Effects of Elevated Downstream Pressure and the Role of Smooth Muscle Cell Coupling through Connexin45 on Lymphatic Pacemaking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Mice and Tamoxifen-Dependent Cre-Recombinase
2.3. Solutions and Chemicals
2.4. In Vivo Recording of Contractions of Popliteal Lymphatic Vessels
2.5. Vessel Isolation, Pressure Myography, and Data Acquisition
2.6. Assessment of Contractile Function of Isolated Lymphatic Vessels
2.7. Assessment of the Initiation and Propagation of Contraction Waves using Space Time Maps (STMs)
2.8. Calculation of Contractile and Pacemaking Parameters
2.9. Fluorescence Activated Cell Sorting (FACS) and End-point RT-PCR
2.10. Statistical Analysis
3. Results
3.1. In Vivo and Ex Vivo Assessement of the Initiation Sites of Spontaneous Contractions and Measurement of the Direction and Speed of the Associated Propagating Contraction Wave in Lymphatic Vessels
3.2. Lymphatic Contractions Display a Higher Probability of Initiating Downstream in the Lymphatic Network
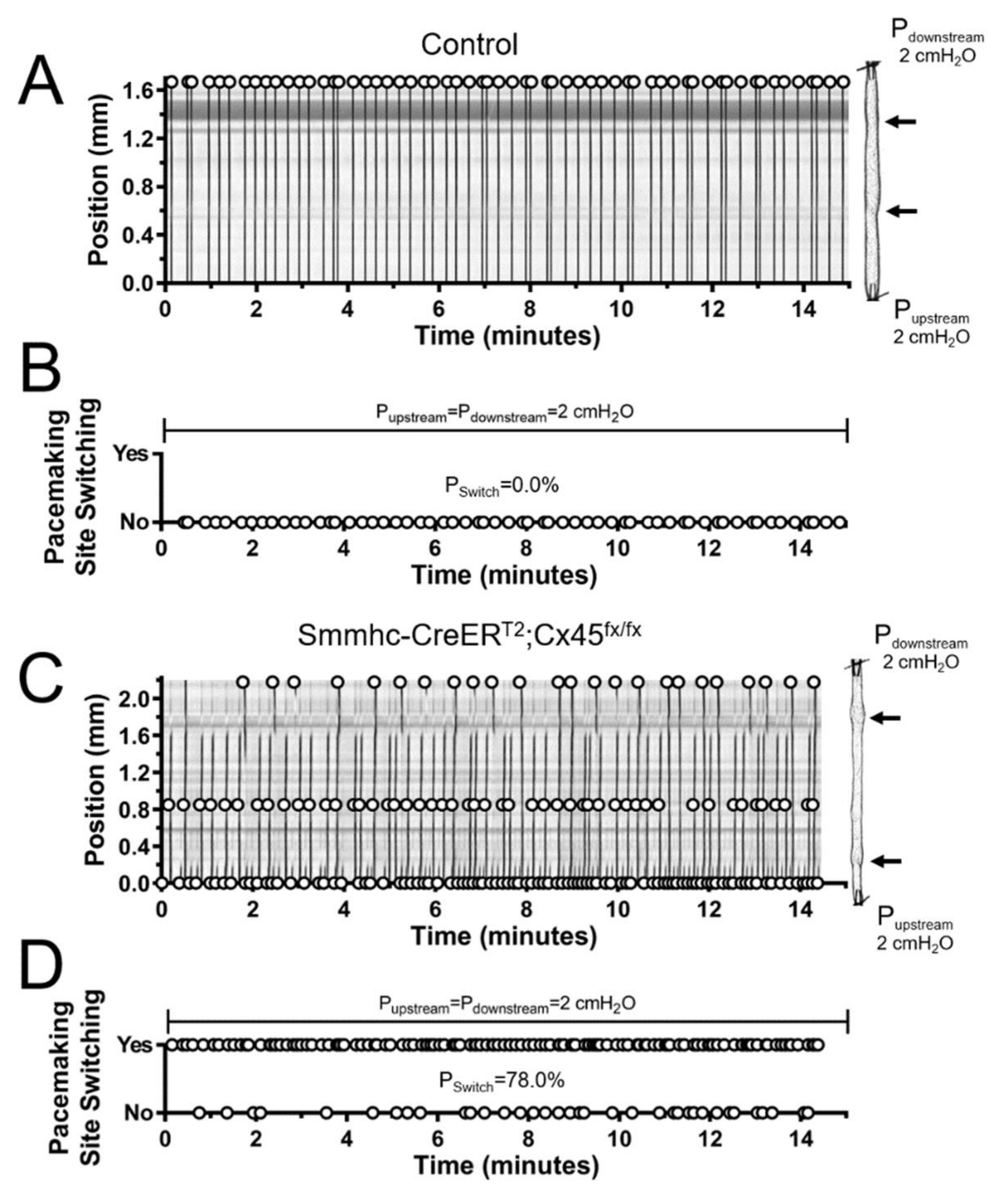
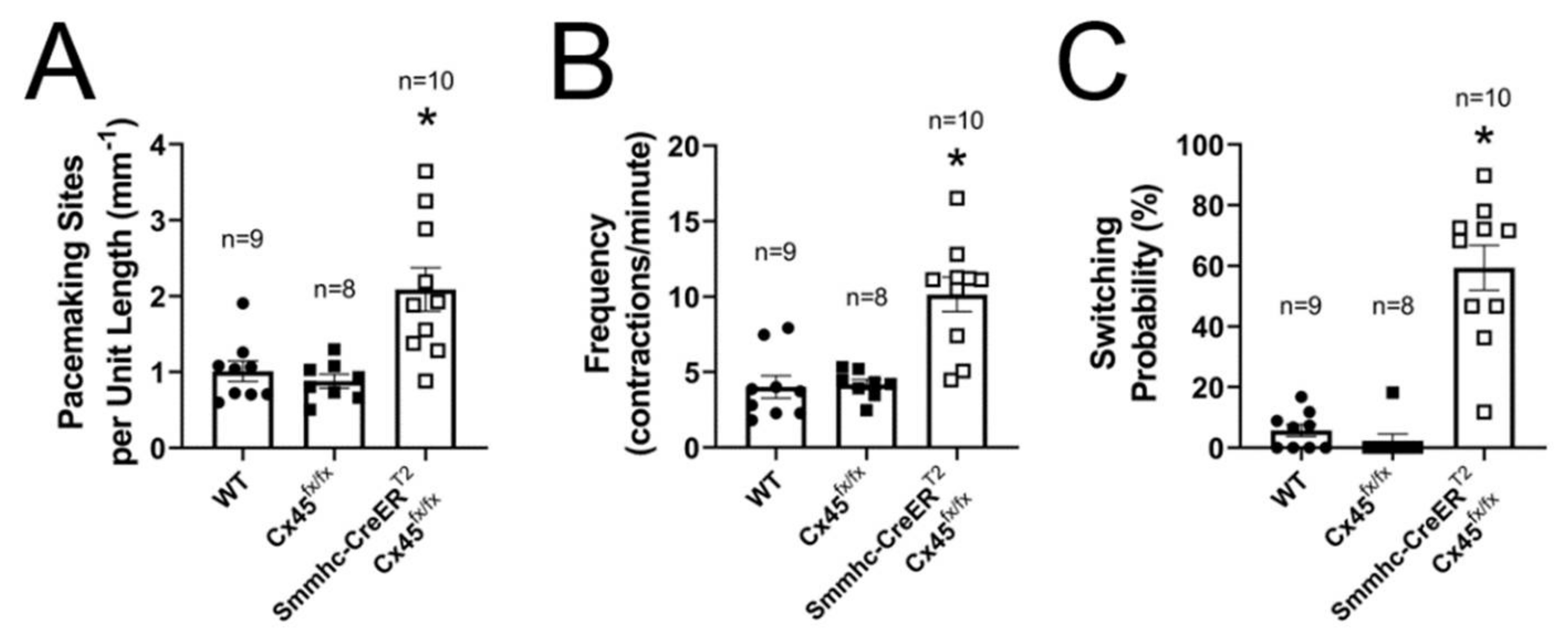
3.3. Efficient Entrainment of Lymphatic Contractions Requires a Dominant Pacemaker and Strong Electrical Coupling between LMCs through Connexin 45-Containing Gap Junctions
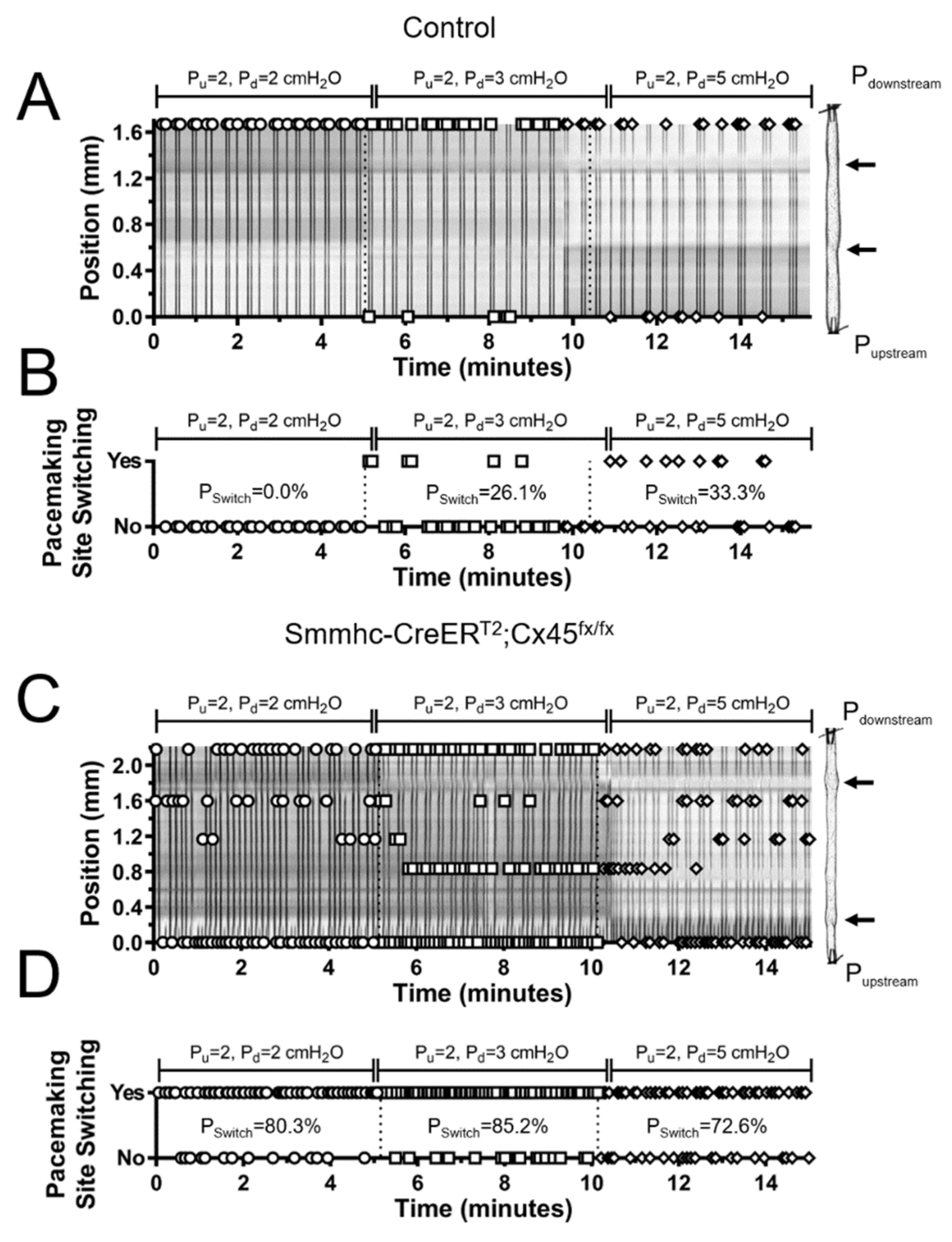
3.4. Lymphatic Pacemaking can be Regulated by Localized Changes in Intraluminal Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Engeset, A.; Olszewski, W.; Jaeger, P.M.; Sokolowski, J.; Theodorsen, L. Twenty-four hour variation in flow and composition of leg lymph in normal men. Acta Physiol. Scand. 1977, 99, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Rahbar, E.; Gashev, A.A.; Zawieja, D.C.; Moore, J.E., Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H48–H60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhoffer, J.; Kagal, A.; Klein, T.; Johnston, M.G. Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc. Res. 1995, 49, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schonbein, G.W. Microlymphatics and lymph flow. Physiol. Rev. 1990, 70, 987–1028. [Google Scholar] [CrossRef] [PubMed]
- Von der Weid, P.Y.; Zawieja, D.C. Lymphatic smooth muscle: The motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004, 36, 1147–1153. [Google Scholar] [CrossRef]
- Castorena-Gonzalez, J.A.; Zawieja, S.D.; Li, M.; Srinivasan, R.S.; Simon, A.M.; de Wit, C.; de la Torre, R.; Martinez-Lemus, L.A.; Hennig, G.W.; Davis, M.J. Mechanisms of Connexin-Related Lymphedema. Circ. Res. 2018, 123, 964–985. [Google Scholar] [CrossRef]
- Crowe, M.J.; von der Weid, P.Y.; Brock, J.A.; Van Helden, D.F. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J. Physiol. 1997, 500 (Pt. 1), 235–244. [Google Scholar] [CrossRef]
- Akl, T.J.; Nepiyushchikh, Z.V.; Gashev, A.A.; Zawieja, D.C.; Cot, G.L. Measuring contraction propagation and localizing pacemaker cells using high speed video microscopy. J. Biomed. Opt. 2011, 16, 026016. [Google Scholar] [CrossRef] [Green Version]
- Zawieja, D.C.; Davis, K.L.; Schuster, R.; Hinds, W.M.; Granger, H.J. Distribution, propagation, and coordination of contractile activity in lymphatics. Am. J. Physiol. 1993, 264, H1283–H1291. [Google Scholar] [CrossRef]
- Zweifach, B.W.; Lipowsky, H.H. Pressure-Flow Relations in Blood and Lymph Microcirculation In Handbook of Physiology, Sect. 2 (The Cardiovascular System); American Physiological Society: Bethesda, MD, USA, 1984; Volume IV, pp. 231–307. [Google Scholar]
- Hargens, A.R.; Millard, R.W.; Pettersson, K.; Johansen, K. Gravitational haemodynamics and oedema prevention in the giraffe. Nature 1987, 329, 59–60. [Google Scholar] [CrossRef]
- Gashev, A.A.; Davis, M.J.; Delp, M.D.; Zawieja, D.C. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 2004, 11, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Davis, M.J. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J. Physiol. 2013, 591, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Wolpers, J.H.; Muthuchamy, M.; Zawieja, D.C.; Gashev, A.A.; Davis, M.J. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H809–H824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scallan, J.P.; Wolpers, J.H.; Davis, M.J. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J. Physiol. 2013, 591, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Scallan, J.P.; Wolpers, J.H.; Muthuchamy, M.; Gashev, A.A.; Zawieja, D.C. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H795–H808. [Google Scholar] [CrossRef] [Green Version]
- Zawieja, S.D.; Castorena, J.A.; Gui, P.; Li, M.; Bulley, S.A.; Jaggar, J.H.; Rock, J.R.; Davis, M.J. Ano1 mediates pressure-sensitive contraction frequency changes in mouse lymphatic collecting vessels. J. Gen. Physiol. 2019, 151, 532–554. [Google Scholar] [CrossRef] [Green Version]
- Scallan, J.P.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Davis, M.J. Lymphatic pumping: Mechanics, mechanisms and malfunction. J. Physiol. 2016, 594, 5749–5768. [Google Scholar] [CrossRef]
- Zawieja, S.D.; Castorena-Gonzalez, J.A.; Dixon, B.; Davis, M.J. Experimental Models Used to Assess Lymphatic Contractile Function. Lymphat Res. Biol. 2017, 15, 331–342. [Google Scholar] [CrossRef]
- Castorena-Gonzalez, J.A.; Scallan, J.P.; Davis, M.J. Methods for Assessing the Contractile Function of Mouse Lymphatic Vessels Ex Vivo. Methods Mol. Biol. 2018, 1846, 229–248. [Google Scholar] [CrossRef]
- Zawieja, S.D.; Castorena-Gonzalez, J.A.; Scallan, J.P.; Davis, M.J. Differences in L-type Ca(2+) channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H991–H1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.J.; Zawieja, D.C.; Gashev, A.A. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res. Biol. 2006, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Kim, H.J.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Gui, P.; Li, M.; Saunders, B.T.; Zinselmeyer, B.H.; Randolph, G.J.; Remedi, M.S.; et al. Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function. J. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hald, B.O.; Castorena-Gonzalez, J.A.; Zawieja, S.D.; Gui, P.; Davis, M.J. Electrical Communication in Lymphangions. Biophys. J. 2018, 115, 936–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanady, J.D.; Dellinger, M.T.; Munger, S.J.; Witte, M.H.; Simon, A.M. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 2011, 354, 253–266. [Google Scholar] [CrossRef]
- Olszewski, W.L.; Engeset, A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am. J. Physiol. 1980, 239, H775–H783. [Google Scholar] [CrossRef]
- To, K.H.T.; Gui, P.; Li, M.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Davis, M.J. T-type, but not L-type, voltage-gated calcium channels are dispensable for lymphatic pacemaking and spontaneous contractions. Sci. Rep. 2020, 10, 70. [Google Scholar] [CrossRef]
- Gasheva, O.Y.; Trzeciakowski, J.P.; Gashev, A.A.; Zawieja, D.C. Temporal Dynamics of the Rat Thoracic Duct Contractility in the Presence of Imposed Flow. Lymphat Res. Biol. 2017, 15, 324–330. [Google Scholar] [CrossRef]
- Kornuta, J.A.; Nepiyushchikh, Z.; Gasheva, O.Y.; Mukherjee, A.; Zawieja, D.C.; Dixon, J.B. Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1122–R1134. [Google Scholar] [CrossRef]
- Bertram, C.D.; Macaskill, C.; Moore, J.E., Jr. Inhibition of contraction strength and frequency by wall shear stress in a single-lymphangion model. J. Biomech. Eng. 2019. [Google Scholar] [CrossRef]
- McHale, N.G.; Meharg, M.K. Co-ordination of pumping in isolated bovine lymphatic vessels. J. Physiol. 1992, 450, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.G.; Tran, C.H.T.; Hald, B.O.; Sancho, M. The Conducted Vasomotor Response: Function, Biophysical Basis, and Pharmacological Control. Annu. Rev. Pharm. Toxicol. 2018, 58, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Dongaonkar, R.M.; Stewart, R.H.; Laine, G.A.; Davis, M.J.; Zawieja, D.C.; Quick, C.M. Venomotion modulates lymphatic pumping in the bat wing. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H2015–H2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalak, T.C.; Schmid-Schonbein, G.W.; Zweifach, B.W. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc. Res. 1984, 28, 95–112. [Google Scholar] [CrossRef]
- Schmidt, V.J.; Jobs, A.; von Maltzahn, J.; Worsdorfer, P.; Willecke, K.; de Wit, C. Connexin45 is expressed in vascular smooth muscle but its function remains elusive. PLoS ONE 2012, 7, e42287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanady, J.D.; Munger, S.J.; Witte, M.H.; Simon, A.M. Combining Foxc2 and Connexin37 deletions in mice leads to severe defects in lymphatic vascular growth and remodeling. Dev. Biol. 2015, 405, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapinski, P.E.; Lubeck, B.A.; Chen, D.; Doosti, A.; Zawieja, S.D.; Davis, M.J.; King, P.D. RASA1 regulates the function of lymphatic vessel valves in mice. J. Clin. Invest. 2017, 127, 2569–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabine, A.; Bovay, E.; Demir, C.S.; Kimura, W.; Jaquet, M.; Agalarov, Y.; Zangger, N.; Scallan, J.P.; Graber, W.; Gulpinar, E.; et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J. Clin. Invest. 2015, 125, 3861–3877. [Google Scholar] [CrossRef] [Green Version]
- Raines, J.K.; O’Donnell, T.F., Jr.; Kalisher, L.; Darling, R.C. Selection of patients with lymphedema for compression therapy. Am. J. Surg. 1977, 133, 430–437. [Google Scholar] [CrossRef]
- Sejersted, O.M.; Hargens, A.R.; Kardel, K.R.; Blom, P.; Jensen, O.; Hermansen, L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 287–295. [Google Scholar] [CrossRef]





| Accession No. | Protein | Strand | Sequence | Amplicon (bp) |
|---|---|---|---|---|
| NM_080454 | Cx47 | s | AGC TCT GCC TTG TGC ATC TC | 216 |
| as | CGT GTT GCA GGT GAA CTT GG | |||
| NM_175452 | Cx47 | s | GAG AGG ATC AGC ATC CAG CC | 262 |
| as | CGT GTT GCA GGT GAA CTT GG | |||
| NM_008122 | Cx45 | s | GGT AAC AGG AGT TCT GGT GAA | 140 |
| as | TCG AAA GAC AAT CAG CAC AGT | |||
| NM_010288 | Cx43 | s | TGA GAG CCC GAA CTC TCC TT | 258 |
| as | AGG CAG ACT GTT CAT CAC CC | |||
| NM_008121 | Cx40 | s | CCA GAG CCT GAA GAA GCC AA | 143 |
| NM_001271628 | as | CCG ATG ACT GTG GAG TGC TT | 200 | |
| NM_008120 | Cx37 | s | GCT GCG CGC TAT TTA AGG C | 131 |
| as | CAT GTT TCC AGG GCC TCT CT | |||
| NM_001302497 | Cx32 | s | CAT GAG ACC ATA GGG GAG CTG | 291 |
| as | ACG TGG GAG ATG GGG AAA AA | |||
| NM_178596 | Cx30.2 | s | CGT CAT CTA CTC CAT GCA CCA | 316 |
| as | GAC GGC GAA GTA GAA GAC CAC | |||
| NM_001010937 | Cx30 | s | GAT CCC AAC GAG TGC CCT AAT | 430 |
| as | CTG GAC ATC AGC AGC GGT AG | |||
| NM_008125 | Cx26 | s | CAT TTC GGA CCA ACC CAG GA | 148 |
| as | TGC CCC AAT CCA TCT TGT CC | |||
| NM_008937 | Prox1 | s | GTA AGA CAT CAC CGC GTG C | 218 |
| as | TCA TGG TCA GGC ATC ACT GG | |||
| NM_009868 | VE-Cadherin | s | CTT CCT TAC TGC CCT CAT TGT | 313 |
| as | CTG TTT CTC TCG GTC CAA GTT | |||
| NM_008713 | e-NOS | s | CTG CCA CCT GAT CCT AAC TTG | 143 |
| as | CAG CCA AAC ACC AAA GTC ATG | |||
| NM_007392 | α-Actin | s | GAG CTA CGA ACT GCC TGA C | 129 |
| as | CTG TTA TAG GTG GTT TCG TGG A | |||
| NM_013607 | Myh11 | s | AAG CTG CGG CTA GAG GTC A | 238 |
| as | CCC TCC CTT TGA TGG CTG AG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castorena-Gonzalez, J.A.; Li, M.; Davis, M.J. Effects of Elevated Downstream Pressure and the Role of Smooth Muscle Cell Coupling through Connexin45 on Lymphatic Pacemaking. Biomolecules 2020, 10, 1424. https://doi.org/10.3390/biom10101424
Castorena-Gonzalez JA, Li M, Davis MJ. Effects of Elevated Downstream Pressure and the Role of Smooth Muscle Cell Coupling through Connexin45 on Lymphatic Pacemaking. Biomolecules. 2020; 10(10):1424. https://doi.org/10.3390/biom10101424
Chicago/Turabian StyleCastorena-Gonzalez, Jorge A., Min Li, and Michael J. Davis. 2020. "Effects of Elevated Downstream Pressure and the Role of Smooth Muscle Cell Coupling through Connexin45 on Lymphatic Pacemaking" Biomolecules 10, no. 10: 1424. https://doi.org/10.3390/biom10101424
APA StyleCastorena-Gonzalez, J. A., Li, M., & Davis, M. J. (2020). Effects of Elevated Downstream Pressure and the Role of Smooth Muscle Cell Coupling through Connexin45 on Lymphatic Pacemaking. Biomolecules, 10(10), 1424. https://doi.org/10.3390/biom10101424






