 18, 15. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.Z.; Bourque, G.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.G.; Wishart, D.S. MSEA: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.W.; Wang, Z.; Zhang, K.L.; Chi, Z.X.; Xu, T.; Jiang, D.L.; Chen, S.; Li, W.X.; Yang, X.Y.; Zhang, X.; et al. One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol. Cell 2019, 75, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Khan, M.H.; Panda, S.K. Hydrogen peroxide induces oxidative stress in detached leaves of Oryza sativa L. Gen. Appl. Plant Physiol. 2007, 33, 83–96. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ai, L.; Li, Z.H.; Xie, Z.X.; Tian, X.L.; Eneji, A.E.; Duan, L.S. Coronatine alleviates polyethylene glycol-induced water stress in two rice (Oryza sativa L.) cultivars. J. Agron. Crop Sci. 2008, 194, 360–368. [Google Scholar] [CrossRef]
- Li, X.W.; Shen, X.F.; Li, J.M.; Eneji, A.E.; Li, Z.H.; Tian, X.L.; Duan, L.S. Coronatine Alleviates Water Deficiency Stress on Winter Wheat Seedlings. J. Integr. Plant Biol. 2010, 52, 616–625. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.L.; Jensen, C.R.; Andersen, M.N. Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: Its implication in altering pod set. Field Crop. Res. 2004, 86, 1–13. [Google Scholar] [CrossRef]
- Couee, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Bishi, S.K.; Singh, A.L.; Zala, P.V.; Mahatma, M.K.; Kalariya, K.A.; Jat, R.A. Rapid induction of small heat shock proteins improves physiological adaptation to high temperature stress in peanut. J. Agron. Crop Sci. 2018, 204, 285–297. [Google Scholar] [CrossRef]
- Nam, K.H.; Shin, H.J.; Pack, I.S.; Park, J.H.; Kim, H.B.; Kim, C.G. Metabolomic changes in grains of well-watered and drought-stressed transgenic rice. J. Sci. Food Agric. 2016, 96, 807–814. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.J.; Peng, Y.; Huang, B.R. Metabolic pathways regulated by abscisic acid, salicylic acid and -aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Goswami, L.; Sangma, S.; Mukherjee, A.; Mukherjee, R.; Roy, N.; Basak, P.; Majumder, A.L. Manipulation of inositol metabolism for improved plant survival under stress: A “network engineering approach”. J. Plant Biochem. Biotechnol. 2012, 21, 15–23. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Timpa, J.D.; Burke, J.J.; Quisenberry, J.E.; Wendt, C.W. Effects of water-stress on the organic-acid and carbohydrate compositions of cotton plants. Plant Physiol. 1986, 82, 724–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.F.; Gong, Z.H.; Wu, M.B.; Chan, H.L.; Yuan, Y.J.; Tang, N.; Zhang, Q.; Miao, M.J.; Chang, W.; Li, Z.; et al. Integrative comparative analyses of metabolite and transcript profiles uncovers complex regulatory network in tomato (Solanum lycopersicum L.) fruit undergoing chilling injury. Sci. Rep. 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.P.; Dai, H.L.; Liu, L.L.; Xu, C.; Yin, Y.X.; Yi, J.L.; Bielec, M.D.; Han, Y.C.; Li, S.P. Citrate reduced oxidative damage in stem cells by regulating cellular redox signaling pathways and represent a potential treatment for oxidative stress-induced diseases. Redox Biol. 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.Z.; Yong, B.; Liu, T.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Liu, W.; Nie, G. Metabolomics and physiological analyses reveal beta-sitosterol as an important plant growth regulator inducing tolerance to water stress in white clover. Planta 2019, 250, 2033–2046. [Google Scholar] [CrossRef]
- Berglund, T.; Ohlsson, A.B. Defensive and secondary metabolism in plant tissue cultures, with special reference to nicotinamide, glutathione and oxidative stress. Plant Cell Tiss. Organ Cult. 1995, 43, 137–145. [Google Scholar] [CrossRef]
- Azooz, M. Proteins, sugars and ion leakage as a selection criterion for the salt tolerance of three sorghum cultivars at seedling stage grown under NaCl and nicotinamide. Int. J. Agric. Biol. 1560, 8530, 27–35. [Google Scholar]
- Tramontano, W.A.; Jouve, D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 1997, 44, 1037–1040. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lightfoot, D.A.; Wood, A.J. Trigonelline concentrations in salt stressed leaves of cultivated Glycine max. Phytochemistry 1999, 52, 1235–1238. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Aspinall, D.; Jones, G.P.; Paleg, L.G. Stress metabolism. IX. Effect of salt stress on trigonelline accumulation in tomato. Can. J. Plant Sci. 2001, 81, 487–498. [Google Scholar] [CrossRef]
- Cho, Y.; Njiti, V.N.; Chen, X.; Lightfoot, D.A.; Wood, A.J. Trigonelline concentration in field-grown soybean in response to irrigation. Biol. Plant. 2003, 46, 405–410. [Google Scholar] [CrossRef]
- Xie, Z.X.; Duan, L.S.; Li, Z.H.; Wang, X.D.; Liu, X.J. Dose-Dependent Effects of Coronatine on Cotton Seedling Growth Under Salt Stress. J. Plant Growth Regul. 2015, 34, 651–664. [Google Scholar] [CrossRef]
- Shoji, T.; Ogawa, T.; Hashimoto, T. Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol. 2008, 49, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Hashimoto, T. Tobacco MYC2 Regulates Jasmonate-Inducible Nicotine Biosynthesis Genes Directly and By Way of the NIC2-Locus ERF Genes. Plant Cell Physiol. 2011, 52, 1117–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.T.; Zhang, Y.; Du, Y.Y.; Chen, S.Y.; Tang, H.R. Dynamic Metabonomic Responses of Tobacco (Nicotiana tabacum) Plants to Salt Stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Murzello, C.; Sun, Y.; Kim, M.S.; Xie, X.T.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Pare, P.W. Choline and Osmotic-Stress Tolerance Induced in Arabidopsis by the Soil Microbe Bacillus subtilis (GB03). Mol. Plant-Microbe Interact. 2010, 23, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Nikiforova, V.J.; Bielecka, M.; Gakiere, B.; Krueger, S.; Rinder, J.; Kempa, S.; Morcuende, R.; Scheible, W.R.; Hesse, H.; Hoefgen, R. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids 2006, 30, 173–183. [Google Scholar] [CrossRef]
- Lugan, R.; Niogret, M.F.; Leport, L.; Guegan, J.P.; Larher, F.R.; Savoure, A.; Kopka, J.; Bouchereau, A. Metabolome and water homeostasis analysis of Thellungiella salsuginea suggests that dehydration tolerance is a key response to osmotic stress in this halophyte. Plant J. 2010, 64, 215–229. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Bhandari, K.; Kumar, S.; Kumar, J.; Prasad, P.V.V.; Siddique, K.H.M.; Nayyar, H. Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant Cell Environ. 2019, 42, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, L.; Li, J.; Sun, P.; Lu, M.; Hu, J. Comparative metabolomics analysis reveals different metabolic responses to drought in tolerant and susceptible poplar species. Physiol. Plant. 2019. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widodo; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roessner, U.; Patterson, J.H.; Forbes, M.G.; Fincher, G.B.; Langridge, P.; Bacic, A. An investigation of boron toxicity in barley using metabolomics. Plant Physiol. 2006, 142, 1087–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, C.; Purdy, S.; Christ, A.; Morot-Gaudry, J.F.; Wingler, A.; Masclaux-Daubresse, C.L. Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis. A metabolic profiling approach. Plant Physiol. 2005, 138, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Q.; Li, Z.H.; Eneji, A.E.; Tian, X.L.; Zhai, Z.X.; Li, J.M.; Duan, L.S. Effects of coronatine on growth, gas exchange traits, chlorophyll content, antioxidant enzymes and lipid peroxidation in maize (Zea mays L.) seedlings under simulated drought stress. Plant Prod. Sci. 2008, 11, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Shang, H.T.; Cao, S.F.; Yang, Z.F.; Cai, Y.T.; Zheng, Y.H. Effect of Exogenous gamma-Aminobutyric Acid Treatment on Proline Accumulation and Chilling Injury in Peach Fruit after Long-Term Cold Storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]



| Treatment | FW (g/Plant) | DW (g/Plant) |
|---|---|---|
| CK | 17.06 ± 0.59 a | 1.72 ± 0.04 a |
| COR | 17.20 ± 0.49 a | 1.72 ± 0.03 a |
| PEG | 11.27 ± 0.46 c | 1.43 ± 0.05 c |
| PEG+COR | 14.09 ± 0.51 b | 1.60 ± 0.04 b |
| Mode | Metabolite | VIP | FC | p Value |
|---|---|---|---|---|
| Sugar and sugar derivatives | ||||
| ESI (−) | D-Allose 1 | 2.22 | 2.07 | 2.0 × 10−3 |
| ESI (+) | Galactinol 1 | 2.70 | 2.15 | 9.0 × 10−7 |
| ESI (−) | α-D-Glucose 2 | 1.56 | 2.13 | 6.3 × 10−4 |
| ESI (−) | D-Fructose 2 | 1.68 | 2.33 | 2.9 × 10−4 |
| ESI (+) | D-Mannose 2 | 2.21 | 6.62 | 1.7 × 10−8 |
| ESI (−) | D-Quinovose 2 | 1.80 | 2.48 | 2.4 × 10−7 |
| ESI (−) | Maltitol 2 | 2.16 | 3.69 | 2.7 × 10−7 |
| ESI (+) | Myo-Inositol 2 | 1.57 | 2.60 | 3.6 × 10−8 |
| Organic acids | ||||
| ESI (−) | α-Ketoglutarate 1 | 2.16 | 2.08 | 2.9 × 10−2 |
| ESI (−) | Chlorogenic acid 1 | 1.84 | 1.59 | 7.2 × 10−6 |
| ESI (+) | Citrate 1 | 2.28 | 1.79 | 2.0 × 10−4 |
| ESI (−) | α-Ketoglutarate 2 | 1.58 | 2.57 | 4.5 × 10−3 |
| ESI (−) | Citrate 2 | 2.06 | 3.45 | 4.8 × 10−6 |
| ESI (−) | Glyceric acid 2 | 1.30 | 1.76 | 5.0 × 10−4 |
| ESI (−) | Quinic acid 2 | 2.55 | 6.32 | 2.1 × 10−7 |
| Amino acids | ||||
| ESI (+) | D-Proline 2 | 1.92 | 4.10 | 5.1 × 10−8 |
| ESI (+) | GABA 2 | 1.19 | 1.80 | 4.5 × 10−5 |
| ESI (+) | L-Arginine 2 | 1.53 | 0.38 | 2.0 × 10−5 |
| ESI (+) | L-Asparagine 2 | 1.52 | 0.39 | 1.9 × 10−5 |
| ESI (+) | L-Histidine 2 | 1.18 | 0.55 | 7.3 × 10−5 |
| ESI (+) | L-Isoleucine 2 | 1.98 | 0.22 | 1.7 × 10−9 |
| ESI (+) | L-Leucine 2 | 1.95 | 0.23 | 2.4 × 10−10 |
| ESI (−) | L-Lysine 2 | 1.91 | 0.33 | 1.5 × 10−3 |
| ESI (−) | L-Norleucine 2 | 2.61 | 0.15 | 8.0 × 10−7 |
| ESI (−) | L-Threonine 2 | 1.94 | 0.27 | 5.2 × 10−4 |
| ESI (−) | L-Tryptophan 2 | 2.41 | 0.20 | 1.7 × 10−9 |
| Others | ||||
| ESI (+) | Nicotinamide 1 | 3.25 | 3.12 | 4.8 × 10−4 |
| ESI (+) | 2-Ethoxyethanol 2 | 1.17 | 0.52 | 1.0 × 10−3 |
| ESI (+) | 4-Hydroxybutanoic acid lactone 2 | 1.07 | 0.61 | 4.7 × 10−4 |
| ESI (+) | Choline 2 | 1.36 | 2.07 | 1.1 × 10−6 |
| ESI (+) | L-Nicotine 2 | 1.29 | 2.07 | 7.4 × 10−4 |
| ESI (+) | Nicotinamide 2 | 1.45 | 2.89 | 5.9 × 10−3 |
| ESI (+) | Phosphorylcholine 2 | 1.43 | 2.46 | 4.5 × 10−3 |
| ESI (+) | Trigonelline 2 | 1.04 | 1.88 | 9.5 × 10−3 |
| ID | KEGG Pathway | Compounds |
|---|---|---|
| map00970 | Aminoacyl-tRNA biosynthesis | C00152, C00135, C00062, C00047, C00407, C00123, C00188, C00078 |
| map00052 | Galactose metabolism | C00267, C01235, C00137, C00159 |
| map00260 | Glycine, serine, and threonine metabolism | C00114, C00258, C00188, C00078 |
| map00290 | Valine, leucine, and isoleucine biosynthesis | C00123, C00188, C00407 |
| map00250 | Alanine, aspartate, and glutamate metabolism | C00152, C00026, C00334 |
| map00966 | Glucosinolate biosynthesis | C00078, C00123, C00407 |
| map00220 | Arginine biosynthesis | C00026, C00062 |
| map00051 | Fructose and mannose metabolism | C00267, C00159 |
| map00020 | Citrate cycle (tricarboxylic acid (TCA) cycle) | C00158, C00026 |
| map00330 | Arginine and proline metabolism | C00062, C00334 |
| map00280 | Valine, leucine, and isoleucine degradation | C00407, C00123 |
| map00564 | Glycerophospholipid metabolism | C00114, C00588 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhou, Y.; Xu, Z.; Chen, Z.; Duan, L. Combining Physiological and Metabolomic Analysis to Unravel the Regulations of Coronatine Alleviating Water Stress in Tobacco (Nicotiana tabacum L.). Biomolecules 2020, 10, 99. https://doi.org/10.3390/biom10010099
Xu J, Zhou Y, Xu Z, Chen Z, Duan L. Combining Physiological and Metabolomic Analysis to Unravel the Regulations of Coronatine Alleviating Water Stress in Tobacco (Nicotiana tabacum L.). Biomolecules. 2020; 10(1):99. https://doi.org/10.3390/biom10010099
Chicago/Turabian StyleXu, Jiayang, Yuyi Zhou, Zicheng Xu, Zheng Chen, and Liusheng Duan. 2020. "Combining Physiological and Metabolomic Analysis to Unravel the Regulations of Coronatine Alleviating Water Stress in Tobacco (Nicotiana tabacum L.)" Biomolecules 10, no. 1: 99. https://doi.org/10.3390/biom10010099





