Antitumour Activity of the Ribonuclease Binase from Bacillus pumilus in the RLS40 Tumour Model Is Associated with the Reorganisation of the miRNA Network and Reversion of Cancer-Related Cascades to Normal Functioning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Binase Preparation and Purification
2.2. Cell Culture
2.3. Mice
2.4. Viability of RLS40 Cells
2.5. Treatment of RLS40 Cells with Binase for miRNA Analysis
2.6. Annexin V FITC/PI Apoptosis Detection
2.7. Determination of Caspase-3/-7 Activity and Caspase-3/-7-Positive Cells
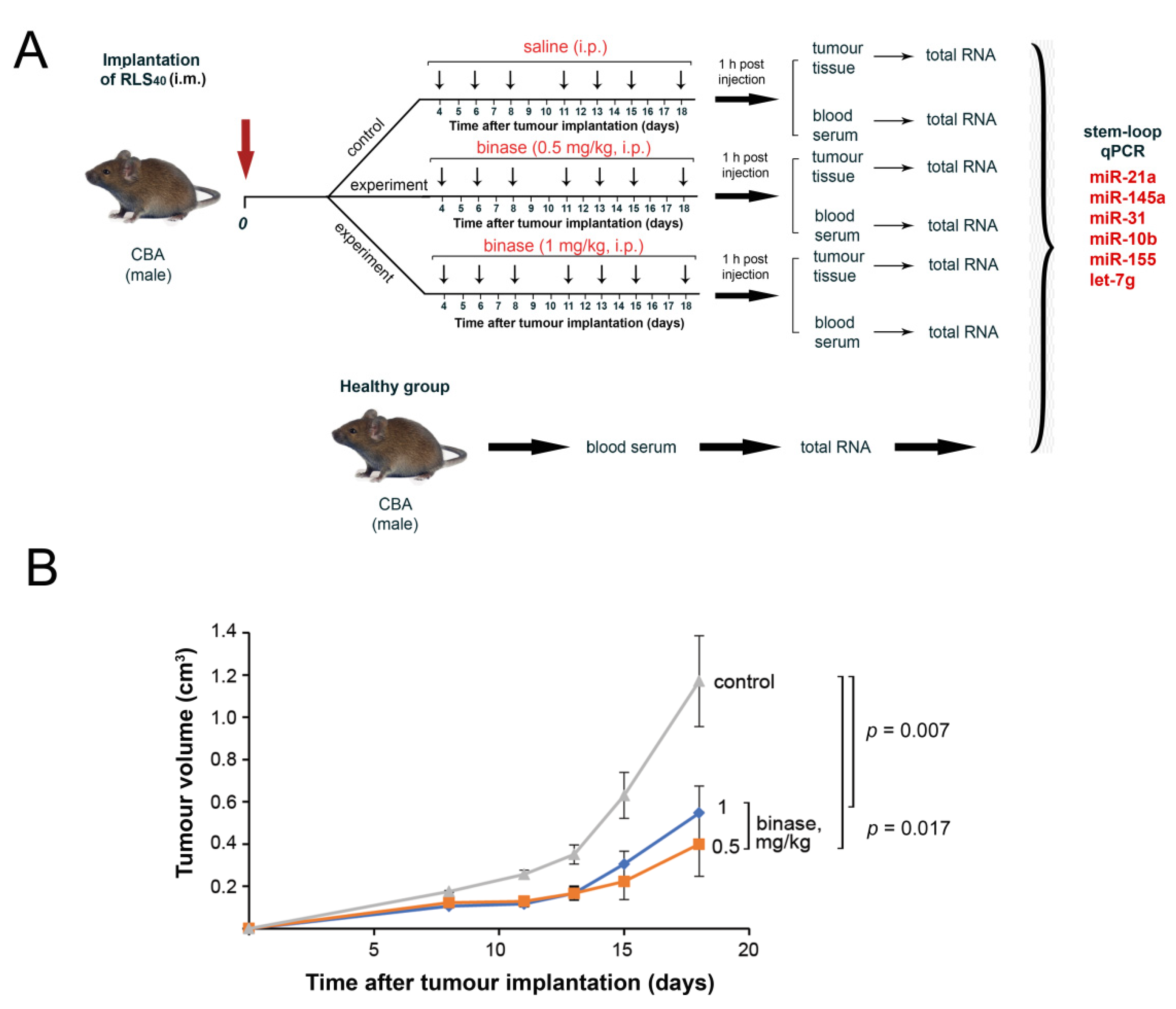
2.8. Tumour Transplantation and the Design of the Animal Experiments
2.9. Sample Processing and RNA Extraction
2.10. qPCR
2.11. Histology and Morphometry
2.12. Blood Biochemistry
2.13. miRNA Target Prediction and Functional Analysis
2.14. Statistics
3. Results
3.1. The Choice of the Tumour Model
3.2. The Effect of Binase on the Proliferation of RLS40 Cells and Apoptosis Induction
3.3. The Effect of Binase on miRNA Expression Levels in RLS40 Cells
3.4. The Effect of Binase on RLS40 Tumour Growth and Metastasis Development In Vivo
3.5. Toxicity and Immunomodulatory Effect of Binase in RLS40-Bearing Mice
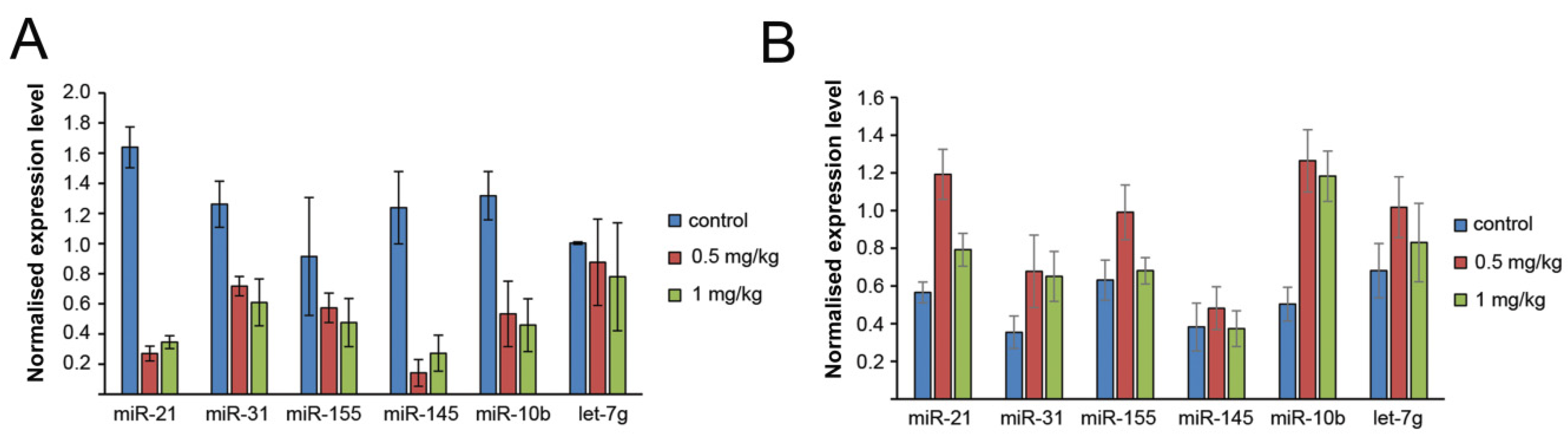
3.6. The Effect of Binase on miRNA Profile of the Tumour Tissue and Blood Serum of Mice with RLS40
3.7. Pathways Controlled by the Revealed Binase-Susceptible miRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Jin, Y. MicroRNA in cell differentiation and development. Sci. China C Life Sci. 2009, 52, 205–211. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taby, R.; Issa, J.P. Cancer epigenetics. CA Cancer J. Clin. 2010, 60, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Jeyapalan, Z.; Deng, Z.; Shatseva, T.; Fang, L.; He, C.; Yang, B.B. Expression of CD44 3′-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res. 2011, 39, 3026–3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.X.; Jiao, J.Q.; Li, Q.; Long, B.; Wang, K.; Liu, J.-P.; Li, Y.-R.; Li, P.-F. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011, 17, 71–78. [Google Scholar] [CrossRef]
- Pichiorri, F.; Suh, S.S.; Rocci, A.; De Luca, L.; Taccioli, C.; Santhanam, R.; Zhou, W.; Benson, D.M., Jr.; Hofmainster, C.; Alder, H.; et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 2010, 18, 367–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.W.; Cheng, Y.W.; Wang, J.; Chen, C.Y.; Lee, H. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res. 2010, 70, 10392–10401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Xian, R.R.; Li, Y.; Polony, T.S.; Beemon, K.L. Telomerase reverse transcriptase expression elevated by avian leukosis virus integration in B cell lymphomas. Proc. Natl. Acad. Sci. USA 2007, 104, 18952–18957. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, N. Cancer: Small losses, big gains with microRNAs. Nat. Rev. Genet. 2010, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Mironova, N.; Vlassov, V. Surveillance of tumour development: The relationship between tumour-associated RNAs and ribonucleases. Front. Pharmacol. 2019, 10, 1019. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y.; Huber, P.W.; Wool, I.G. The ribonuclease activity of the cytotoxin alpha-sarcin. The characteristics of the enzymatic activity of alpha-sarcin with ribosomes and ribonucleic acids as substrates. J. Biol. Chem. 1983, 258, 2662–2667. [Google Scholar] [PubMed]
- Kao, R.; Shea, J.E.; Davies, J.; Holden, D.W. Probing the active site of mitogillin, a fungal ribotoxin. Mol. Microbiol. 1998, 29, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Lacadena, J.; Alvarez-Garcia, E.; Carreras-Sangra, N.; Herrero-Galan, E.; Alegre-Cebollada, J.; Garcia-Ortega, L.; Oñaderra, M.; Gavilanes, J.G.; Martínez del Pozo, A. Fungal ribotoxins: Molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 2007, 31, 212–237. [Google Scholar] [CrossRef] [Green Version]
- Pouckova, P.; Zadinova, M.; Hlouskova, D.; Strohalm, J.; Plocová, D.; Spunda, M.; Olejár, T.; Zitko, M.; Matousek, J.; Ulbrich, K.; et al. Polymer-conjugated bovine pancreatic and seminal ribonucleases inhibit growth of human tumors in nude mice. J. Control. Release 2004, 95, 83–92. [Google Scholar] [CrossRef]
- Lee, I.; Lee, Y.H.; Mikulski, S.M.; Lee, J.; Covone, K.; Shogen, K. Tumoricidal effects of onconase on various tumors. J. Surg. Oncol. 2000, 73, 164–171. [Google Scholar] [CrossRef]
- Patutina, O.A.; Mironova, N.L.; Ryabchikova, E.I.; Popova, N.A.; Nikolin, V.P.; Kaledin, V.I.; Vlassov, V.V.; Zenkova, M.A. Tumoricidal activity of RNase A and DNase I. Acta Naturae 2010, 2, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Patutina, O.; Mironova, N.; Ryabchikova, E.; Popova, N.; Nikolin, V.; Kaledin, V.; Vlassov, V.V.; Zenkova, M.A. Inhibition of metastasis development by daily administration of ultralow doses of RNase A and DNase I. Biochimie 2011, 93, 689–696. [Google Scholar] [CrossRef]
- Prior, T.; Kunwar, S.; Pastan, I. Studies on the activity of barnase toxins in vitro and in vivo. Bioconjugate Chem. 1996, 7, 23–29. [Google Scholar] [CrossRef]
- Makarov, A.A.; Ilinskaya, O.N. Cytotoxic ribonucleases: Molecular weapons and their targets. FEBS Lett. 2003, 540, 15–20. [Google Scholar] [CrossRef]
- Giancola, C.; Ercole, C.; Fotticchia, I.; Spadaccini, R.; Pizzo, E.; D’Alessio, G.; Picone, D. Structure-cytotoxicity relationships in bovine seminal ribonuclease: New insights from heat and chemical denaturation studies on variants. FEBS J. 2011, 278, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Mitkevich, V.A.; Kretova, O.V.; Petrushanko, I.Y.; Burnysheva, K.M.; Sosin, D.V.; Simonenko, O.V.; Ilinskaya, O.N.; Tchurikov, N.A.; Makarov, A.A. Ribonuclease binase apoptotic signature in leukemic Kasumi-1 cells. Biochimie 2013, 95, 1344–1349. [Google Scholar] [CrossRef]
- Makarov, A.A.; Kolchinsky, A.; Ilinskaya, O.N. Binase and other microbial RNases as potential anticancer agents. BioEssays 2008, 30, 781–790. [Google Scholar] [CrossRef]
- Ardelt, W.; Ardelt, B.; Darzynkiewicz, Z. Ribonucleases as potential modalities in anticancer therapy. Eur. J. Pharmacol. 2009, 625, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, E.F.; Ng, T.B. Ribonucleases of different origins with a wide spectrum of medicinal applications. Biochim. Biophys. Acta 2011, 1815, 65–74. [Google Scholar] [CrossRef]
- Ulyanova, V.; Vershinina, V.; Ilinskaya, O. Barnase and binase: Twins with distinct fates. FEBS J. 2011, 278, 3633–3643. [Google Scholar] [CrossRef]
- Mironova, N.L.; Petrushanko, I.Y.; Patutina, O.A.; Sen’kova, A.V.; Simonenko, O.V.; Mitkevich, V.A.; Markov, O.V.; Zenkova, M.A.; Makarov, A.A. Ribonuclease binase inhibits primary tumor growth and metastases via apoptosis induction in tumor cells. Cell Cycle 2013, 12, 2120–2131. [Google Scholar] [CrossRef]
- Garipov, A.R.; Nesmelov, A.A.; Cabrera-Fuentes, H.A.; Ilinskaya, O.N. Bacillus intermedius ribonuclease (BINASE) induces apoptosis in human ovarian cancer cells. Toxicon 2014, 92, 54–59. [Google Scholar] [CrossRef]
- Shah Mahmud, R.; Ilinskaya, O.N. Antiviral activity of binase against the pandemic influenza A (H1N1) virus. Acta Naturae 2013, 5, 44–51. [Google Scholar] [CrossRef]
- Shah Mahmud, R.; Mostafa, A.; Müller, C.; Kanrai, P.; Ulyanova, V.; Sokurenko, Y.; Dzieciolowski, J.; Kuznetsova, I.; Ilinskaya, O.; Pleschka, S. Bacterial ribonuclease binase exerts an intra-cellular anti-viral mode of action targeting viral RNAs in influenza a virus-infected MDCK-II cells. Virol. J. 2018, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Kurinenko, B.M.; Sobchuk, L.I.; Khaĭbullina, S.A.; Bulgakova, R.S. Experimental research on the antitumor effectiveness of Bac. intermedius RNAse. Eksp Onkol. 1988, 10, 54–57. [Google Scholar] [PubMed]
- Kurinenko, B.M.; Sergeeva, E.V.; Sobchuk, L.I.; Bulgakova, R.S.; Khaĭbullina, S.A. In vitro and in vivo studies of RNAse of Bacillus intermedius. Antibiot Khimioter 1989, 34, 266–270. [Google Scholar] [PubMed]
- Mitkevich, V.A.; Petrushanko, I.Y.; Spirin, P.V.; Fedorova, T.V.; Kretova, O.V.; Tchurikov, N.A.; Prassolov, V.S.; Ilinskaya, O.N.; Makarov, A.A. Sensitivity of acute myeloid leukemia Kasumi-1 cells to binase toxic action depends on the expression of KIT and AML1-ETO oncogenes. Cell Cycle 2011, 10, 4090–4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mit’kevich, V.A.; Orlova, N.N.; Petrushanko, I.; Simonenko, O.V.; Spirin, P.V.; Prokofeva, M.M.; Gornostaeva, A.S.; Stocking, C.; Makarov, A.A.; Prasolov, V.S. Expression of FLT3-ITD oncogene confers mice progenitor B-cells BAF3 sensitivity to the ribonuclease binase cytotoxic action. Mol. Biol. 2013, 47, 249252. [Google Scholar]
- Mitkevich, V.A.; Tchurikov, N.A.; Zelenikhin, P.V.; Petrushanko, I.Y.; Makarov, A.A.; Ilinskaya, O.N. Binase cleaves cellular noncoding RNAs and affects coding mRNAs. FEBS J. 2010, 277, 186–196. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, 20. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Kutmon, M.; Kelder, T.; Mandaviya, P.; Evelo, C.T.A.; Coort, S.L. CyTargetLinker: A cytoscape app to integrate regulatory interactions in network analysis. PLoS ONE 2013, 8, e82160. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.D.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y.; et al. MiRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genecards®. The Human Gene Database. Available online: https://www.genecards.org (accessed on 19 October 2020).
- KEGG. Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.kegg.jp/ (accessed on 19 October 2020).
- Nakanishi, H.; Takenaga, K.; Oguri, K.; Yoshida, A.; Okayama, M. Morphological characteristics of tumours formed by Lewis lung carcinoma-derived cloned cell lines with different metastatic potentials: Structural differences in their basement membranes formed in vivo. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bobek, V.; Kolostova, K.; Pinterova, D.; Kacprzak, G.; Adamiak, J.; Kolodziej, J.; Boubelik, M.; Kubecova, M.; Hoffman, R.M. A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Res. 2010, 30, 4799–4803. [Google Scholar]
- Mironova, N.; Patutina, O.; Brenner, E.; Kurilshikov, A.; Vlassov, V.; Zenkova, M. MicroRNA drop in the bloodstream and microRNA boost in the tumour caused by treatment with ribonuclease A leads to an attenuation of tumour malignancy. PLoS ONE 2013, 8, e83482. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.P.; Nolde, M.; Slack, F.J. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature 2010, 467, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Eis, P.S.; Tam, W.; Sun, L.; Chadburn, A.; Li, Z.; Gomez, M.F.; Lund, E.; Dahlberg, J.E. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA 2005, 102, 3627–3632. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Fuentes, H.A.; Aslam, M.; Saffarzadeh, M.; Kolpakov, A.; Zelenikhin, P.; Preissner, K.T.; Ilinskaya, O.N. Internalization of Bacillus intermedius ribonuclease (BINASE) induces human alveolar adenocarcinoma cell death. Toxicon 2013, 69, 219–226. [Google Scholar] [CrossRef]
- Qiao, M.; Zu, L.D.; He, X.H.; Shen, R.L.; Wang, Q.C.; Liu, M.F. Onconase downregulates microRNA expression through targeting microRNA precursors. Cell. Res. 2012, 22, 1199–1202. [Google Scholar] [CrossRef] [Green Version]
- Goparaju, C.M.; Blasberg, J.D.; Volinia, S.; Palatini, J.; Ivanov, S.; Donington, J.S.; Croce, C.; Carbone, M.; Yang, H.; Pass, H.I. Onconase mediated NFKb downregulation in malignant pleural mesothelioma. Oncogene 2011, 30, 2767–2777. [Google Scholar] [CrossRef] [Green Version]
- Ilinskaya, O.N.; Singh, I.; Dudkina, E.; Ulyanova, V.; Kayumov, A.; Barreto, G. Direct inhibition of oncogenic KRAS by Bacillus pumilus ribonuclease (binase). Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 1559–1567. [Google Scholar] [CrossRef]
- Mironova, N.; Patutina, O.; Brenner, E.; Kurilshikov, A.; Vlassov, V.; Zenkova, M. The systemic tumor response to RNase A treatment affects the expression of genes involved in maintaining cell malignancy. Oncotarget 2017, 8, 78796–78810. [Google Scholar] [CrossRef] [Green Version]
- Al-Haj, L.; Blackshear, P.J.; Khabar, K.S.A. Regulation of p21/CIP1/WAF-1 mediated cell-cycle arrest by RNase L and tristetraprolin, and involvement of AU-rich elements. Nucleic Acids Res. 2012, 40, 7739–7752. [Google Scholar] [CrossRef] [Green Version]
- Fang, E.F.; Zhang, C.Z.Y.; Zhang, L.; Fong, W.P.; Ng, T.B. In vitro and in vivo anticarcinogenic effects of RNase MC2, a ribonuclease isolated from dietary bitter gourd, toward human liver cancer cells. Int. J. Biochem. Cell Biol. 2012, 44, 1351–1360. [Google Scholar] [CrossRef]
- Makeeva, A.; Rodriguez-Montesinos, J.; Zelenikhin, P.; Nesmelov, A.; Preissner, K.T.; Cabrera-Fuentes, H.A.; Ilinskaya, O.N. Antitumor macrophage response to bacillus pumilus ribonuclease (Binase). Mediators Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Zelenikhin, P.V.; Ead Mohamed, I.S.; Nadyrova, A.I.; Sirotkina, A.A.; Ulyanova, V.V.; Mironova, N.L.; Mit’kevich, V.A.; Makarov, A.A.; Zenkova, M.A.; Ilinskaya, O.N. Bacillus pumilus ribonuclease inhibits migration of human duodenum adenocarcinoma HuTu 80 cells. Mol. Biology (Moskow) 2020, 54, 146–152. [Google Scholar] [CrossRef]




| Number in the Library According to RPKM * | Top miRNA (LLC NGS Data) [46] | miRNA sequence, 5′→3′ | The Number of Guanine Residues # | Expression Level of miRNAs in the RLS40 cells ## | The Alteration of the miRNA Levels after the Binase Treatment ## |
|---|---|---|---|---|---|
| 1 | mir-21a | UAGCUUAUCAGACUGAUGUUGA | 5 | 1.7 | 2.0↓ |
| 10 | mir-145a | GUCCAGUUUUCCCAGGAAUCCCU | 4 | 1.4 | no effect |
| 15 | mir-31 | AGGCAAGAUGCUGGCAUAGCUG | 7 | 0.85 | 1.6↓ |
| 29 | mir-10b | UACCCUGUAGAACCGAAUUUGUG | 4 | 1.5 | no effect |
| 46 | let-7g | UGAGGUAGUAGUUUGUACAGUU | 7 | 1.8 | 1.6↓ |
| 47 | miR-155 | UUAAUGCUAAUUGUGAUAGGGGU | 7 | 1.9 | 1.8↓ |
| U6 snRNA ** | 0.40 | ||||
| Morphological Parameter | Control | Binase (0.5 mg/kg) | Binase (1 mg/kg) |
|---|---|---|---|
| Unchanged tumour tissue, Vv (%) | 71.2 ± 1.3 | 74.5 ± 1.7 # | 74.4 ± 1 # |
| Lymphoid infiltration, Vv (%) | 19.5 ± 1.4 | 14.3 ± 0.9 # | 15 ± 0.9 # |
| Necrotic changes, Vv (%) | 9 ± 0.9 | 10.9 ± 2 | 10.3 ± 0.8 |
| Mitotic cells, Nv | 5.6 ± 0.4 | 1.1 ± 0.3 # | 3.2 ± 1 # |
| PCNA positive cells, Vv (%) | 62.7 ± 3.8 | 21.4 ± 2.3 # | 44.7 ± 9.5 # |
| Caspase-7 positive cells, Nv | 5.3 ± 0.5 | 25.9 ± 2.3 # | 17.8 ± 3.5 # |
| Event | Target Gene | Description | miRNAs | Pathway |
|---|---|---|---|---|
| Apoptosis | CTSB | Cathepsin C | miR-10b/let-7g | Apoptosis and autophagy Apoptosis modulation and signalling Caspase activation via an extrinsic apoptotic signalling pathway |
| KRAS | KRAS Proto-Oncogene, GTPase | miR-155/miR-145a | Apoptosis pathway | |
| FADD | Fas Associated via Death Domain | miR-155/miR-10b | Apoptosis modulation and signalling Apoptosis and autophagy | |
| TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | Cellular apoptosis pathway Mitochondrial apoptosis | |
| Proliferation | KIF4A | Kinesin Family Member 4A | miR-10b/miR-145a | L1CAM interactions |
| TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | TGF-beta pathway | |
| Cancer-related pathways | IFNAR1 | Interferon Alpha and Beta Receptor Subunit 1 | miR-10b/let-7g | JAK/STAT signalling pathway PI3K/Akt signalling pathway |
| PIAS3 | Protein Inhibitor of Activated STAT 3 | miR-155/miR-21a | JAK/STAT signalling pathway | |
| KRAS | KRAS Proto-Oncogene, GTPase | miR-155/miR-145a | MAPK Erk pathway Ras signalling pathway Oncogenic MAPK signalling | |
| RHOA | Ras Homolog Family Member A | miR-155/miR-31 | ERK signalling Wnt signalling pathway AGE/RAGE pathway | |
| FADD | Fas Associated Via Death Domain | miR-155/miR-10b | PI3K/Akt signalling pathway | |
| KCNK6 | Potassium Two-Pore Domain Channel Subfamily K Member 6 | miR10b/miR-21a | PKA signalling | |
| TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | TGF-β pathway | |
| Immunity/ inflammation | PIAS3 | Protein Inhibitor of Activated STAT 3 | miR-155/miR-21a | IL-6-mediated signalling |
| RHOA | Ras Homolog Family Member A | miR-155/miR-31 | CCR5 pathway in macrophages (chemokine signalling) | |
| FADD | Fas Associated via Death Domain | miR-155/miR-10b | Toll-like receptor 4 (TLR4) cascade | |
| TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | Toll-like receptor signalling pathway Plasmin signalling | |
| Angiogenesis | TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | Angiogenesis |
| Adhesion | RHOA | Ras Homolog Family Member A | miR-155/miR-31 | Cytoskeleton remodelling (cell adhesion and migration) Adherens junction |
| TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | Cell adhesion | |
| miRNA in cancer | KRAS | KRAS Proto-Oncogene, GTPase | miR-155/miR-145a | Silencing of tumour suppressor genes |
| MSI2 | Musashi RNA Binding Protein 2 | miR-155/let-7g | mRNA surveillance pathway | |
| TGFB2 | Transforming Growth Factor Beta 2 | miR-145a/miR-31 | MicroRNAs in cancer | |
| House-keeping functions | GNL3L | G Protein Nuclear 3 Like | miR-10b/let-7g | Ribosome biogenesis in eukaryotes |
| KCNK6 | Potassium Two-Pore Domain Channel Subfamily K Member 6 | miR-10b/miR-21a | Potassium channels Hepatic ABC transporters | |
| PIAS3 | Protein Inhibitor of Activated STAT 3 | miR-155/miR-21a | Transcription-coupled nucleotide excision repair (TC-NER) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, I.S.E.; Sen’kova, A.V.; Nadyrova, A.I.; Savin, I.A.; Markov, A.V.; Mitkevich, V.A.; Makarov, A.A.; Ilinskaya, O.N.; Mironova, N.L.; Zenkova, M.A. Antitumour Activity of the Ribonuclease Binase from Bacillus pumilus in the RLS40 Tumour Model Is Associated with the Reorganisation of the miRNA Network and Reversion of Cancer-Related Cascades to Normal Functioning. Biomolecules 2020, 10, 1509. https://doi.org/10.3390/biom10111509
Mohamed ISE, Sen’kova AV, Nadyrova AI, Savin IA, Markov AV, Mitkevich VA, Makarov AA, Ilinskaya ON, Mironova NL, Zenkova MA. Antitumour Activity of the Ribonuclease Binase from Bacillus pumilus in the RLS40 Tumour Model Is Associated with the Reorganisation of the miRNA Network and Reversion of Cancer-Related Cascades to Normal Functioning. Biomolecules. 2020; 10(11):1509. https://doi.org/10.3390/biom10111509
Chicago/Turabian StyleMohamed, Islam Saber Ead, Aleksandra V. Sen’kova, Alsu I. Nadyrova, Innokenty A. Savin, Andrey V. Markov, Vladimir A. Mitkevich, Aleksander A. Makarov, Olga N. Ilinskaya, Nadezhda L. Mironova, and Marina A. Zenkova. 2020. "Antitumour Activity of the Ribonuclease Binase from Bacillus pumilus in the RLS40 Tumour Model Is Associated with the Reorganisation of the miRNA Network and Reversion of Cancer-Related Cascades to Normal Functioning" Biomolecules 10, no. 11: 1509. https://doi.org/10.3390/biom10111509





