Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives
Abstract
:1. Introduction
2. Role of Natural Products in Cancer Therapy
3. Chrysin: An Overview of Chemistry, Sources, and Pharmacokinetics
4. Chrysin and Its Pharmacological Activities
5. Chrysin and Cancer
5.1. Breast Cancer
5.2. Lung Cancer
5.3. Prostate Cancer
5.4. Ovarian Cancer
5.5. Gastric Cancer
5.6. Cervical Cancer
5.7. Liver Cancer
5.8. Melanoma
5.9. Bladder Cancer
5.10. Colorectal Cancer
6. Chrysin, Chemotherapy and Drug Resistance
7. Chrysin-Loaded Nanoparticles in Cancer Therapy
8. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ER | endoplasmic reticulum |
| ROS | reactive oxygen species |
| EMT | epithelial-to-mesenchymal transition |
| NAFLD | non-alcoholic fatty liver disease |
| TNF-α | tumor necrosis factor-α |
| IL | interleukin |
| I/R | ischemic/reperfusion |
| PD | Parkinson’s disease |
| TBI | traumatic brain injury |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| EGFR | epidermal growth factor receptor |
| VEGF | vascular endothelial growth factor |
| HIF-1 | hypoxia-inducible factor-1 |
| STAT3 | signal transducer and activator of transcription 3 |
| TLRs | toll-like receptors |
| NF-κB | nuclear factor-kappaB |
| PC | prostate cancer |
| UPR | unfolded protein response |
| PERK | PRKR-like ER kinase |
| elF2α | eukaryotic translation initiation factor 2α |
| GRP78 | 78 kDa glucose-regulated protein |
| OC | ovarian cancer |
| GC | gastric cancer |
| 5mC | 5-methylcytosine |
| 5hmC | 5-hydroxymethylcytosine |
| miR | microRNA |
| TGF-β | transforming growth factor-beta |
| HK | hexokinase |
| HCC | hepatocellular carcinoma |
| ECM | extracellular matrix |
| 5-FU | 5-Fluorouracil |
| CRC | colorectal cancer |
| LC-3II | light chain-3II |
| mTOR | mammalian target of rapamycin |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| CYP | cytochrome C |
| DTX | docetaxel |
| FDA | Food and Drug Administration |
| TIMPs | tissue inhibitors of metalloproteinases |
| lncRNAs | long non-coding RNAs |
| circRNAs | circular RNAs |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, B.; Pal, B.; Bhuyan, R.; Li, H.; Sarma, A.; Gayan, S.; Talukdar, J.; Sandhya, S.; Bhuyan, S.; Gogoi, G.; et al. MYC Regulates the HIF2α Stemness Pathway via Nanog and Sox2 to Maintain Self-Renewal in Cancer Stem Cells versus Non-Stem Cancer Cells. Cancer Res. 2019, 79, 4015–4025. [Google Scholar] [CrossRef]
- Fang, Z.; Li, T.; Chen, W.; Wu, D.; Qin, Y.; Liu, M.; Wu, G.; He, L.; Li, H.; Gu, H. Gab2 promotes cancer stem cell like properties and metastatic growth of ovarian cancer via downregulation of miR-200c. Exp. Cell Res. 2019, 382, 111462. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B.; et al. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Xiao, X.; Cheng, W.; Hu, L.; Yao, W.; Qian, Z.; Wu, W.; Chang, W. MiR-204 inhibits hepatocellular cancer drug resistance and metastasis through targeting NUAK1. Biochem. Cell Biol. 2019, 97, 563–570. [Google Scholar] [CrossRef]
- Sheng, X.; Li, Y.; Li, Y.; Liu, W.; Lu, Z.; Zhan, J.; Xu, M.; Chen, L.; Luo, X.; Cai, G.; et al. PLOD2 contributes to drug resistance in laryngeal cancer by promoting cancer stem cell-like characteristics. BMC Cancer 2019, 19, 840. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Yang, S.-F.; Weng, C.-J.; Sethi, G.; Hu, D.-N. Natural Bioactives and Phytochemicals Serve in Cancer Treatment and Prevention. Evid.-Based Complement. Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Yang, S.-F.; Sethi, G.; Hu, D.-N. Natural Bioactives in Cancer Treatment and Prevention. BioMed Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Líšková, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Wu, S.-Y.; Chen, H.-C.; Chu, C.-A.; Tang, H.-H.; Liu, H.-S.; Hong, Y.-R.; Huang, C.-Y.; Huang, G.-C.; Su, A.C.-L. Curcumin functions as a MEK inhibitor to induce a synthetic lethal effect on KRAS mutant colorectal cancer cells receiving targeted drug regorafenib. J. Nutr. Biochem. 2019, 74, 108227. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Du, S.; Liu, Y.; Lv, C.; Song, Y.; Chen, X.; Zhang, B.; Li, D.; Gao, S.; Cui, W.; et al. Mucoadhesive-to-penetrating controllable peptosomes-in-microspheres co-loaded with anti-miR-31 oligonucleotide and Curcumin for targeted colorectal cancer therapy. Theranostics 2020, 10, 3594–3611. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F.; Shanmugam, S.W.M.K. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [Green Version]
- Merarchi, M.; Sethi, G.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Ahn, K.S. Role of Natural Products in Modulating Histone Deacetylases in Cancer. Molecules 2019, 24, 1047. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.-H.; Sethi, G.; Ahn, K.S. β-caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2013, 53, 793–806. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.-Y.; Du, H.; Li, S.-Z.; Tu, R.; Jia, Y.-F.; Zheng, Z.; Song, X.-M.; Du, R.-L.; Zhang, X.-D. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019, 26, 2300–2313. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-C.; Chueh, F.-S.; Hsiao, Y.-T.; Cheng, Z.-Y.; Lien, J.-C.; Liu, K.-C.; Peng, S.-F.; Chung, J.-G. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef] [PubMed]
- Montazerabadi, A.; Beik, J.; Irajirad, R.; Attaran, N.; Khaledi, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Folate-modified and curcumin-loaded dendritic magnetite nanocarriers for the targeted thermo-chemotherapy of cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Attar, T.; Madihally, S.V. Targeted cancer treatment using a combination of siRNA-liposomes and resveratrol-electrospun fibers in co-cultures. Int. J. Pharm. 2019, 569, 118599. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chin, Y.-T.; Shih, Y.-J.; Chen, Y.-R.; Chung, Y.-Y.; Lin, C.-Y.; Hsiung, C.-N.; Whang-Peng, J.; Lee, S.; Lin, H.-Y.; et al. Resveratrol antagonizes thyroid hormone-induced expression of checkpoint and proliferative genes in oral cancer cells. J. Dent. Sci. 2019, 14, 255–262. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [Green Version]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [Green Version]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.-J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2013, 5, 634–648. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhang, P. EGFR-mediated autophagy in tumourigenesis and therapeutic resistance. Cancer Lett. 2019, 469, 207–216. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yoo, E.-S.; Woo, J.-S.; Han, S.-H.; Lee, J.-H.; Jung, S.-H.; Kim, H.-J.; Jung, J.-Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019, 860, 172568. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Fimognari, C. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2017, 128, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chiang, S.Y.; Nam, D.; Chung, W.-S.; Lee, J.; Na, Y.-S.; Sethi, G.; Ahn, K.S. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014, 345, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Venkata, K.C.N.; Bhattacharyya, P.; Sethi, G.; Bishayee, A. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Semin. Cancer Biol. 2016, 40–41, 100–115. [Google Scholar] [CrossRef]
- Ko, J.; Yang, M.H.; Baek, S.H.; Nam, D.; Jung, S.H.; Ahn, K.S. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA-MB-231 cells. Phytother. Res. 2019, 33, 1934–1942. [Google Scholar] [CrossRef]
- Cheng, J.-T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.; Sethi, G.; Xin, H.-W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [Green Version]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Zhou, M.; Huang, D.; Wasan, H.S.; Zhang, K.; Sun, L.; Huang, H.; Ma, S.; Shen, M.; Ruan, S. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial- mesenchymal transition via the AKT/GSK-3β/Snail signaling pathway. Mol. Med. Rep. 2019, 20, 2783–2795. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Shanmugam, M.K.; Rangappa, S.; Sethi, G.; Siveen, K.S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Basappa, S.; et al. Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie 2020, 175, 58–68. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Ali, Z.; Baek, S.H.; Mirza, B.; Ahn, K.S. Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin. Biomedicines 2020, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Naidu, V.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules 2020, 25, 2278. [Google Scholar] [CrossRef]
- Hwang, S.T.; Um, J.-Y.; Chinnathambi, A.; Alharbi, S.A.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Ahn, K.S. Evodiamine Mitigates Cellular Growth and Promotes Apoptosis by Targeting the c-Met Pathway in Prostate Cancer Cells. Molecules 2020, 25, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, I.; Ramchandani, S.; Khan, R.A.; Yang, M.H.; Ahn, K.S. Anticancer Potential of Raddeanin A, a Natural Triterpenoid Isolated from Anemone raddeana Regel. Molecules 2020, 25, 1035. [Google Scholar] [CrossRef] [Green Version]
- Pingili, R.; Pawar, A.K.; Challa, S.R.; Kodali, T.; Koppula, S.; Toleti, V. A comprehensive review on hepatoprotective and nephroprotective activities of chrysin against various drugs and toxic agents. Chem. Interact. 2019, 308, 51–60. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Pyrgelis, E.-S.; Piperi, C. Neuroprotective potential of chrysin in Parkinson’s disease: Molecular mechanisms and clinical implications. Neurochem. Int. 2020, 132, 104612. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; Plygun, S.; Heydari, M.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef] [PubMed]
- Bajgai, S.P.; Prachyawarakorn, V.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Hybrid flavan-chalcones, aromatase and lipoxygenase inhibitors, from Desmos cochinchinensis. Phytochemistry 2011, 72, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Hadjmohammadi, M.R.; Nazari, S.S.S.J. Separation optimization of quercetin, hesperetin and chrysin in honey by micellar liquid chromatography and experimental design. J. Sep. Sci. 2010, 33, 3144–3151. [Google Scholar] [CrossRef]
- Canini, A.; Pichichero, E.; Cicconi, R.; Mattei, M.; Muzi, M.G. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int. J. Oncol. 2010, 37, 973–981. [Google Scholar] [CrossRef] [Green Version]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef]
- Balta, C.; Herman, H.; Boldura, O.; Gasca, I.; Rosu, M.; Ardelean, A.; Hermenean, A. Chrysin attenuates liver fibrosis and hepatic stellate cell activation through TGF-β/Smad signaling pathway. Chem. Interact. 2015, 240, 94–101. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Galijatovic, A.; Otake, Y.; Walle, U.K.; Walle, T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica 1999, 29, 1241–1256. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar]
- Walle, U.; Galijatovic, A.; Walle, T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 1999, 58, 431–438. [Google Scholar] [CrossRef]
- Ferrado, J.B.; Perez, A.A.; Visentini, F.F.; Islan, G.A.; Castro, G.R.; Santiago, L.G. Formation and characterization of self-assembled bovine serum albumin nanoparticles as chrysin delivery systems. Colloids Surf. B Biointerfaces 2019, 173, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Belhan, S.; Yıldırım, S.; Karasu, A.; Kömüroğlu, A.U.; Özdek, U. Investigation of the protective role of chrysin within the framework of oxidative and inflammatory markers in experimental testicular ischaemia/reperfusion injury in rats. Andrologia 2020, 13714. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Boubaker, J.; Loussaief, A.; Jomaa, K.; Ghedira, K.; Chekir-Ghedira, L. Protective Effect of Chrysin, a Dietary Flavone against Genotoxic and Oxidative Damage Induced by Mitomycin C in Balb/C Mice. Nutr. Cancer 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jiang, M.; Zhang, Y.; Liu, X.; Li, Y.; Feng, G. Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma. Int. Immunopharmacol. 2016, 32, 24–31. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kang, M.-K.; Kim, Y.-H.; Kim, D.Y.; Oh, H.; Kim, S.-I.; Oh, S.Y.; Kang, Y.-H. Dietary Chrysin Suppresses Formation of Actin Cytoskeleton and Focal Adhesion in AGE-Exposed Mesangial Cells and Diabetic Kidney: Role of Autophagy. Nutrients 2019, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Bortolotto, V.C.; Araujo, S.M.; Pinheiro, F.C.; Poetini, M.R.; De Paula, M.T.; Meichtry, L.B.; De Almeida, F.P.; Musachio, E.A.S.; Guerra, G.P.; Prigol, M. Modulation of glutamate levels and Na+, K+-ATPase activity contributes to the chrysin memory recovery in hypothyroidism mice. Physiol. Behav. 2020, 222, 112892. [Google Scholar] [CrossRef]
- Song, Y.; Wu, W.; Sheng, L.; Jiang, B.; Li, X.; Cai, K. Chrysin ameliorates hepatic steatosis induced by a diet deficient in methionine and choline by inducing the secretion of hepatocyte nuclear factor 4α-dependent very low-density lipoprotein. J. Biochem. Mol. Toxicol. 2020, 34, e22497. [Google Scholar] [CrossRef]
- Yang, M.; Xiong, J.; Zou, Q.; Wang, D.-D.; Huang, C. Chrysin attenuates interstitial fibrosis and improves cardiac function in a rat model of acute myocardial infarction. J. Mol. Histol. 2018, 49, 555–565. [Google Scholar] [CrossRef]
- Choi, J.H.; Yun, J.W. Chrysin induces brown fat–like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrients 2016, 32, 1002–1010. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.-S.; Juvekar, A.R. Chrysin ameliorates nonalcoholic fatty liver disease in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Kazemi, S.; Hosseini, M.; Varzi, H.N.; Feyzi, F.; Morakabati, P.; Moghadamnia, A.A.; Mohammadi, A. Chrysin Effect in Prevention of Acetaminophen-Induced Hepatotoxicity in Rat. Chem. Res. Toxicol. 2019, 32, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shi, H.; Liu, D. Chrysin protects against renal ischemia reperfusion induced tubular cell apoptosis and inflammation in mice. Exp. Ther. Med. 2019, 17, 2256–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melekoglu, R.; Çiftçi, O.; Eraslan, S.; Alan, S.; Başak, N. The Protective Effects of Glycyrrhetinic Acid and Chrysin against Ischemia-Reperfusion Injury in Rat Ovaries. BioMed Res. Int. 2018, 2018, 5421308. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, L.; Xiao, J.; Wang, C.; Jiang, W.; Zhang, R.; Hao, J. Chrysin Protects against Focal Cerebral Ischemia/Reperfusion Injury in Mice through Attenuation of Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2014, 15, 20913–20926. [Google Scholar] [CrossRef] [Green Version]
- Saliba-Gustafsson, P.; Pedrelli, M.; Gertow, K.; Werngren, O.; Janas, V.; Pourteymour, S.; Baldassarre, D.; Tremoli, E.; Veglia, F.; Rauramaa, E.; et al. Subclinical atherosclerosis and its progression are modulated by PLIN2 through a feed-forward loop between LXR and autophagy. J. Intern. Med. 2019, 286, 660–675. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, A.; Sevanan, M.; Mani, S.; Balu, M.; Balaji, S.; Ramajayan, P. Chrysin restores MPTP induced neuroinflammation, oxidative stress and neurotrophic factors in an acute Parkinson’s disease mouse model. Neurosci. Lett. 2019, 709, 134382. [Google Scholar] [CrossRef]
- Rashno, M.; Ghaderi, S.; Nesari, A.; Khorsandi, L.; Farbood, Y.; Sarkaki, A. Chrysin attenuates traumatic brain injury-induced recognition memory decline, and anxiety/depression-like behaviors in rats: Insights into underlying mechanisms. Psychopharmacology 2020, 237, 1–13. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, J.; Zhu, S.; Lan, T.; Yang, J.; Li, L. Chrysin Alleviates Chronic Hypoxia–Induced Pulmonary Hypertension by Reducing Intracellular Calcium Concentration in Pulmonary Arterial Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2019, 74, 426–435. [Google Scholar] [CrossRef]
- Li, H.-J.; Wu, N.-L.; Pu, C.-M.; Hsiao, C.-Y.; Chang, D.-C.; Hung, C.-F. Chrysin alleviates imiquimod-induced psoriasis-like skin inflammation and reduces the release of CCL20 and antimicrobial peptides. Sci. Rep. 2020, 10, 2932. [Google Scholar] [CrossRef] [Green Version]
- Koc, F.; Tekeli, M.Y.; Kanbur, M.; Karayigit, M.Ö.; Liman, B.C. The effects of chrysin on lipopolysaccharide-induced sepsis in rats. J. Food Biochem. 2020, e13359. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, L.; De Gomes, M.G.; Souza, L.C.; Goes, A.R.; Boeira, S.P.; Oliveira, M.S.; Furian, A.F.; Jesse, C.R. Chrysin suppress immune responses and protects from experimental autoimmune encephalomyelitis in mice. J. Neuroimmunol. 2019, 335, 577007. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, Q.; Tang, G.; Song, C.; Wang, G.; Zhang, Y.; Xiao, Y.; Zeng, X.; Wang, Z.; Xiao, J.; et al. Synthesis and Anti-Thyroid Cancer Effect of Iodo-Chrysin Derivatives. Med. Chem. 2015, 12, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Jabir, M.S.; Hameed, A.H. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Andrade, S.; Silva, C.; Rodrigues, I.; Guardão, L.; Guimarães, J.T.; Keating, E.; Martel, F. Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats. Eur. J. Nutr. 2019, 59, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Prajit, R.; Sritawan, N.; Suwannakot, K.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats. Nutrients 2020, 12, 1100. [Google Scholar] [CrossRef]
- Shooshtari, M.K.; Sarkaki, A.; Mansouri, S.M.T.; Badavi, M.; Khorsandi, L.; Dehcheshmeh, M.G.; Farbood, Y. Protective effects of Chrysin against memory impairment, cerebral hyperemia and oxidative stress after cerebral hypoperfusion and reperfusion in rats. Metab. Brain Dis. 2019, 35, 401–412. [Google Scholar] [CrossRef]
- Khezri, S.; Sabzalipour, T.; Jahedsani, A.; Azizian, S.; Atashbar, S.; Salimi, A. Chrysin ameliorates aluminum p hosphide-induced oxidative stress and mitochondrial damages in rat cardiomyocytes and isolated mitochondria. Environ. Toxicol. 2020. [Google Scholar] [CrossRef]
- Baykalir, B.G.; Arslan, A.S.; Mutlu, S.I.; Ak, T.P.; Seven, I.; Seven, P.T.; Yaman, M.; Gul, H.F. The protective effect of chrysin against carbon tetrachloride-induced kidney and liver tissue damage in rats. Int. J. Vitam. Nutr. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 325–337. [Google Scholar] [CrossRef]
- Liao, Z.-Y.; Liang, I.-C.; Li, H.-J.; Wu, C.-C.; Lo, H.-M.; Chang, D.-C.; Hung, C.-F. Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation. Int. J. Mol. Sci. 2020, 21, 5541. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, W.; Zych, M.; Borymski, S.; Kaczmarczyk-Sedlak, I. Chrysin Reduces Oxidative Stress but Does Not Affect Polyol Pathway in the Lenses of Type 1 Diabetic Rats. Antioxidants 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagundes, F.L.; Piffer, G.D.M.; Périco, L.L.; Rodrigues, V.P.; Lima, C.A.H.; Santos, R.D.C.D. Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing. Int. J. Mol. Sci. 2020, 21, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, C.E.; Bryan, L.; Jemal, A. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kar, S.; Lai, X.; Cai, W.; Arfuso, F.; Sethi, G.; Lobie, P.E.; Goh, B.C.; Lim, L.H.K.; Hartman, M.; et al. Triple negative breast cancer in Asia: An insider’s view. Cancer Treat. Rev. 2018, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anti-Cancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef]
- Haque, M.; Desai, K.V. Pathways to Endocrine Therapy Resistance in Breast Cancer. Front. Endocrinol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Pandey, K.; An, H.-J.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef] [Green Version]
- Bhuvanalakshmi, G.; Rangappa, K.S.; Dharmarajan, A.; Sethi, G.; Kumar, A.P.; Warrier, S. Basappa Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt β-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front. Pharmacol. 2017, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yuan, Y.; Kar, S.; Kanchi, M.M.; Arora, S.; Kim, J.E.; Koh, P.F.; Yousef, E.; Samy, R.P.; Shanmugam, M.K.; et al. PPARγ Ligand–induced Annexin A1 Expression Determines Chemotherapy Response via Deubiquitination of Death Domain Kinase RIP in Triple-negative Breast Cancers. Mol. Cancer Ther. 2017, 16, 2528–2542. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, C.; Tao, Z.; Zhao, L.; Zhu, Z.; Wu, W.; He, Y.; Chen, H.; Zheng, B.; Huang, X.; et al. Curcumin derivative WZ35 inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Jiang, H.; Chen, Y.; Ren, F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J. Cell. Biochem. 2019, 120, 16283–16292. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor–negative breast cancer cells. J. Cell. Biochem. 2018, 120, 4504–4513. [Google Scholar] [CrossRef]
- Maasomi, Z.J.; Soltanahmadid, Y.P.; Dadashpour, M.; Alipour, S.; Abolhasani, S.; Zarghami, N. Synergistic Anticancer Effects of Silibinin and Chrysin in T47D Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18, 1283–1287. [Google Scholar]
- Kolibaba, K.S.; Druker, B.J. Protein tyrosine kinases and cancer. Biochim. Biophys. Acta (BBA) Bioenergy 1997, 1333, F217–F248. [Google Scholar] [CrossRef]
- Lacouture, M.E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat. Rev. Cancer 2006, 6, 803–812. [Google Scholar] [CrossRef]
- Choowongkomon, K.; Sawatdichaikul, O.; Songtawee, N.; Limtrakul, J. Receptor-Based Virtual Screening of EGFR Kinase Inhibitors from the NCI Diversity Database. Molecules 2010, 15, 4041–4054. [Google Scholar] [CrossRef] [Green Version]
- Debnath, S.; Kanakaraju, M.; Islam, M.; Yeeravalli, R.; Sen, D.; Das, A. In silico design, synthesis and activity of potential drug-like chrysin scaffold-derived selective EGFR inhibitors as anticancer agents. Comput. Biol. Chem. 2019, 83, 107156. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, J.H.; Lee, S.H. Hypoxia-induced PGC-1α Regulates Mitochondrial Function and Tumorigenesis of Colorectal Cancer Cells. Anticancer Res. 2019, 39, 4865–4876. [Google Scholar] [CrossRef]
- Ren, S.; Liu, J.; Feng, Y.; Li, Z.; He, L.; Li, L.; Cao, X.; Wang, Z.; Zhang, Y. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J. Exp. Clin. Cancer Res. 2019, 38, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Multhoff, G.; Vaupel, P. Hypoxia Compromises Anti-Cancer Immune Responses. Adv. Exp. Med. Biol. 2020, 131–143. [Google Scholar] [CrossRef]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Bean, J.M.; Prosnitz, L.R.; Dewhirst, M.W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996, 56, 941–943. [Google Scholar] [PubMed]
- Hockel, M.; Schlenger, K.; Aral, B.; Mitze, M.; Schaffer, U.; Vaupel, P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996, 56, 4509–4515. [Google Scholar]
- Minet, E.; Michel, G.; Mottet, D.; Raes, M.; Michiels, C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic. Biol. Med. 2001, 31, 847–855. [Google Scholar] [CrossRef]
- Jung, J.E.; Lee, H.-G.; Cho, I.-H.; Chung, D.H.; Yoon, S.-H.; Yang, Y.M.; Lee, J.W.; Choi, S.; Park, J.-W.; Ye, S.-K.; et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005, 19, 1296–1298. [Google Scholar] [CrossRef]
- Barr, M.P.; Bouchier-Hayes, D.J.; Harmey, J.J. Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. Int. J. Oncol. 2008, 32, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lu, A.; Beech, D.; Jiang, B.; Lu, Y. Suppression of breast cancer metastasis through the inhibition of VEGF-mediated tumor angiogenesis. Cancer Ther. 2007, 5, 273–286. [Google Scholar]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Maruyama, T.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Yagita, H.; Ruchirawat, S.; et al. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol. Rep. 2013, 30, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, Y.; Park, E.H.; Lee, S.; Kim, H.; Kim, A.; Lee, S.B.; Shim, S.; Jang, H.; Myung, J.K.; et al. Effects of metformin and phenformin on apoptosis and epithelial-mesenchymal transition in chemoresistant rectal cancer. Cancer Sci. 2019, 110, 2834–2845. [Google Scholar] [CrossRef]
- Xue, J.; Li, L.; Li, N.; Li, F.; Qin, X.; Li, T.; Liu, M. Metformin suppresses cancer cell growth in endometrial carcinoma by inhibiting PD-L1. Eur. J. Pharmacol. 2019, 859, 172541. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, S.; Zarghami, N. Synergistic Growth Inhibitory Effects of Chrysin and Metformin Combination on Breast Cancer Cells through hTERT and Cyclin D1 Suppression. Asian Pac. J. Cancer Prev. 2018, 19, 977–982. [Google Scholar] [PubMed]
- International Agency for Research on Cancer. Chromium, Nickel and Welding. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1990; Volume 49. [Google Scholar]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine Attenuates Tumor Growth and Deactivates STAT5 Signaling in a Lung Cancer Xenograft Model. Cancers 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, C.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Ophiopogonin D modulates multiple oncogenic signaling pathways, leading to suppression of proliferation and chemosensitization of human lung cancer cells. Phytomedicine 2018, 40, 165–175. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Wong, R.-H.; Lin, J.-Y.; Lai, J.-C.; Lee, H. Accumulation of Chromium and Nickel Metals in Lung Tumors from Lung Cancer Patients in Taiwan. J. Toxicol. Environ. Health Part A 2006, 69, 1337–1344. [Google Scholar] [CrossRef]
- Huang, H.-H.; Huang, J.-Y.; Lung, C.-C.; Wu, C.-L.; Ho, C.-C.; Sun, Y.-H.; Ko, P.-C.; Su, S.-Y.; Chen, S.-C.; Wang, B.-Y. Cell-type specificity of lung cancer associated with low-dose soil heavy metal contamination in Taiwan: An ecological study. BMC Public Health 2013, 13, 330. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-H.; Tang, S.C.; Wang, P.-H.; Lee, H.; Ko, J.-L. Nickel-induced Epithelial-Mesenchymal Transition by Reactive Oxygen Species Generation and E-cadherin Promoter Hypermethylation. J. Biol. Chem. 2012, 287, 25292–25302. [Google Scholar] [CrossRef] [Green Version]
- Chiou, Y.-H.; Liou, S.-H.; Wong, R.-H.; Chen, C.-Y.; Lee, H. Nickel may contribute to EGFR mutation and synergistically promotes tumor invasion in EGFR-mutated lung cancer via nickel-induced microRNA-21 expression. Toxicol. Lett. 2015, 237, 46–54. [Google Scholar] [CrossRef]
- Guo, H.; Deng, H.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Wu, B.; Chen, K. Nickel chloride (NiCl2)-caused inflammatory responses via activation of NF-κB pathway and reduction of anti-inflammatory mediator expression in the kidney. Oncotarget 2015, 6, 28607–28620. [Google Scholar] [CrossRef] [Green Version]
- Basith, S.; Manavalan, B.; Yoo, T.H.; Kim, S.G.; Choi, S. Roles of toll-like receptors in Cancer: A double-edged sword for defense and offense. Arch. Pharmacal Res. 2012, 35, 1297–1316. [Google Scholar] [CrossRef]
- Yesudhas, D.; Gosu, V.; Anwar, M.A.; Choi, S. Multiple Roles of Toll-Like Receptor 4 in Colorectal Cancer. Front. Immunol. 2014, 5, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.C.; Chan, S.T.; Chang, C.N.; Yu, P.S.; Chuang, C.H.; Yeh, S.L. Quercetin and chrysin inhibit nickel-induced invasion and migration by downregulation of TLR4/NF-κB signaling in A549 cells. Chem. Biol. Interact. 2018, 292, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.-H.; Lee, S.G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2016, 8, 17700–17711. [Google Scholar] [CrossRef]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.K.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid. Redox Signal. 2016, 24, 575–589. [Google Scholar] [CrossRef]
- Mandair, D.; Rossi, R.E.; Pericleous, M.; Whyand, T.; Caplin, M. Prostate cancer and the influence of dietary factors and supplements: A systematic review. Nutr. Metab. 2014, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Potosky, A.L.; Haque, R.; Cassidy-Bushrow, A.E.; Yood, M.U.; Jiang, M.; Tsai, H.-T.; Luta, G.; Keating, N.L.; Smith, M.R.; Eeden, S.K.V.D. Effectiveness of Primary Androgen-Deprivation Therapy for Clinically Localized Prostate Cancer. J. Clin. Oncol. 2014, 32, 1324–1330. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Sun, L.; Chu, Y.; Li, T.; Lei, C.; Wang, X.; Jiang, M.; Min, Y.; Lu, Y.; Zhao, X.; et al. DDIT4 promotes gastric cancer proliferation and tumorigenesis through the p53 and MAPK pathways. Cancer Commun. 2018, 38, 45. [Google Scholar] [CrossRef] [Green Version]
- Presti, D.; Quaquarini, E. The PI3K/AKT/mTOR and CDK4/6 Pathways in Endocrine Resistant HR+/HER2- Metastatic Breast Cancer: Biological Mechanisms and New Treatments. Cancers 2019, 11, 1242. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Di Grazia, A.; De Simone, V.; Cherubini, F.; Colantoni, A.; Ortenzi, A.; Franzè, E.; DiNallo, V.; Di Fusco, D.; Monteleone, I.; et al. Induction of endoplasmic reticulum stress and inhibition of colon carcinogenesis by the anti-helmintic drug rafoxanide. Cancer Lett. 2019, 462, 1–11. [Google Scholar] [CrossRef]
- Okubo, K.; Isono, M.; Asano, T.; Sato, A. Lopinavir-Ritonavir Combination Induces Endoplasmic Reticulum Stress and Kills Urological Cancer Cells. Anticancer Res. 2019, 39, 5891–5901. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Z.; Wang, J.; Wu, Y.; Chen, W.; Zheng, L.; Xi, T.; Wang, A.; Lu, Y. MicroRNA-9 and breast cancer. Biomed. Pharmacother. 2019, 122, 109687. [Google Scholar] [CrossRef]
- Niyonizigiye, I.; Ngabire, D.; Patil, M.P.; Singh, A.A.; Kim, G.-D. In vitro induction of endoplasmic reticulum stress in human cervical adenocarcinoma HeLa cells by fucoidan. Int. J. Biol. Macromol. 2019, 137, 844–852. [Google Scholar] [CrossRef]
- Jessmon, P.; Boulanger, T.; Zhou, W.; Patwardhan, P. Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev. Anticancer. Ther. 2017, 17, 427–437. [Google Scholar] [CrossRef]
- Guo, X.; Mei, J.; Jing, Y.; Wang, S. Curcumin-Loaded Nanoparticles with Low-Intensity Focused Ultrasound-Induced Phase Transformation as Tumor-Targeted and pH-Sensitive Theranostic Nanoplatform of Ovarian Cancer. Nanoscale Res. Lett. 2020, 15, 73. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Yang, Y.; Liu, T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agents Cancer 2019, 14, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2019, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Ryu, S.; Bazer, F.W.; Kim, S.-M.; Song, G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J. Cell. Physiol. 2017, 233, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Yu, H.; Chen, T. Inhibition of esophageal cancer growth through the suppression of PI3K/AKT/mTOR signaling pathway. OncoTargets Ther. 2019, 12, 7637–7647. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Ma, Z.; Zhang, G.; Yang, X.; Du, Q.; Wang, W. CSNK2A1 Promotes Gastric Cancer Invasion Through the PI3K-Akt-mTOR Signaling Pathway. Cancer Manag. Res. 2019, 11, 10135–10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef]
- Hwang, S.T.; Kim, C.; Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.; Sethi, G.; Ahn, K.S. Cycloastragenol can negate constitutive STAT3 activation and promote paclitaxel-induced apoptosis in human gastric cancer cells. Phytomedicine 2019, 59, 152907. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2013, 92, 267–276. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-F.; Li, B.-Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-Mediated Formation of 5-Carboxylcytosine and Its Excision by TDG in Mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef] [Green Version]
- Yatagai, N.; Saito, T.; Akazawa, Y.; Hayashi, T.; Yanai, Y.; Tsuyama, S.; Ueyama, H.; Murakami, T.; Watanabe, S.; Nagahara, A.; et al. TP53 inactivation and expression of methylation-associated proteins in gastric adenocarcinoma with enteroblastic differentiation. Virchows Arch. 2018, 474, 315–324. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, X.; Huang, C.; Gao, Y.; Yang, C.; Zeng, P.; Chen, Z. The FENDRR/miR-214-3P/TET2 axis affects cell malignant activity via RASSF1A methylation in gastric cancer. Am. J. Transl. Res. 2018, 10, 3211–3223. [Google Scholar] [PubMed]
- Du, C.; Kurabe, N.; Matsushima, Y.; Suzuki, M.; Kahyo, T.; Ohnishi, I.; Tanioka, F.; Tajima, S.; Goto, M.; Yamada, H.; et al. Robust quantitative assessments of cytosine modifications and changes in the expressions of related enzymes in gastric cancer. Gastric Cancer 2014, 18, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells. OncoTargets Ther. 2020, 13, 3277–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Zhang, J.; Zhang, K.; Zhao, Y. Curcumin inhibits cell viability, migration, and invasion of thymic carcinoma cells via downregulation of microRNA-27a. Phytother. Res. 2020, 34, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zheng, Z.; Huang, J.; Ma, X.; Huang, C.; Wu, R.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; et al. Modulation of miR-34a in curcumin-induced antiproliferation of prostate cancer cells. J. Cell. Biochem. 2019, 120, 15616–15624. [Google Scholar] [CrossRef]
- Boubaker, N.S.; Spagnuolo, M.; Trabelsi, N.; Said, R.; Gurtner, A.; Regazzo, G.; Ayed, H.; Blel, A.; Karray, O.; Saadi, A.; et al. miR-143 expression profiles in urinary bladder cancer: Correlation with clinical and epidemiological parameters. Mol. Biol. Rep. 2019, 47, 1283–1292. [Google Scholar] [CrossRef]
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.H.; Mohammadi, A.; Gjerstorff, M.F.; Baradaran, B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Jiang, W.; Zhang, R.; Zhang, B.; Silayiding, A.; Duan, X. MicroRNA-135a promotes proliferation, migration, invasion and induces chemoresistance of endometrial cancer cells. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 5, 100103. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Zhao, S.; Jin, B.; Jia, R.; Ge, J.; Xu, H. MicroRNA-186-5p Inhibits Proliferation And Metastasis Of Esophageal Cancer By Mediating HOXA9. OncoTargets Ther. 2019, 12, 8905–8914. [Google Scholar] [CrossRef] [Green Version]
- Mohammadian, F.; Soltanahmadid, Y.P.; Zarghami, F.; Akbarzadeh, A.; Zarghami, N. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1201–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, H.; Li, C.; Zhang, T.; Meng, X.; Zhang, Y.; Qian, H. Spheres from cervical cancer cells display stemness and cancer drug resistance. Oncol. Lett. 2016, 12, 2184–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.-M.; Shen, C.-J.; Chang, C.-C.; Chou, C.-Y.; Tsai, C.-C.; Hsu, Y.-C. Inducement of apoptosis by cucurbitacin E, a tetracyclic triterpenes, through death receptor 5 in human cervical cancer cell lines. Cell Death Discov. 2017, 3, 17014. [Google Scholar] [CrossRef] [PubMed]
- Ningegowda, R.; Shivananju, N.S.; Rajendran, P.; Rangappa, K.S.; Chinnathambi, A.; Li, F.; Achar, R.R.; Shanmugam, M.K.; Bist, P.; Alharbi, S.A.; et al. A novel 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivative inhibits tumor cell invasion and potentiates the apoptotic effect of TNFα by abrogating NF-κB activation cascade. Apoptosis 2016, 22, 145–157. [Google Scholar] [CrossRef]
- Rashid, S.; Labani, S.; Das, B.C. Knowledge, Awareness and Attitude on HPV, HPV Vaccine and Cervical Cancer among the College Students in India. PLoS ONE 2016, 11, e0166713. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, E.; Santiago-López, L.; Cruz-Domínguez, V.B.; Toledo-Guzmán, M.E.; Hernández-Cueto, D.; Muñiz-Hernández, S.; Garrido, E.; De León, D.C.; García-Carrancá, A. Characterization of cervical cancer stem cell-like cells: Phenotyping, stemness, and human papilloma virus co-receptor expression. Oncotarget 2016, 7, 31943–31954. [Google Scholar] [CrossRef] [Green Version]
- Vishnoi, K.; Mahata, S.; Tyagi, A.; Pandey, A.; Verma, G.; Jadli, M.; Singh, T.; Singh, S.M.; Bharti, A.C. Cross-talk between Human Papillomavirus Oncoproteins and Hedgehog Signaling Synergistically Promotes Stemness in Cervical Cancer Cells. Sci. Rep. 2016, 6, 34377. [Google Scholar] [CrossRef] [Green Version]
- Hazelbag, S.; Fleuren, G.J.; Baelde, H.J.; Schuuring, E.; Kenter, G.G.; Gorter, A. Cytokine Profile of Cervical Cancer Cells. Gynecol. Oncol. 2001, 83, 235–243. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Walayat, S.; Chen, H.W.; Wang, Y. Association between cytokine gene polymorphisms and cervical cancer in a Chinese population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 330–333. [Google Scholar] [CrossRef]
- Yang, B.; Lu, Y.; Zhang, A.; Zhou, A.; Zhang, L.; Zhang, L.; Gao, L.; Zang, Y.; Tang, X.; Sun, L. Doxycycline Induces Apoptosis and Inhibits Proliferation and Invasion of Human Cervical Carcinoma Stem Cells. PLoS ONE 2015, 10, e0129138. [Google Scholar]
- Dong, W.; Chen, A.; Chao, X.; Li, X.; Cui, Y.; Xu, C.; Cao, J.; Ning, Y.; Cao, X. Chrysin Inhibits Proinflammatory Factor-Induced EMT Phenotype and Cancer Stem Cell-Like Features in HeLa Cells by Blocking the NF-κB/Twist Axis. Cell. Physiol. Biochem. 2019, 52, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Pokhrel, S.; Vaidya, B.N.; Joshee, N. In vitro micropropagation and callus induction in Scutellaria discolor colebr.–A medicinally important plant of Nepal. Indian J. Plant Genet. Resour. 1998, 12, 219–223. [Google Scholar]
- Laishram, S.; Moirangthem, D.S.; Borah, J.C.; Pal, B.C.; Suman, P.; Gupta, S.K.; Kalita, M.C.; Talukdar, N.C. Chrysin rich Scutellaria discolor Colebr. induces cervical cancer cell death via the induction of cell cycle arrest and caspase-dependent apoptosis. Life Sci. 2015, 143, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Líšková, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—Concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009, 19, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, G.J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015, 34, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rho, M.; Kim, J.; Jee, C.D.; Lee, Y.M.; Lee, H.E.; Kim, M.A.; Lee, H.S.; Kim, W.H. Expression of type 2 hexokinase and mitochondria-related genes in gastric carcinoma tissues and cell lines. Anticancer Res. 2007, 27, 251–258. [Google Scholar]
- Suh, D.H.; Kim, M.A.; Kim, H.; Kim, M.K.; Kim, H.-S.; Chung, H.H.; Kim, Y.B.; Song, Y.S. Association of overexpression of hexokinase II with chemoresistance in epithelial ovarian cancer. Clin. Exp. Med. 2013, 14, 345–353. [Google Scholar] [CrossRef]
- Palmieri, D.; Fitzgerald, D.; Shreeve, S.M.; Hua, E.; Bronder, J.L.; Weil, R.J.; Davis, S.; Stark, A.M.; Merino, M.J.; Kurek, R.; et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 2009, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo-Qing, P.; Yuan, Y.; Cai-Gao, Z.; Hongling, Y.; Gonghua, H.; Yan, T. A study of association between expression of hOGG1, VDAC1, HK-2 and cervical carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seydi, E.; Rahimpour, Z.; Salimi, A.; Pourahmad, J. Selective toxicity of chrysin on mitochondria isolated from liver of a HCC rat model. Bioorg. Med. Chem. 2019, 27, 115163. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Cheung, S.T. STAT3: An Emerging Therapeutic Target for Hepatocellular Carcinoma. Cancers 2019, 11, 1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, S.G.; Kameshwar, V.H.; Shubha, P.B.; Looi, C.Y.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Sethi, G.; Swamy, S.N.; Bishayee, A. Targeting multiple oncogenic pathways for the treatment of hepatocellular carcinoma. Target. Oncol. 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z.; et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–833. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Chang, Y.-C.; Kumar, D.; et al. Ascochlorin Enhances the Sensitivity of Doxorubicin Leading to the Reversal of Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Chen, Y.; Dong, T.; Yue, M.; Zhang, Y.; An, T.; Zhang, J.; Liu, P.; Yang, X. Polydatin inhibits hepatocellular carcinoma via the AKT/STAT3-FOXO1 signaling pathway. Oncol. Lett. 2019, 17, 4505–4513. [Google Scholar] [CrossRef] [Green Version]
- Mohan, C.D.; Bharathkumar, H.; Bulusu, K.C.; Pandey, V.; Rangappa, S.; Fuchs, J.E.; Shanmugam, M.K.; Dai, X.; Li, F.; Deivasigamani, A.; et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. [Google Scholar] [CrossRef] [Green Version]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma. Anti-Cancer Drugs 2015, 26, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, F.; Xiao, X.; Pan, W.; Yuan, Q.; Cao, J. Chrysin inhibits sphere formation in SMMC-7721 cells via modulation of SHP-1/STAT3 signaling pathway. Cancer Manag. Res. 2019, 11, 2977–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuphal, S.; Bosserhoff, A.-K. Recent progress in understanding the pathology of malignant melanoma. J. Pathol. 2009, 219, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Eilber, F.R.; Malmgren, R.A.; Wood, W.C. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery 1970, 68, 158. [Google Scholar]
- Bittner, M.; Meltzer, P.; Chen, Y.; Jiang, Y.; Seftor, E.; Hendrix, M.; Radmacher, M.; Simon, R.; Yakhini, Z.; Ben-Dor, A.; et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000, 406, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Ghazaeian, M.; Khorsandi, K.; Hosseinzadeh, R.; Naderi, A.; Abrahamse, H. Curcumin–silica nanocomplex preparation, hemoglobin and DNA interaction and photocytotoxicity against melanoma cancer cells. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef]
- Yang, H.-Z.; Zhang, J.; Zeng, J.; Liu, S.; Zhou, F.; Zhang, F.; Giampieri, F.; Cianciosi, D.; Forbes-Hernandez, T.Y.; Ansary, J.; et al. Resveratrol inhibits the proliferation of melanoma cells by modulating cell cycle. Int. J. Food Sci. Nutr. 2019, 71, 84–93. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, G.-X.; Wu, Y.; Xiao, J.; Liu, J.; Qiu, P.; Liu, X.; Sun, L.; Du, B.; et al. Synergistic inhibitory effect of resveratrol and TK/GCV therapy on melanoma cells. J. Cancer Res. Clin. Oncol. 2020, 146, 1489–1499. [Google Scholar] [CrossRef]
- Sassi, A.; Maatouk, M.; El Gueder, D.; Bzéouich, I.M.; Hatira, S.A.-B.; Jemni-Yacoub, S.; Ghedira, K.; Chekir, L. Chrysin, a natural and biologically active flavonoid suppresses tumor growth of mouse B16F10 melanoma cells: In vitro and In vivo study. Chem. Interact. 2018, 283, 10–19. [Google Scholar] [CrossRef]
- Folgueras, A.R.; Pendas, A.M.; Sánchez, L.M.; López-Otín, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004, 48, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babykutty, S.; Suboj, P.; Srinivas, P.; Nair, A.S.; Chandramohan, K.; Gopala, S. Insidious role of nitric oxide in migration/invasion of colon cancer cells by upregulating MMP-2/9 via activation of cGMP-PKG-ERK signaling pathways. Clin. Exp. Metastasis 2012, 29, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.D.; Feng, C.C.; Kuo, W.W.; Pai, P.; Chung, L.C.; Chang, S.H.; Hsu, H.H.; Tsai, F.J.; Lin, Y.M.; Huang, C.Y. Suppression of plasminogen activators and the MMP-2/-9 pathway by a Zanthoxylum avicennae extract to inhibit the HA22T human hepatocellular carcinoma cell migration and invasion effects in vitro and in vivo via phosphatase 2A activation. Biosci. Biotechnol. Biochem. 2013, 77, 1814–1821. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, F.; Chen, W.; Zhang, J.; Li, H. miRNAs: A Promising Target in the Chemoresistance of Bladder Cancer. OncoTargets Ther. 2019, 12, 11805–11816. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Li, H.; Wu, S.; Qu, J.; Yuan, H.; Zhou, Y.; Lu, Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int. Urol. Nephrol. 2019, 51, 1771–1779. [Google Scholar] [CrossRef]
- Almeida, T.C.; Guerra, C.C.C.; De Assis, B.L.G.; Soares, R.D.O.A.; Garcia, C.C.M.; Lima, A.A.; Da Silva, G.N. Antiproliferative and toxicogenomic effects of resveratrol in bladder cancer cells with different TP53 status. Environ. Mol. Mutagen. 2019, 60, 740–751. [Google Scholar] [CrossRef]
- Sun, N.; Liang, Y.; Chen, Y.; Wang, L.; Li, D.; Liang, Z.; Sun, L.; Wang, Y.; Niu, H. Glutamine affects T24 bladder cancer cell proliferation by activating STAT3 through ROS and glutaminolysis. Int. J. Mol. Med. 2019, 44, 2189–2200. [Google Scholar] [CrossRef]
- Korac-Prlic, J.; Degoricija, M.; Vilović, K.; Haupt, B.; Ivanišević, T.; Franković, L.; Grivennikov, S.; Terzić, J. Targeting Stat3 signaling impairs the progression of bladder cancer in a mouse model. Cancer Lett. 2020, 490, 89–99. [Google Scholar] [CrossRef]
- Anand, V.; Khandelwal, M.; Appunni, S.; Gupta, N.; Seth, A.; Singh, P.; Mathur, S.; Sharma, A. CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: Novel therapeutic approach. J. Cancer Res. Clin. Oncol. 2019, 145, 2649–2661. [Google Scholar] [CrossRef]
- Xu, Y.; Tong, Y.; Ying, J.; Lei, Z.; Wan, L.; Zhu, X.; Ye, F.; Mao, P.; Wu, X.; Pan, R.; et al. Chrysin induces cell growth arrest, apoptosis, and ER stress and inhibits the activation of STAT3 through the generation of ROS in bladder cancer cells. Oncol. Lett. 2018, 15, 9117–9125. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Berger, M.D.; Naseem, M.; Tokunaga, R.; Battaglin, F.; Cao, S.; Hanna, D.L.; McSkane, M.; Soni, S.; Zhang, W.; et al. Colorectal cancer: Epigenetic alterations and their clinical implications. Biochim. Biophys. Acta (BBA) Bioenergy 2017, 1868, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Coppedè, F.; Lopomo, A.; Spisni, R.; Migliore, L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J. Gastroenterol. 2014, 20, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef]
- Crea, F.; Nobili, S.; Paolicchi, E.; Perrone, G.; Napoli, C.; Landini, I.; Danesi, R.; Mini, E. Epigenetics and chemoresistance in colorectal cancer: An opportunity for treatment tailoring and novel therapeutic strategies. Drug Resist. Updat. 2011, 14, 280–296. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Chen, C.-I.; Hsiang, Y.-P.; Hsu, Y.-C.; Cheng, K.-C.; Chien, P.-H.; Pan, H.-L.; Lu, C.-C.; Chen, Y.-J. Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin. Int. J. Mol. Sci. 2018, 19, 1763. [Google Scholar] [CrossRef] [Green Version]
- Eghtedardoost, M.; Ghazanfari, T.; Sadeghipour, A.; Hassan, Z.M.; Ghanei, M.; Ghavami, S. Delayed effects of sulfur mustard on autophagy suppression in chemically-injured lung tissue. Int. Immunopharmacol. 2020, 80, 105896. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Linder, B.; Kögel, D. Autophagy in Cancer Cell Death. Biology 2019, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Teng, J.-F.; Qin, D.-L.; Mei, Q.-B.; Qiu, W.-Q.; Pan, R.; Xiong, R.; Zhao, Y.; Law, B.Y.-K.; Wong, V.K.-W.; Tang, Y.; et al. Polyphyllin VI, a saponin from Trillium tschonoskii Maxim. induces apoptotic and autophagic cell death via the ROS triggered mTOR signaling pathway in non-small cell lung cancer. Pharmacol. Res. 2019, 147, 104396. [Google Scholar] [CrossRef]
- Majeed, T.; Wani, I.A.; Hamdani, A.M.; Bhat, N.A. Effect of sonication and γ-irradiation on the properties of pea (Pisum sativum) and vetch (Vicia villosa) starches: A comparative study. Int. J. Biol. Macromol. 2018, 114, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Jenjob, A.; Uthairatanakij, A.; Jitareerat, P.; Wongs-Aree, C.; Aiamla-Or, S. Effect of harvest seasonal and gamma irradiation on the physicochemical changes in pineapple fruit cv. Pattavia during stimulated sea shipment. Food Sci. Nutr. 2017, 5, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.A.; Song, H.-Y.; Byun, E.-H.; Ahn, N.-G.; Kim, H.-M.; Nam, Y.R.; Lee, G.H.; Jang, B.-S.; Choi, D.S.; Lee, D.-E.; et al. Gamma-irradiated black ginseng extract inhibits mast cell degranulation and suppresses atopic dermatitis-like skin lesions in mice. Food Chem. Toxicol. 2018, 111, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Y.; Kim, H.-M.; Mushtaq, S.; Kim, W.S.; Kim, Y.J.; Lim, S.-T.; Byun, E.-B. Gamma-Irradiated Chrysin Improves Anticancer Activity in HT-29 Colon Cancer Cells Through Mitochondria-Related Pathway. J. Med. Food 2019, 22, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Fruchart, J.-C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [Green Version]
- Sikka, S.; Chen, L.; Sethi, G.; Kumar, A.P. Targeting PPARγ Signaling Cascade for the Prevention and Treatment of Prostate Cancer. PPAR Res. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin Inhibits Proliferation and Invasion and Induces Apoptosis through the Modulation of Peroxisome Proliferator-activated Receptor γ Activation Pathway in Gastric Cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef] [Green Version]
- Berthold, D.R.; Pond, G.R.; Roessner, M.; De Wit, R.; Eisenberger, M.; Tannock, A.I.F. Treatment of Hormone-Refractory Prostate Cancer with Docetaxel or Mitoxantrone: Relationships between Prostate-Specific Antigen, Pain, and Quality of Life Response and Survival in the TAX-327 Study. Clin. Cancer Res. 2008, 14, 2763–2767. [Google Scholar] [CrossRef] [Green Version]
- Grau, R.; Punzón, C.; Fresno, M.; Iñiguez, M.A. Peroxisome-proliferator-activated receptor α agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochem. J. 2006, 395, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Waxman, D.J. P450 Gene Induction by Structurally Diverse Xenochemicals: Central Role of Nuclear Receptors CAR, PXR, and PPAR. Arch. Biochem. Biophys. 1999, 369, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Danielson, P.á. The Cytochrome P450 Superfamily: Biochemistry, Evolution and Drug Metabolism in Humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Winter, S.; Klumpp, B.; Turpeinen, M.; Klein, K.; Schwab, M.; Zanger, U.M. Peroxisome proliferator-activated receptor alpha, PPARα, directly regulates transcription of cytochrome P450 CYP2C8. Front. Pharmacol. 2015, 6, 261. [Google Scholar] [CrossRef] [Green Version]
- Khor, C.Y.; Khoo, B.Y. PPARα plays an important role in the migration activity, and the expression of CYP2S1 and CYP1B1 in chrysin-treated HCT116 cells. Biotechnol. Lett. 2020, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ji, F.; Guo, J.; Wang, J.; Li, Y.; Bao, Y. Directional modification of chrysin for exerting apoptosis and enhancing significantly anti-cancer effects of 10-hydroxy camptothecin. Biomed. Pharmacother. 2016, 82, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Chen, Y.H.; Fang, Y.L.; Zhong, L.Y.; Yuan, Q.Q.; Xu, X.Y.; Cao, J.G. Effects of chrysin on sphere formation and CK2α expression of ovarian cancer stem-like cells derived from SKOV3 cell line. Zhonghua Yi Xue Za Zhi 2016, 96, 2013–2016. [Google Scholar] [PubMed]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Al-Oudat, B.A.; Alqudah, M.A.; Audat, S.A.; Al-Balas, Q.A.; El-Elimat, T.; Hassan, M.A.; Frhat, I.N.; Azaizeh, M.M. Design, synthesis, and biologic evaluation of novel chrysin derivatives as cytotoxic agents and caspase-3/7 activators. Drug Des. Devel. Ther. 2019, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Peng, X. Effects of chrysin on the apoptosis in oral squamous carcinoma KB cell line and the underlying mechanisms. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 522–527. [Google Scholar]
- Ni, Z.; He, J.; Wu, Y.; Hu, C.; Dai, X.; Yan, X.; Li, B.; Li, X.; Xiong, H.; Li, Y.; et al. AKT-mediated phosphorylation of ATG4B impairs mitochondrial activity and enhances the Warburg effect in hepatocellular carcinoma cells. Autophagy 2018, 14, 685–701. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh, M.; Samarghandian, S.; Azimi-Nezhad, M.; Borji, A.; Jabbari, F.; Farkhondeh, T.; Samini, M. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacogn. Mag. 2016, 12, 436–S440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.-Z.; Wang, Z.-C.; Zhu, X.-H.; Zhu, D.; Li, Z.; Shen, F.-Q.; Duan, Y.-T.; Cao, H.; Zhao, J.; Zhu, H.-L. Design and biological evaluation of novel hybrids of 1, 5-diarylpyrazole and Chrysin for selective COX-2 inhibition. Bioorg. Med. Chem. 2018, 26, 4264–4275. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-M.; Phan, T.; Patel, P.N.; Jaskula-Sztul, R.; Chen, H.; Bs, P.N.P. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer 2012, 119, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, M.; Hashimoto, R.; Inoue, Y.; Ishiuchi, K.; Matsuno, M.; Itoh, Y.; Tokugawa, M.; Ohoka, N.; Morishita, D.; Mizukami, H.; et al. Anti-Tumorigenic Activity of Chrysin from Oroxylum indicum via Non-Genotoxic p53 Activation through the ATM-Chk2 Pathway. Molecules 2018, 23, 1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celinska-Janowicz, K.; Zareba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.; Park, S.; Lim, W.; Song, G. Chrysin disrupts intracellular homeostasis through mitochondria-mediated cell death in human choriocarcinoma cells. Biochem. Biophys. Res. Commun. 2018, 503, 3155–3161. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.; Baharara, J.; Amini, E. Anticancer Properties of Chrysin on Colon Cancer Cells, In vitro and In vivo with Modulation of Caspase-3, -9, Bax and Sall4. Iran. J. Biotechnol. 2016, 14, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Kahroba, H.; Shirmohamadi, M.; Hejazi, M.S.; Samadi, N. The Role of Nrf2 signaling in cancer stem cells: From stemness and self-renewal to tumorigenesis and chemoresistance. Life Sci. 2019, 239, 116986. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zang, F.; Zhang, S.-J. RBCK1 contributes to chemoresistance and stemness in colorectal cancer (CRC). Biomed. Pharmacother. 2019, 118, 109250. [Google Scholar] [CrossRef]
- Kirtonia, A.; Gala, K.; Fernandes, S.G.; Pandya, G.; Pandey, A.K.; Sethi, G.; Khattar, E.; Garg, M. Repurposing of drugs: An attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Paciello, F.; Fetoni, A.R.; Mezzogori, D.; Rolesi, R.; Di Pino, A.; Paludetti, G.; Grassi, C.; Troiani, D. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San, T.T.; Khaenam, P.; Prachayasittikul, V.; Sripa, B.; Kunkeaw, N.; Chan-On, W. Curcumin enhances chemotherapeutic effects and suppresses ANGPTL4 in anoikis-resistant cholangiocarcinoma cells. Heliyon 2020, 6, e03255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, V.K.; Shukla, D.; Kumar, A.; Vishvakarma, N.K. Curcumin circumvent lactate-induced chemoresistance in hepatic cancer cells through modulation of hydroxycarboxylic acid receptor-1. Int. J. Biochem. Cell Biol. 2020, 123, 105752. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, X.; Chen, L.; Zhao, Y.; Huang, Z.; Zhou, H.; Shi, M. MiR-181a Promotes Apoptosis and Reduces Cisplatin Resistance by Inhibiting Osteopontin in Cervical Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 559–565. [Google Scholar] [CrossRef]
- Zou, A.; Wu, A.; Luo, M.; Zhou, C.; Lu, Y.; Yu, X. SHCBP1 promotes cisplatin induced apoptosis resistance, migration and invasion through activating Wnt pathway. Life Sci. 2019, 235, 116798. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, S.; Li, H.; Wang, J.; Wei, C.; Liu, Q. SNHG16 promotes osteosarcoma progression and enhances cisplatin resistance by sponging miR-16 to upregulate ATG4B expression. Biochem. Biophys. Res. Commun. 2019, 518, 127–133. [Google Scholar] [CrossRef]
- Tan, L.-M.; Li, X.; Qiu, C.-F.; Zhu, T.; Hu, C.-P.; Yin, J.-Y.; Zhang, W.; Zhou, H.-H.; Liu, Z.-Q. CLEC4M is associated with poor prognosis and promotes cisplatin resistance in NSCLC patients. J. Cancer 2019, 10, 6374–6383. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.L.; Charneira, C.; Gandin, V.; Silva, J.L.A.F.; Justino, G.C.; Telo, J.P.; Vieira, A.J.S.C.; Marzano, C.; Antunes, A.M.M. Selenium-Containing Chrysin and Quercetin Derivatives: Attractive Scaffolds for Cancer Therapy. J. Med. Chem. 2015, 58, 4250–4265. [Google Scholar] [CrossRef]
- Kodumudi, K.N.; Woan, K.; Gilvary, D.L.; Sahakian, E.; Wei, S.; Djeu, J. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 2010, 16, 4583–4594. [Google Scholar] [CrossRef] [Green Version]
- Sekino, Y.; Han, X.; Kawaguchi, T.; Babasaki, T.; Goto, K.; Inoue, S.; Hayashi, T.; Teishima, J.; Shiota, M.; Yasui, W.; et al. TUBB3 Reverses Resistance to Docetaxel and Cabazitaxel in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 3936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaudommongkon, I.; Tanaudommongkon, A.; Prathipati, P.; Nguyen, J.T.; Keller, E.T.; Dong, X. Curcumin Nanoparticles and Their Cytotoxicity in Docetaxel-Resistant Castration-Resistant Prostate Cancer Cells. Biomedicines 2020, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-K.; Kim, K.M.; Jeong, S.-Y.; Choi, E.K.; Jung, J. Chrysin Increases the Therapeutic Efficacy of Docetaxel and Mitigates Docetaxel-Induced Edema. Integr. Cancer Ther. 2016, 16, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aylon, Y.; Oren, M. Living with p53, Dying of p53. Cell 2007, 130, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Nag, S.A.; Qin, J.-J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-p53 pathway revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar] [CrossRef]
- Moll, U.M.; Wolff, S.; Speidel, D.; Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 2005, 17, 631–636. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.-M.; Wang, J.-N.; Xiong, X.-K.; Yang, X.-F.; Zou, F. Combination of chrysin and cisplatin promotes the apoptosis of Hep G2 cells by up-regulating p53. Chem. Interact. 2015, 232, 12–20. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Li, J.; Dashwood, R. Emerging crosstalk between long non-coding RNAs and Nrf2 signaling. Cancer Lett. 2020. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chang, X.; Zhan, H.; Zhang, Q.; Li, C.; Gao, Q.; Yang, M.; Luo, Z.; Li, S.; Sun, Y. Curcumin and Baicalin ameliorate ethanol-induced liver oxidative damage via the Nrf2/HO-1 pathway. J. Food Biochem. 2020, e13425. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, R.; Chen, J.; Xue, Q.; Moghal, N.; Tsao, M.-S. PIDD interaction with KEAP1 as a new mutation-independent mechanism to promote NRF2 stabilization and chemoresistance in NSCLC. Sci. Rep. 2019, 9, 12437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhang, L.; Li, H.; Wu, S.; Liu, Z. Nrf2 induced cisplatin resistance in ovarian cancer by promoting CD99 expression. Biochem. Biophys. Res. Commun. 2019, 518, 698–705. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Q.; Ye, T.; Liu, Y.; Liu, D.; Song, S.; Zheng, C. NRF2/ABCB1-mediated efflux and PARP1-mediated dampening of DNA damage contribute to doxorubicin resistance in chronic hypoxic HepG2 cells. Fundam. Clin. Pharmacol. 2020, 34, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucci, M.A.; Grattarola, M.; Dianzani, C.; Damia, G.; Ricci, F.; Roetto, A.; Trotta, F.; Barrera, G.; Pizzimenti, S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radic. Biol. Med. 2020, 150, 125–135. [Google Scholar] [CrossRef]
- Gao, A.M.; Ke, Z.P.; Shi, F.; Sun, G.C.; Chen, H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013, 206, 100–108. [Google Scholar] [CrossRef]
- Laredj-Bourezg, F.; Bolzinger-Thevenin, M.A.; Pelletier, J.; Chevalier, Y. Pickering emulsions stabilized by biodegradable block copolymer micelles for controlled topical drug delivery. Int. J. Pharm. 2017, 531, 134–142. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Ghamkhari, A.; Pouyafar, A.; Salehi, R.; Rahbarghazi, R. Chrysin and Docetaxel Loaded Biodegradable Micelle for Combination Chemotherapy of Cancer Stem Cell. Pharm. Res. 2019, 36, 165. [Google Scholar] [CrossRef] [PubMed]
- Davaran, S.; Fazeli, H.; Ghamkhari, A.; Rahimi, F.; Molavi, O.; Anzabi, M.; Salehi, R. Synthesis and characterization of novel P(HEMA-LA-MADQUAT) micelles for co-delivery of methotrexate and Chrysin in combination cancer chemotherapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Restani, R.B.; Morgado, P.I.; Ribeiro, M.P.; Correia, I.J.; Aguiar-Ricardo, A.; Bonifácio, V.D.B. Biocompatible Polyurea Dendrimers with pH-Dependent Fluorescence. Angew. Chem. 2012, 124, 5252–5255. [Google Scholar] [CrossRef]
- Palakurthi, S.; Yellepeddi, V.; Vangara, K.K. Recent trends in cancer drug resistance reversal strategies using nanoparticles. Expert Opin. Drug Deliv. 2012, 9, 287–301. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Santos, I.; Ramos, C.; Mendes, C.; Sequeira, C.O.; Tomé, C.S.; Fernandes, D.G.; Mota, P.; Pires, R.F.; Urso, D.; Hipólito, A.; et al. Targeting Glutathione and Cystathionine β-Synthase in Ovarian Cancer Treatment by Selenium-Chrysin Polyurea Dendrimer Nanoformulation. Nutrients 2019, 11, 2523. [Google Scholar] [CrossRef] [Green Version]
- Eatemadi, A.; Darabi, M.; Afraidooni, L.; Zarghami, N.; Daraee, H.; Eskandari, L.; Mellatyar, H.; Akbarzadeh, A. Comparison, synthesis and evaluation of anticancer drug-loaded polymeric nanoparticles on breast cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–10. [Google Scholar] [CrossRef]
- Seidi, K. Nanomagnet-Based Detoxifying Machine: An Alternative/Complementary Approach in HIV therapy. J. AIDS Clin. Res. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 410–422. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mellatyar, H.; Akbarzadeh, A.; Rahmati, M.; Ghalhar, M.G.; Eatemadi, A.; Nejati-Koshki, K.; Zarghami, N.; Barkhordari, A. Comparison of Inhibitory Effect of 17-DMAG Nanoparticles and Free 17-DMAG in HSP90 Gene Expression in Lung Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 8693–8698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eatemadi, A.; Daraee, H.; Aiyelabegan, H.T.; Negahdari, B.; Rajeian, B.; Zarghami, N. Synthesis and Characterization of Chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed. Pharmacother. 2016, 84, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- DiMarco-Crook, C.; Rakariyatham, K.; Li, Z.; Du, Z.; Zheng, J.; Wu, X.; Xiao, H. Synergistic anticancer effects of curcumin and 3’,4’-didemethylnobiletin in combination on colon cancer cells. J. Food Sci. 2020, 85, 1292–1301. [Google Scholar] [CrossRef]
- Verma, A.H.; Kumar, T.S.S.; Madhumathi, K.; Rubaiya, Y.; Ramalingan, M.; Doble, M. Curcumin Releasing Eggshell Derived Carbonated Apatite Nanocarriers for Combined Anti-Cancer, Anti-Inflammatory and Bone Regenerative Therapy. J. Nanosci. Nanotechnol. 2019, 19, 6872–6880. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Sanaat, Z.; Zarghami, N. Synergistic Effect of Free and Nano-encapsulated Chrysin-Curcumin on Inhibition of hTERT Gene Expression in SW480 Colorectal Cancer Cell Line. Drug Res. 2018, 68, 335–343. [Google Scholar] [CrossRef]
- Kazemi-Lomedasht, F.; Rami, A.; Zarghami, N. Comparison of Inhibitory Effect of Curcumin Nanoparticles and Free Curcumin in Human Telomerase Reverse Transcriptase Gene Expression in Breast Cancer. Adv. Pharm. Bull. 2013, 3, 127–130. [Google Scholar]
- Pourhassan, M.; Zarghami, N.; Rahmati, M.; Alibakhshi, A.; Ranjbari, J.; Mohammad, P.; Nosratollah, Z.; Abbas, A.; Javad, R. The inhibitory effect of Curcuma longa extract on telomerase activity in A549 lung cancer cell line. Afr. J. Biotechnol. 2010, 9, 912–919. [Google Scholar] [CrossRef]
- Tavakoli, F.; Jahanban-Esfahlan, R.; Seidi, K.; Jabbari, M.; Behzadi, R.; Soltanahmadid, Y.P.; Zarghami, N. Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs and TIMPs gene expression in mouse B16F10 melanoma tumour model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Khokha, R. Suppression of the Tumorigenic and Metastatic Abilities of Murine B16-F10 Melanoma Cells In Vivo by the Overexpression of the Tissue Inhibitor of the Metalloproteinases-1. J. Natl. Cancer Inst. 1994, 86, 299–304. [Google Scholar] [CrossRef]
- Komath, S.; Garg, A.; Wahajuddin, M. Development and evaluation of Chrysin-Phospholipid complex loaded solid lipid nanoparticles-storage stability and in vitro anti-cancer activity. J. Microencapsul. 2018, 35, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Zak, M.S.; Seidi, K.; Barati, M.; Akbarzadeh, A.; Zarghami, N. The Effect of Chrysin Loaded PLGA-PEG on Metalloproteinase Gene Expression in Mouse 4T1 Tumor Model. Drug Res. 2017, 67, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, S.; Jafari, S.; Hamidi, S.; Molavi, O.; Davaran, S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-Fluorouracil and Chrysin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1107–1126. [Google Scholar] [CrossRef] [PubMed]
- Ferrado, J.B.; Perez, A.A.; Ruiz, M.C.; León, I.E.; Santiago, L.G.; Rubin, A.A.P. Chrysin-loaded bovine serum albumin particles as bioactive nanosupplements. Food Funct. 2020, 11, 6007–6019. [Google Scholar] [CrossRef]
- Lotfi-Attari, J.; Soltanahmadid, Y.P.-; Dadashpour, M.; Alipour, S.; Farajzadeh, R.; Javidfar, S.; Zarghami, N. Co-Delivery of Curcumin and Chrysin by Polymeric Nanoparticles Inhibit Synergistically Growth and hTERT Gene Expression in Human Colorectal Cancer Cells. Nutr. Cancer 2017, 69, 1290–1299. [Google Scholar] [CrossRef]
- Mohammadinejad, S.; Akbarzadeh, A.; Rahmati-Yamchi, M.; Hatam, S.; Kachalaki, S.; Zohreh, S.; Zarghami, N. Preparation and Evaluation of Chrysin Encapsulated in PLGA-PEG Nanoparticles in the T47-D Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2015, 16, 3753–3758. [Google Scholar] [CrossRef] [Green Version]
- Anari, E.; Akbarzadeh, A.; Zarghami, N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1410–1416. [Google Scholar] [CrossRef]
- Javan, N.; Ansari, M.H.K.; Dadashpour, M.; Khojastehfard, M.; Bastami, M.; Rahmati-Yamchi, M.; Zarghami, N. Synergistic Antiproliferative Effects of Co-nanoencapsulated Curcumin and Chrysin on MDA-MB-231 Breast Cancer Cells Through Upregulating miR-132 and miR-502c. Nutr. Cancer 2019, 71, 1201–1213. [Google Scholar] [CrossRef]
- Kim, K.M.; Lim, H.K.; Shim, S.H.; Jung, J. Improved chemotherapeutic efficacy of injectable chrysin encapsulated by copolymer nanoparticles. Int. J. Nanomed. 2017, 12, 1917–1925. [Google Scholar] [CrossRef] [Green Version]
- Baidya, D.; Kushwaha, J.; Mahadik, K.; Patil, S. Chrysin-loaded folate conjugated PF127-F68 mixed micelles with enhanced oral bioavailability and anticancer activity against human breast cancer cells. Drug Dev. Ind. Pharm. 2019, 45, 852–860. [Google Scholar] [CrossRef]
- Mohammadian, F.; Soltanahmadid, Y.P.; Mofarrah, M.; Dastani-Habashi, M.; Zarghami, N. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Singh, J.D. Synthesis and preliminary biological evaluation for the anticancer activity of organochalcogen (S/se) tethered chrysin-based organometallic RuII(η6-p-cymene) complexes. J. Biomol. Struct. Dyn. 2018, 37, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Zhou, Y.-Y.; Liu, X.-L.; Zhang, W.-H.; Chen, S.; Zhou, Y.; Liu, X.-W. Regioselective synthesis and evaluation of 2-amino 3-cyano chromene-chrysin hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett. 2020, 30, 127087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Chen, S.; Liu, X.-L.; Lin, B.; Zhou, Y. Study on antitumor activities of the chrysin-chromene-spirooxindole on Lewis lung carcinoma C57BL/6 mice in vivo. Bioorg. Med. Chem. Lett. 2020, 30, 127410. [Google Scholar] [CrossRef] [PubMed]
- Al-Oudat, B.A.; Ramapuram, H.; Malla, S.; Audat, S.A.; Hussein, N.; Len, J.M.; Kumari, S.; Bedi, M.F.; Ashby, J.C.R.; Tiwari, A.K. Novel Chrysin-De-Allyl PAC-1 Hybrid Analogues as Anticancer Compounds: Design, Synthesis, and Biological Evaluation. Molecules 2020, 25, 3063. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, P.; Ábrányi-Balogh, P.; Szigetvári, Á.; Dékány, M.; Szántay, J.C.; Hazai, L. Synthesis and In Vitro Anticancer Evaluation of Novel Chrysin and 7-Aminochrysin Derivatives. Molecules 2020, 25, 888. [Google Scholar] [CrossRef] [Green Version]
- Xuan, H.; Zhang, J.-H.; Wang, Y.-H.; Fu, C.-L.; Zhang, W. Anti-tumor activity evaluation of novel chrysin–organotin compound in MCF-7 cells. Bioorg. Med. Chem. Lett. 2016, 26, 570–574. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Antonoglou, O.; Hatzidimitriou, A.; Sagnou, M.; Pantazaki, A.A.; Litsardakis, G.; Pelecanou, M. Structurally characterized gallium–chrysin complexes with anticancer potential. Dalton Trans. 2020, 49, 2734–2746. [Google Scholar] [CrossRef]
- Zhang, H.M.; Li, H.; Wang, G.X.; Wang, J.; Xiang, Y.; Huang, Y.; Shen, C.; Dai, Z.T.; Li, J.P.; Zhang, T.C.; et al. MKL1/miR-5100/CAAP1 loop regulates autophagy and apoptosis in gastric cancer cells. Neoplasia 2020, 22, 220–230. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Song, L.; Zhai, H.; Chang, C. MAP7 promotes migration and invasion and progression of human cervical cancer through modulating the autophagy. Cancer Cell Int. 2020, 20, 17–18. [Google Scholar] [CrossRef]
- Liang, G.; Ling, Y.; Mehrpour, M.; Saw, P.E.; Liu, Z.; Tan, W.; Tian, Z.; Zhong, W.; Lin, W.; Luo, Q.; et al. Autophagy-associated circRNA circCDYL augments autophagy and promotes breast cancer progression. Mol. Cancer 2020, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wu, C.; Li, Y.; Li, H.; An, Y.; Wang, G.; Dai, J.; Wang, Q. LncRNA DANCR silence inhibits SOX5-medicated progression and autophagy in osteosarcoma via regulating miR-216a-5p. Biomed. Pharmacother. 2019, 122, 109707. [Google Scholar] [CrossRef] [PubMed]
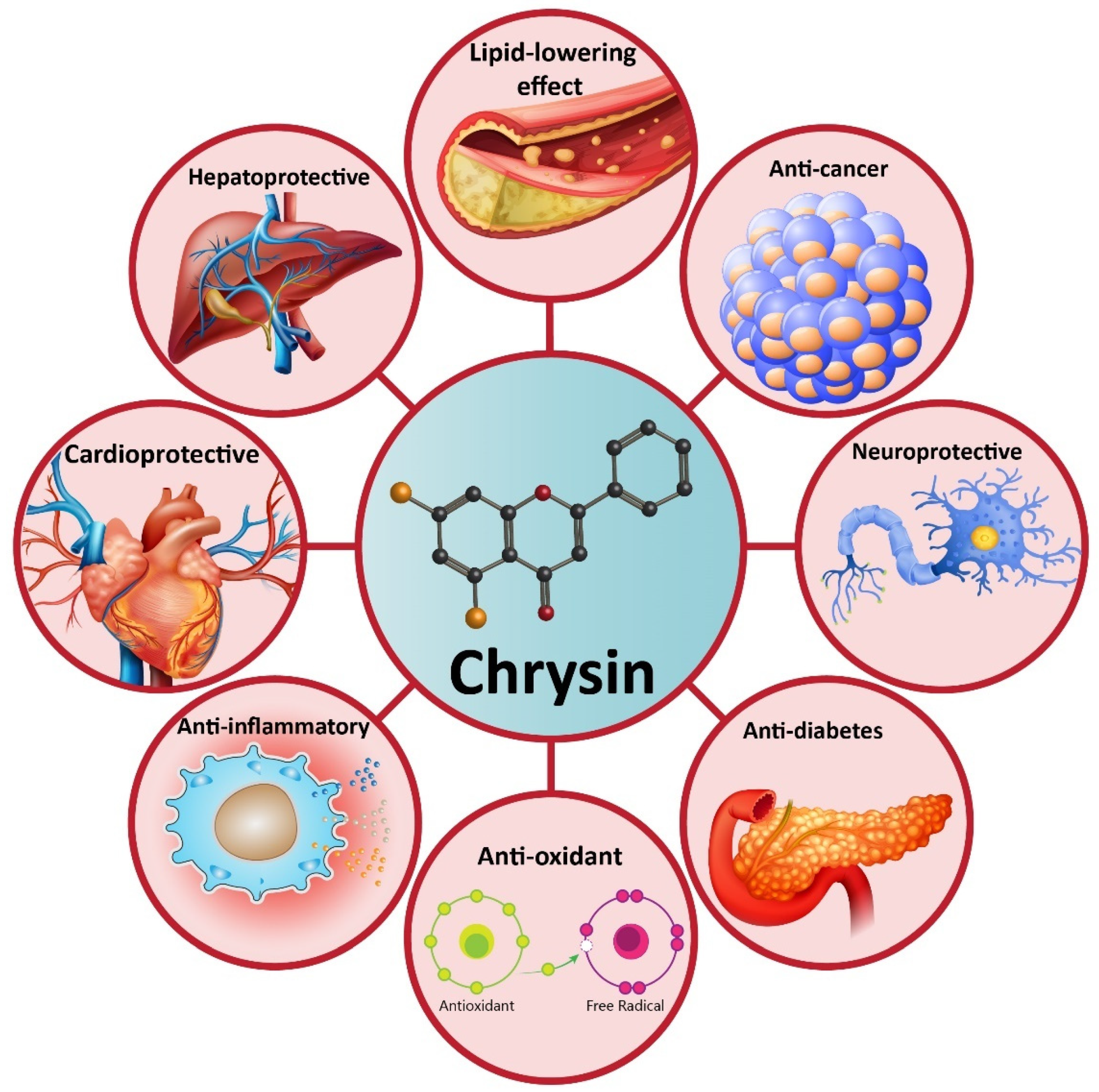

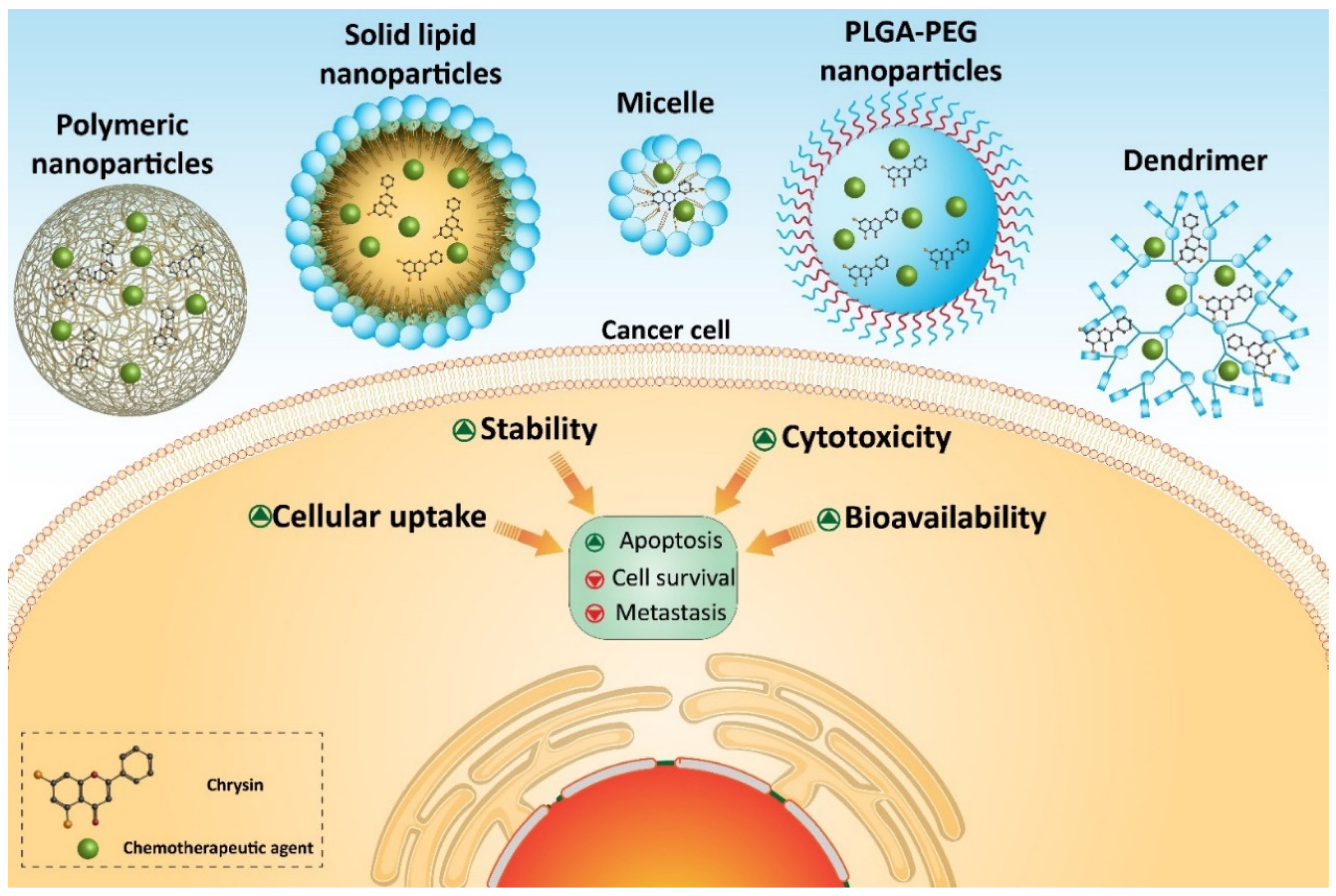
| Therapeutic Effect/Disease | In Vitro/In Vivo | Cell Line/Animal Model | Dose (In Vivo)/Concentration (In Vitro) | Duration of Experiment | Administration Route | Outcomes | Refs |
|---|---|---|---|---|---|---|---|
| Anti-hypertension | In vivo | Rat | 100 mg/kg | 18 weeks | Oral administration | Decreasing systolic and diastolic pressures Reducing insulin, angiotensin II and tiacylglycerols levels | [85] |
| Neuroprotective | In vivo | Rat | 10 and 30 mg/kg | 8 weeks | Oral gavage | Improving memory impairment Enhancing neuronal cell survival Reducing hippocampal neurogenesis depletion | [86] |
| Neuroprotective | In vivo | Rat | 10, 30, and 100 mg/kg | 3 weeks | Oral administration | Enhancing GPx activity and number of surviving cells in hippocampus Reducing MDA, NO and PGE2 levels Improving passive avoidance memory | [87] |
| Cardioprotective | In vitro | Cardiomyocyte | 10, 50, and 100 µM | 3 h | - | Decreasing aluminium-phosphide-mediated oxidative stress Reducing mitochondrial damage Improving mitochondrial function | [88] |
| Renoprotective Hepatoprotective | In vivo | Rat | 100 mg/kg | - | - | Reinforcing antioxidant defense system via up-regulating GSH and SOD activities Reducing lipid peroxidation Decreasing inflammation via TNF-α down-regulation | [89] |
| Renoprotective Hepatoprotective | In vivo | Rat | 25 and 50 mg/kg | 7 days | Oral administration | Reducing AST, ALT, ALP, urea, creatinine, MDA and hepatorenal deterioration Enhancing SOD, CAT, and GPx activities Apoptosis inhibition via Bcl-2 up-regulation and Bax down-regulation Reducing inflammation via NF-κB down-regulation | [90] |
| Anti-diabetic | In vitro | Chorioretinal endothelial cells | 1, 3, 10, 30, and 50 µM | 24 h | - | Reducing Akt, ERK, MMP-2, and VEGF expressions | [91] |
| Anti-diabetic | In vivo | Rat model of type I diabetes | 50 and 100 mg/kg | 28 days | Oral gavage | Reducing oxidative stress index Enhancing glutathione levels | [92] |
| Gastric healing | In vivo | Mouse model of gastric ulcer via ethanol | 10, 50, and 100 mg/kg | 7 and 14 days | Oral administration | Apoptosis inhibition via caspase-3 down-regulation Reducing macroscopic lesions Enhancing catalase activity Improving inflammation via COX-2 down-regulation | [93] |
| Cancer Type | In Vitro/In Vivo | Cell Line/Animal Model | Dose (In Vivo)/Concentration (In Vitro) | Period of Experiment | Administration Route | Outcomes | Refs |
|---|---|---|---|---|---|---|---|
| Prostate cancer | In vitro | DU145 and PC-3 cell lines | 12.5, 25 and 50 µM | - | - | Induction of mitochondrion- and ER-mediated apoptosis Cell cycle arrest Down-regulation of MAPK and PI3K/Akt signaling pathways Impairing proliferation of PC cells | [144] |
| Gastric cancer | In vitro | MKN45 cells Mouse model of GC (created by CRIPSR/Cas9) | 10, 20, 40, 80 and 160 µM 20 mg/kg | 12, 24 and 45 h 14 days | Oral gavage | Suppressing migration Apoptosis induction Enhancing TET1 expression | [165] |
| Lung cancer | In vitro | A549 cells | 2 and 5 µM | 4 h | - | Down-regulation of MyD88 and TLR4 Inhibition of inflammation via NF-κB down-regulation Suppressing survival and metastasis | [134] |
| Cervical cancer | In vitro | HeLa cells | 5, 10, 20 and 40 µM | 0.5, 3, 6, 12 and 24 h | - | Down-regulation of NF-κB signaling pathway Inhibition of Twist/EMT axis Suppressing metastasis of cervical cancer | [182] |
| Breast cancer | In vitro | T47D breast cancer cells | 20, 40, 60, 80, 100 and 120 µM | 48 h | - | Disrupting proliferation of cancer cells via down-regulation of cyclin D1 and hTERT | [105] |
| Hepatocellular carcinoma | In vitro In vivo | Normal human hepatic cell LO2 and HepG2, Hep3B, Huh-7, HCC-LM3, Bel-7402 and SMMC-7721 Tumor xenografts | 15, 30, and 60 µM 30 mg/kg | 24, 48 and 72 h | Intraperitoneal injection | Down-regulation of HK-2 Suppressing glycolysis Apoptosis induction | [194] |
| Breast cancer Cervical cancer | In vitro | HeLa cells MCF-7 cells | 15, 20, 25 and 30 µM | 30 min | - | Significant reduction in survival of cancer cells Inducing both intrinsic and extrinsic apoptotic pathways P53-dependent apoptosis | [246] |
| Ovarian cancer | In vitro | SKOV3 cell line | 5, 10 and 20 µmol/L | - | - | Decreasing the viability of cancer cells in a dose-dependent manner Down-regulation of CK2α, CD133 and CD44 Suppressing sphere formation capability | [247] |
| Breast cancer | In vitro | MDA-MB-231 | 10 µM | 24 and 48 h | - | Inhibition of EGFR Reducing migration, growth and sphere formation ability of cancer cells | [109] |
| Breast cancer | In vitro In vivo | 4T1 mouse breast cancer cells Balb/c mice implanted with 4T1 cells | 60–100 µM 250 mg/kg | 30 min 18 days | Oral administration | Suppressing lung metastasis Down-regulation of VEGF, and STAT3 Inhibiting proliferation | [119] |
| Prostate cancer | In vitro | Human prostate cancer cell line PC-3 | 10, 20, 30, and 40 µM | 24, 48 and 72 h | - | Reducing the viability of cancer cells in a time- and dose-dependent manner Apoptosis induction | [248] |
| Cervical cancer | In vitro | Human cervical epidermoid carcinoma cell line ME180, and human cervical carcinoma cell lines HeLa, BU25TK− and SiHa | 0–160 mg/mL | - | - | Apoptosis induction via caspase-3, caspase-9, and Bax up-regulation Stimulating mitochondrial dysfunction Cell cycle arrest induction | [184] |
| Liver cancer | In vitro | Hepatocellular carcinoma cells | 5–100 µM | 15, 30, 45 and 60 min | - | Mitochondrial dysfunction Cytochrome c release into the cytoplasm Apoptosis induction | [195] |
| Breast cancer | In vitro | MDA-MB-231 and MCF-7 cells | 3–12 µM | - | - | Reducing the viability of cancer cells Apoptosis induction via capase-3 and caspase-7 up-regulation | [249] |
| Melanoma | In vitro In vivo | B16F10 cells Melanoma-bearing mice | 12.5, 25, 50, and 100 µM 50 mg/kg | 24 and 48 h 21 days | - | Induction of cell cycle arrest at G2/m phase Reducing tumor growth in vivo Promoting the anti-tumor activity of immune cells, such as macrophages and natural killer cells | [211] |
| Oral squamous cell carcinoma | In vitro | Oral squamous carcinoma KB cell line | 1, 2, 4, 8, 16, and 32 µmol/L | 24 h | - | Suppressing proliferation in a dose-dependent manner Apoptosis induction via capase-3 and caspase-7 up-regulation Inducing mitochondrial dysfunction Reducing the viability via down-regulation of PI3K/Akt signaling pathways | [250] |
| Bladder cancer | In vitro | Human bladder cancer cell lines T-24 and 5637 and the non-malignant immortalized urothelial SV-HUC-1 cells | 20, 40 and 80 µM | 24 h | - | Induction of ER stress via UPR activation Stimulating intrinsic pathway of apoptosis via caspase-3 and caspase-9 up-regulation Inhibition of STAT3 signaling pathway | [251] |
| Melanoma | In vitro | Human melanoma A375.S2 cell line | 5, 10 and 15 µM | 24 and 48 h | - | Impairing metastasis via VEGF, MMP-2, and N-cadherin down-regulation Enhancing E-cadherin expression Down-regulation of PI3K/Akt and NF-κB pathways in suppressing cancer proliferation | [182] |
| Colorectal cancer | In vitro | SW48, SW480, and SW620 CRC cells | 5–50 µM | 24 h | - | Enhancing ROS generation mTOR down-regulation Elevating LC-3II levels Autophagy induction Impairing cancer cell viability | [227] |
| Breast cancer | In vitro | MCF-7 cells | 20 and 30 µM | 48 and 72 h | - | Anti-proliferative activity in a dose- and time-dependent manner Apoptosis induction | [252] |
| Cervical cancer | In vitro | HeLa cells | 0–10 µM | 12–48 h | - | Stimulating apoptosis and cell cycle arrest Down-regulation of COX-2 expression | [253] |
| Colon cancer | In vitro | HT-29 cells | 12.5, 25, 50, and 100 µg/mL | - | - | Induction of apoptosis via mitochondrial dysfunction Irradiation combined with chrysin exerts a synergistic effect | [235] |
| Thyroid carcinoma | In vitro In vivo | HTh7 and KAT18 cells | 25, 50, and 75 µM 75 mg/kg | 2–6 days 21 days | Oral gavage | Reducing the viability and growth via up-regulation of Notch1 and its down-stream target, Hes1 | [254] |
| Hepatocellular carcinoma | In vitro | SMMC-7721 cells | 10, 20 and 40 µM | 24 and 48 h | - | Reducing sphere formation via STAT3 down-regulation | [203] |
| Breast cancer | In vitro | MCF-7 cells | 40 µM | 8 h | - | Decreasing cell viability by p53 activation through ATM-ChK2 axis Lack of DNA damage | [255] |
| Tongue squamous cell carcinoma | In vitro | CAL-27 cells | 5, 25, 55 and 80 µM | 24 h | - | Apoptosis induction via caspase-3 and caspase-9 up-regulation | [256] |
| Choriocarcinoma cells | In vitro | JAR and JEG3 cells | 0–100 µM | 24 h | - | Suppressing cell viability in a dose-dependent manner Inducing cell death via promoting ROS production and changing mitochondrial membrane potential | [257] |
| Colorectal cancer | In vitro | HCT116 cells | 20, 30, 40 and 50 µM | 36 h | - | Cell cycle arrest Migration inhibition PARPα up-regulation CYP2S1 and CYP1B1 induction | [245] |
| Colon cancer | In vitro In vivo | CT26 cells Allograft colon carcinoma model | 10–200 µg/mL 0–10 mg/kg | 24 and 48 h 28 days | Oral administration | Reducing tumor growth Induction of apoptosis via caspase-3 and caspase-9 up-regulation | [258] |
| Nanovehicle | Cancer Type | In Vitro/In Vivo | Cell Line/Animal Model | Particle Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (%) | Outcomes | Refs |
|---|---|---|---|---|---|---|---|---|
| Micelle | Colorectal cancer | In vitro | Human-derived epithelial colorectal cancer cell lines HT-29 | 72–142 | +10.1 | 77 (Docetaxel) 44 (chrysin) | Enhanced cellular uptake Effective inhibition of cancer stem cell migration | [292] |
| Polymeric micelles | Breast cancer | In vitro | MCF-7 cells | 55 | −2.7 | 87.6 (methotrexate) 86.5 (chrysin) | Enhancing efficacy of chrysin and methotrexate in breast cancer therapy via promoting cellular uptake | [293] |
| Dendrimer | Ovarian cancer | In vitro | Serous carcinoma (OSC) cell lines (OVCAR3 HTB-161™ and OVCAR8 CVCL_1629™) and a clear cell carcinoma (OCCC) cell line (ES2 CRL-1978™) | - | - | - | Selective targeting of cancer cells by folate functionalization of dendrimers High cellular uptake Remarkable decrease in survival of cancer cells | [297] |
| Polymeric nanoparticles | Breast cancer | In vitro | T47D breast cancer cell line | 75 | - | 99.89 | Higher cytotoxicity against breast cancer cells compared to chrysin alone | [304] |
| PLGA-PEG nanoparticles | Breast cancer | In vitro | T47-D breast cancer cell line | 20–75 | - | 70 | High cytotoxicity Excellent cellular uptake and encapsulation efficiency | [317] |
| PLGA-PEG nanoparticles | Colorectal cancer | In vitro | SW480 cells | 50–140 nm | - | Higher cytotoxicity compared to chrysin and curcumin alone hTERT down-regulation | [307] | |
| PLGA-PEG nanoparticles | Melanoma | In vivo | C57B16 mice bearing B16F10 melanoma tumours | 285 | −3.7 | 78.27 (curcumin) 83.5 (chrysin) | Enhancing expression of TIMP-1 and TIMP-2 Down-regulation of MMP-2 and MMP-9 Suppressing metastasis of cancer cells | [310] |
| Solid lipid nanoparticles | Breast cancer | In vitro | MCF-7 cells | Below 500 | −20 to −47 | More than 90% | High stability and promoting the anti-tumor activity of chrysin | [312] |
| PLGA-PEG nanoparticles | Breast cancer | In vitro | T47D cells | 70–300 | - | 99.89 | Accumulation in breast cancer cells High cytotoxicity | [318] |
| PLGA-PEG nanoparticles | Breast cancer | In vitro | MDA-MB-231 cells | 305 | −3.8 | 80.22 (curcumin) 85.25 (chrysin) | Synergistic effect Cell cycle arrest at G2/M phase Apoptosis induction Up-regulation of miR-132 and miR-502c | [319] |
| Copolymer nanoparticle | Lung cancer | In vitro In vivo | A549 cells Mice bearing an A549-derived tumor | 77 | −2.22 | 46.96 | Enhanced cytotoxicity More potential in exerting tumor growth delay | [320] |
| Micelle | Breast cancer | In vitro | MCF-7 cells | 152–420 | −21.6 | 52–89 | Promoting bioavailability of chrysin Exerting a 5-fold increase in anti-tumor activity | [321] |
| PLGA-PEG nanoparticles | Gastric cancer | In vitro | AGS cells | 70–300 | - | 98.6 | Decreasing cell survival via down-regulation of miR-18a, miR-21, and miR-221 | [322] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghadam, E.R.; Ang, H.L.; Asnaf, S.E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A.P. Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules 2020, 10, 1374. https://doi.org/10.3390/biom10101374
Moghadam ER, Ang HL, Asnaf SE, Zabolian A, Saleki H, Yavari M, Esmaeili H, Zarrabi A, Ashrafizadeh M, Kumar AP. Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules. 2020; 10(10):1374. https://doi.org/10.3390/biom10101374
Chicago/Turabian StyleMoghadam, Ebrahim Rahmani, Hui Li Ang, Sholeh Etehad Asnaf, Amirhossein Zabolian, Hossein Saleki, Mohammad Yavari, Hossein Esmaeili, Ali Zarrabi, Milad Ashrafizadeh, and Alan Prem Kumar. 2020. "Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives" Biomolecules 10, no. 10: 1374. https://doi.org/10.3390/biom10101374
APA StyleMoghadam, E. R., Ang, H. L., Asnaf, S. E., Zabolian, A., Saleki, H., Yavari, M., Esmaeili, H., Zarrabi, A., Ashrafizadeh, M., & Kumar, A. P. (2020). Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules, 10(10), 1374. https://doi.org/10.3390/biom10101374







