The Presence of Human Herpesvirus 6 in the Brain in Health and Disease
Abstract
:1. Introduction
2. HHV-6 Can Infect Multiple Neural Cell Types
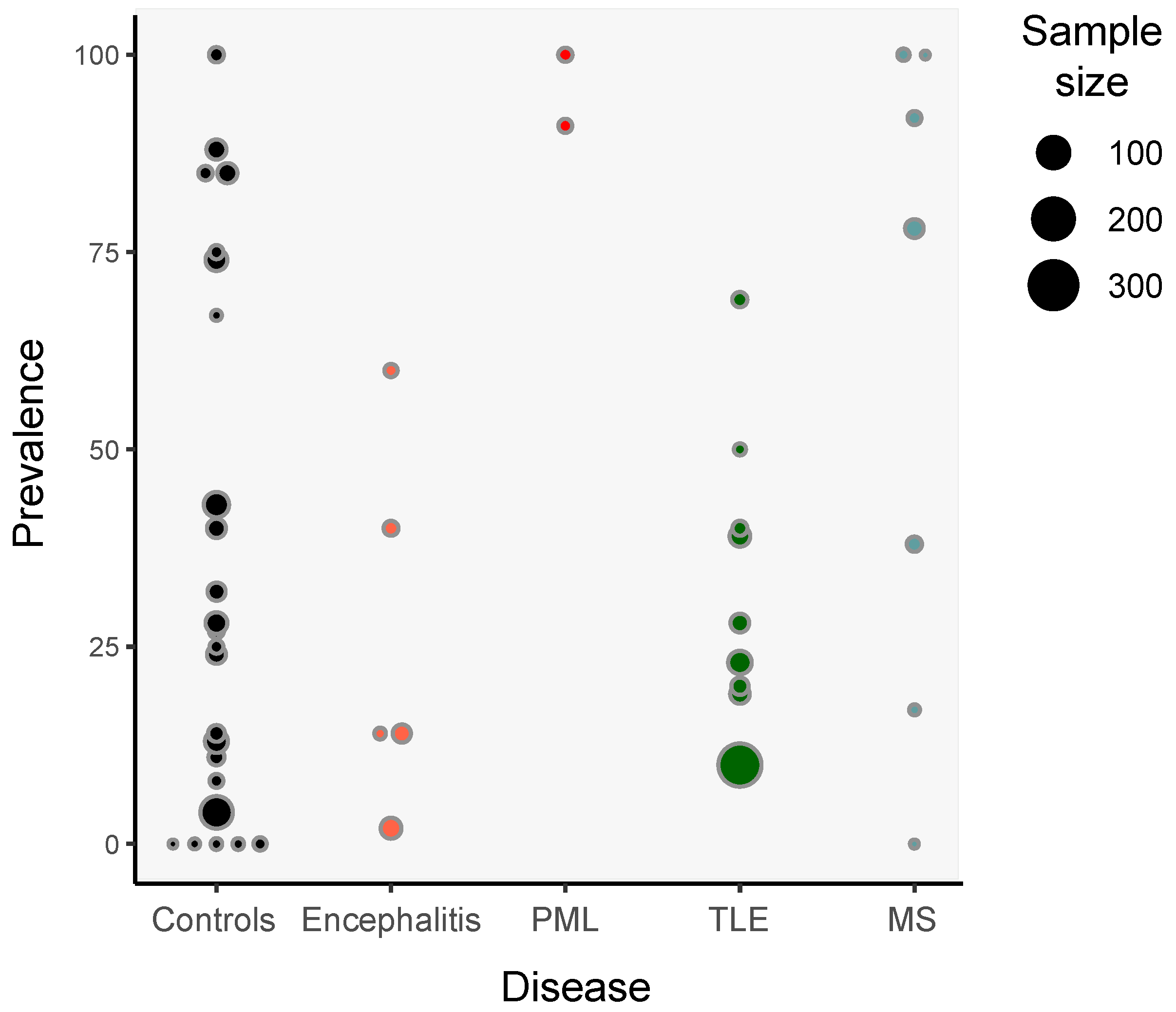
3. Detection of HHV-6 in Non-Pathological Samples
4. Mechanism of Entry of HHV-6 into the Brain Parenchyma
5. HHV-6 and Encephalitis
6. HHV-6 and Epilepsy
7. HHV-6 and Multiple Sclerosis
8. HHV-6 and Alzheimer’s Disease
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ihira, M.; Yoshikawa, T.; Ishii, J.; Nomura, M.; Hishida, H.; Ohashi, M.; Enomoto, Y.; Suga, S.; Iida, K.; Saito, Y.; et al. Serological examination of human herpesvirus 6 and 7 in patients with coronary artery disease. J. Med. Virol. 2002, 67, 534–537. [Google Scholar] [CrossRef]
- Okuno, T.; Takahashi, K.; Balachandra, K.; Shiraki, K.; Yamanishi, K.; Takahashi, M.; Baba, K. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J. Clin. Microbiol. 1989, 27, 651–653. [Google Scholar] [CrossRef] [Green Version]
- Saxinger, C.; Polesky, H.; Eby, N.; Grufferman, S.; Murphy, R.; Tegtmeir, G.; Parekh, V.; Memon, S.; Hung, C. Antibody reactivity with HBLV (HHV-6) in U.S. populations. J. Virol. Methods 1988, 21, 199–208. [Google Scholar] [CrossRef]
- Ablashi, D.; Agut, H.; Alvarez-Lafuente, R.; Clark, D.A.; Dewhurst, S.; DiLuca, D.; Flamand, L.; Frenkel, N.; Gallo, R.; Gompels, U.A.; et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014, 159, 863–870. [Google Scholar] [CrossRef]
- Pantry, S.; Medveczky, P. Latency, Integration, and Reactivation of Human Herpesvirus-6. Viruses 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovitch, E.; Wohler, J.E.; Cummings Macri, S.M.; Motanic, K.; Harberts, E.; Gaitán, M.I.; Maggi, P.; Ellis, M.; Westmoreland, S.; Silva, A.; et al. Novel Marmoset (Callithrix jacchus) Model of Human Herpesvirus 6A and 6B Infections: Immunologic, Virologic and Radiologic Characterization. PLoS Pathog. 2013, 9, e1003138. [Google Scholar] [CrossRef]
- Salahuddin, S.; Ablashi, D.; Markham, P.; Josephs, S.; Sturzenegger, S.; Kaplan, M.; Halligan, G.; Biberfeld, P.; Wong-Staal, F.; Kramarsky, B.; et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 1986, 234, 596–601. [Google Scholar] [CrossRef]
- Downing, R.G.; Sewankambo, N.; Serwadda, D.; Honess, R.; Crawford, D.; Jarrett, R.; Griffin, B.E. Isolation of human lymphotropic herpesviruses from Uganda. Lancet 1987, 2, 390. [Google Scholar] [CrossRef]
- Lopez, C.; Pellett, P.; Stewart, J.; Goldsmith, C.; Sanderlin, K.; Black, J.; Warfield, D.; Feorino, P. Characteristics of Human Herpesvirus-6. J. Infect. Dis. 1988, 157, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Houldcroft, C.J. Human Herpesvirus Sequencing in the Genomic Era: The Growing Ranks of the Herpetic Legion. Pathogens 2019, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Telford, M.; Navarro, A.; Santpere, G. Whole genome diversity of inherited chromosomally integrated HHV-6 derived from healthy individuals of diverse geographic origin. Sci. Rep. 2018, 8, 3472. [Google Scholar] [CrossRef] [Green Version]
- Tweedy, J.; Spyrou, M.A.; Hubacek, P.; Kuhl, U.; Lassner, D.; Gompels, U.A. Analyses of germline, chromosomally integrated human herpesvirus 6A and B genomes indicate emergent infection and new inflammatory mediators. J. Gen. Virol. 2015, 96, 370–389. [Google Scholar] [CrossRef] [PubMed]
- Harberts, E.; Yao, K.; Wohler, J.E.; Maric, D.; Ohayon, J.; Henkin, R.; Jacobson, S. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 13734–13739. [Google Scholar] [CrossRef] [Green Version]
- Arbuckle, J.H.; Medveczky, M.M.; Luka, J.; Hadley, S.H.; Luegmayr, A.; Ablashi, D.; Lund, T.C.; Tolar, J.; De Meirleir, K.; Montoya, J.G.; et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 5563–5568. [Google Scholar] [CrossRef] [Green Version]
- Arbuckle, J.H.; Medveczky, P.G. The molecular biology of human herpesvirus-6 latency and telomere integration. Microbes Infect. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusso, P.; Markham, P.D.; Tschachler, E.; Di Marzo Veronese, F.; Zaki Salahuddin, S.; Ablashi, D.V.; Pahwa, S.; Krohn, K.; Gallo, R.C. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J. Exp. Med. 1988, 167, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Sonoda, S.; Higashi, K.; Kondo, T.; Takahashi, H.; Takahashi, M.; Yamanishi, K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 1989, 63, 3161–3163. [Google Scholar] [CrossRef] [Green Version]
- Albright, A.V.; Lavi, E.; Black, J.B.; Goldberg, S.; O’Connor, M.J.; González-Scarano, F. The effect of human herpesvirus-6 (HHV-6) on cultured human neural cells: Oligodendrocytes and microglia. J. Neurovirol. 1998, 4, 486–494. [Google Scholar] [CrossRef]
- Luka, J.; Okano, M.; Thiele, G. Isolation of human herpesvirus-6 from clinical specimens using human fibroblast cultures. J. Clin. Lab. Anal. 1990, 4, 483–486. [Google Scholar] [CrossRef]
- He, J.; McCarthy, M.; Zhou, Y.; Chandran, B.; Wood, C. Infection of primary human fetal astrocytes by human herpesvirus 6. J. Virol. 1996, 70, 1296–1300. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Popescu, N.; Woodworth, C.; Berneman, Z.; Corbellino, M.; Lusso, P.; Ablashi, D.V.; DiPaolo, J.A. Human herpesvirus 6 infects cervical epithelial cells and transactivates human papillomavirus gene expression. J. Virol. 1994, 68, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Prusty, B.K.; Gulve, N.; Govind, S.; Krueger, G.R.F.; Feichtinger, J.; Larcombe, L.; Aspinall, R.; Ablashi, D.V.; Toro, C.T. Active HHV-6 Infection of Cerebellar Purkinje Cells in Mood Disorders. Front. Microbiol. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Daibata, M.; Taguchi, T.; Sawada, T.; Taguchi, H.; Miyoshi, I. Chromosomal transmission of human herpesvirus 6 DNA in acute lymphoblastic leukaemia. Lancet 1998, 352, 543–544. [Google Scholar] [CrossRef]
- Endo, A.; Watanabe, K.; Ohye, T.; Suzuki, K.; Matsubara, T.; Shimizu, N.; Kurahashi, H.; Yoshikawa, T.; Katano, H.; Inoue, N.; et al. Molecular and Virological Evidence of Viral Activation From Chromosomally Integrated Human Herpesvirus 6A in a Patient With X-Linked Severe Combined Immunodeficiency. Clin. Infect. Dis. 2014, 59, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Gravel, A.; Dubuc, I.; Morissette, G.; Sedlak, R.H.; Jerome, K.R.; Flamand, L. Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc. Natl. Acad. Sci. USA 2015, 112, 8058–8063. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.N.; Hoe, N.L.; Thiruchelvam, A.D.; Atkinson, C.E.; Clark, D.A. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J. Clin. Microbiol. 2007, 45, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Yamanishi, K.; Shiraki, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Asano, Y.; Kurata, T. IDENTIFICATION OF HUMAN HERPESVIRUS-6 AS A CAUSAL AGENT FOR EXANTHEM SUBITUM. Lancet 1988, 331, 1065–1067. [Google Scholar] [CrossRef]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.R.; Phan, T.L.; Ablashi, D.; Hudnall, S.D. Human Herpesvirus 6 and Malignancy: A Review. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Seeley, W.W.; Marty, F.M.; Holmes, T.M.; Upchurch, K.; Soiffer, R.J.; Antin, J.H.; Baden, L.R.; Bromfield, E.B. Post-transplant acute limbic encephalitis: Clinical features and relationship to HHV6. Neurology 2007, 69, 156–165. [Google Scholar] [CrossRef]
- Ishio, T.; Endo, T.; Okada, K.; Shigematsu, A.; Hashino, S.; Teshima, T. Human Herpesvirus-6 Pneumonitis around the Engraftment of Cord Blood Transplantation following Foscarnet Prophylaxis in a Patient with Acute Leukemia. Case Rep. Hematol. 2015, 2015, 949265. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.A.; Myerson, D.; Sedlak, R.H.; Jerome, K.R.; Zerr, D.M. Hepatitis due to human herpesvirus 6B after hematopoietic cell transplantation and a review of the literature. Transpl. Infect. Dis. 2014, 16, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Readhead, B.; Haure-Mirande, J.-V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 2018, 99, 64–82.e7. [Google Scholar] [CrossRef] [Green Version]
- Eimer, W.A.; Vijaya Kumar, D.K.; Navalpur Shanmugam, N.K.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; György, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 2018, 99, 56–63.e3. [Google Scholar] [CrossRef] [Green Version]
- Ablashi, D.V.; Salahuddin, S.Z.; Josephs, S.F.; Imam, F.; Lusso, P.; Gallo, R.C.; Hung, C.; Lemp, J.; Markham, P.D. HBLV (orHHV-6) in human cell lines. Nature 1987, 329, 207. [Google Scholar] [CrossRef]
- Levy, J.A.; Ferro, F.; Lennette, E.T.; Oshiro, L.; Poulin, L. Characterization of a new strain of HHV-6 (HHV-6SF) Recovered from the saliva of an HIV-infected individual. Virology 1990, 178, 113–121. [Google Scholar] [CrossRef]
- Tedder, R.S.; Briggs, M.; Cameron, C.H.; Honess, R.; Robertson, D.; Whittle, H. A NOVEL LYMPHOTROPIC HERPESVIRUS. Lancet 1987, 330, 390–392. [Google Scholar] [CrossRef]
- Luppi, M.; Barozzi, P.; Maiorana, A.; Marasca, R.; Torelli, G. Human Herpesvirus 6 Infection in Normal Human Brain Tissue. J. Infect. Dis. 1994, 169, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Drobyski, W.R.; Knox, K.K.; Majewski, D.; Carrigan, D.R. Fatal Encephalitis Due to Variant B Human Herpesvirus-6 Infection in a Bone Marrow-Transplant Recipient. N. Engl. J. Med. 1994, 330, 1356–1360. [Google Scholar] [CrossRef]
- Opsahl, M.L.; Kennedy, P.G.E. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 2005, 128, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Donati, D.; Akhyani, N.; Fogdell-Hahn, A.; Cermelli, C.; Cassiani-Ingoni, R.; Vortmeyer, A.; Heiss, J.D.; Cogen, P.; Gaillard, W.D.; Sato, S.; et al. Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology 2003, 61, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, J.; Blumberg, B.M.; Roshal, M.; Baker, J.V.; Hurley, S.D.; Mayer-Pröschel, M.; Mock, D.J. Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J. Neurosci. 2004, 24, 4875–4883. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wang, X.; Wang, Y.; Wang, P.; Fan, D.; Chen, S.; Guan, Y.; Li, T.; An, J.; Luan, G. Detection of EBV and HHV6 in the Brain Tissue of Patients with Rasmussen’s Encephalitis. Virol. Sin. 2018, 33, 402–409. [Google Scholar] [CrossRef]
- Wainwright, M.S.; Martin, P.L.; Morse, R.P.; Lacaze, M.; Provenzale, J.M.; Edward Coleman, R.; Morgan, M.A.; Hulette, C.; Kurtzberg, J.; Bushnell, C.; et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann. Neurol. 2001, 50, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Allnutt, M.A.; Johnson, K.; Bennett, D.A.; Connor, S.M.; Troncoso, J.C.; Pletnikova, O.; Albert, M.S.; Resnick, S.M.; Scholz, S.W.; De Jager, P.L.; et al. Human Herpesvirus 6 Detection in Alzheimer’s Disease Cases and Controls across Multiple Cohorts. Neuron 2020. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, P.; Dunn, N.; Beiki, O.; Trinka, E.; Fogdell-Hahn, A. The Viral Hypothesis of Mesial Temporal Lobe Epilepsy—Is Human Herpes Virus-6 the Missing Link? A systematic review and meta-analysis. Seizure Eur. J. Epilepsy 2018, 54, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Warren-Gash, C.; Forbes, H.J.; Williamson, E.; Breuer, J.; Hayward, A.C.; Mavrodaris, A.; Ridha, B.H.; Rossor, M.N.; Thomas, S.L.; Smeeth, L. Human herpesvirus infections and dementia or mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 4743. [Google Scholar] [CrossRef] [Green Version]
- Pellett, P.E.; Sánchez-Martínez, D.; Dominguez, G.; Black, J.B.; Anton, E.; Greenamoyer, C.; Dambaugh, T.R. A strongly immunoreactive virion protein of human herpesvirus 6 variant b strain z29: Identification and characterization of the gene and mapping of a variant-specific monoclonal antibody reactive epitope. Virology 1993, 195, 521–531. [Google Scholar] [CrossRef]
- Agulnick, A.D.; Thompson, J.R.; Iyengar, S.; Pearson, G.; Ablashi, D.; Ricciardi, R.P. Identification of a DNA-binding protein of human herpesvirus 6, a putative DNA polymerase stimulatory factor. J. Gen. Virol. 1993, 74, 1003–1009. [Google Scholar] [CrossRef]
- Balachandran, N.; Amelse, R.E.; Zhou, W.W.; Chang, C.K. Identification of Proteins Specific for Human Herpesvirus 6-Infected Human T Cells. Life Sci. 1989, 14, 2835–2840. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Yan, B.; Lei, D.; Si, Y.; Li, H.; Chen, M.W.; Li, L.; Chen, F.; Zhou, Q.; Zhou, D.; et al. Apolipoprotein 4 may increase viral load and seizure frequency in mesial temporal lobe epilepsy patients with positive human herpes virus 6B. Neurosci. Lett. 2015, 593, 29–34. [Google Scholar] [CrossRef]
- Friedman, J.E.; Lyons, M.J.; Cu, G.; Ablashl, D.V.; Whitman, J.E.; Edgar, M.; Koskiniemi, M.; Vaheri, A.; Zabriskie, J.B. The association of the human herpesvirus-6 and MS. Mult. Scler. 1999, 5, 355–362. [Google Scholar] [CrossRef]
- Skuja, S.; Zieda, A.; Ravina, K.; Chapenko, S.; Roga, S.; Teteris, O.; Groma, V.; Murovska, M. Structural and Ultrastructural Alterations in Human Olfactory Pathways and Possible Associations with Herpesvirus 6 Infection. PLoS ONE 2017, 12, e0170071. [Google Scholar] [CrossRef] [Green Version]
- Katsafanas, G.C.; Schirmer, E.C.; Wyatt, L.S.; Frenkel, N. In vitro activation of human herpesviruses 6 and 7 from latency. Proc. Natl. Acad. Sci. USA 1996, 93, 9788–9792. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, B.M.; Mock, D.J.; Powers, J.M.; Ito, M.; Assouline, J.G.; Baker, J.V.; Chen, B.; Goodman, A.D. The HHV6 paradox: Ubiquitous commensal or insidious pathogen? A two-step in situ PCR approach. J. Clin. Virol. 2000, 16, 159–178. [Google Scholar] [CrossRef]
- Knox, K.K.; Brewer, J.H.; Henry, J.M.; Harrington, D.J.; Carrigan, D.R. Human Herpesvirus 6 and Multiple Sclerosis: Systemic Active Infections in Patients with Early Disease. Clin. Infect. Dis. 2000, 31, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.D.; Mock, D.J.; Powers, J.M.; Baker, J.V.; Blumberg, B.M. Human Herpesvirus 6 Genome and Antigen in Acute Multiple Sclerosis Lesions. J. Infect. Dis. 2003, 187, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Chapenko, S.; Roga, S.; Skuja, S.; Rasa, S.; Cistjakovs, M.; Svirskis, S.; Zaserska, Z.; Groma, V.; Murovska, M. Detection frequency of human herpesviruses-6A, -6B, and -7 genomic sequences in central nervous system DNA samples from post-mortem individuals with unspecified encephalopathy. J. Neurovirol. 2016, 22, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huff, K.; McMasters, R.; Cornford, M.E. Sudden unexpected death associated with HHV-6 in an adolescent with tuberous sclerosis. Pediatr. Neurol. 1999, 21, 488–491. [Google Scholar] [CrossRef]
- Pitkäranta, A.; Piiparinen, H.; Mannonen, L.; Vesaluoma, M.; Vaheri, A. Detection of human herpesvirus 6 and varicella-zoster virus in tear fluid of patients with Bell’s palsy by PCR. J. Clin. Microbiol. 2000, 38, 2753–2755. [Google Scholar] [CrossRef] [Green Version]
- Sakai, R.; Kanamori, H.; Motohashi, K.; Yamamoto, W.; Matsuura, S.; Fujita, A.; Ohshima, R.; Kuwabara, H.; Tanaka, M.; Fujita, H.; et al. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Fida, M.; Hamdi, A.M.; Bryson, A.; Razonable, R.R.; Abu Saleh, O. Long-term Outcomes of Patients With Human Herpesvirus 6 Encephalitis. Open Forum Infect. Dis. 2019, 6. [Google Scholar] [CrossRef]
- Yanagihara, K.; Tanaka-Taya, K.; Itagaki, Y.; Toribe, Y.; Arita, K.; Yamanishi, K.; Okada, S. Human herpesvirus 6 meningoencephalitis with sequelae. Pediatr. Infect. Dis. J. 1995, 14, 240–242. [Google Scholar]
- Howell, K.B.; Tiedemann, K.; Haeusler, G.; MacKay, M.T.; Kornberg, A.J.; Freeman, J.L.; Harvey, A.S. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia 2012, 53, e122–e126. [Google Scholar] [CrossRef]
- Asano, Y.; Yoshikawa, T.; Kajita, Y.; Ogura, R.; Suga, S.; Yazaki, T.; Nakashima, T.; Yamada, A.; Kurata, T. Fatal encephalitis/encephalopathy in primary human herpesvirus-6 infection. Arch. Dis. Child. 1992, 67, 1484–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, K.K.; Harrington, D.P.; Carrigan, D.R. Fulminant human herpesvirus six encephalitis in a human immunodeficiency virus-infected infant. J. Med. Virol. 1995, 45, 288–292. [Google Scholar] [CrossRef]
- Forest, F.; Duband, S.; Pillet, S.; Stachowicz, M.L.; Cornillon, J.; Dumollard, J.M.; Peoc’h, M. Lethal human herpesvirus-6 encephalitis after cord blood transplant. Transpl. Infect. Dis. 2011, 13, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Shahani, L. HHV-6 encephalitis presenting as status epilepticus in an immunocompetent patient. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Novoa, L.J.; Nagra, R.M.; Nakawatase, T.; Edwards-Lee, T.; Tourtellotte, W.W.; Cornford, M.E. Fulminant demyelinating encephalomyelitis associated with productive HHV-6 infection in an immunocompetent adult. J. Med. Virol. 1997, 52, 301–308. [Google Scholar] [CrossRef]
- De Bolle, L.; Naesens, L.; Clercq, E. De Update on Human Herpesvirus 6 Biology, Clinical Features, and Therapy. Clin. Microbiol. Rev. 2005, 18, 217–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevallier, P.; Hebia-Fellah, I.; Planche, L.; Guillaume, T.; Bressolette-Bodin, C.; Coste-Burel, M.; Rialland, F.; Mohty, M.; Imbert-Marcille, B.M. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: A comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant. 2010, 45, 1204–1211. [Google Scholar] [CrossRef]
- Paterson, D.L.; Singh, N.; Gayowski, T.; Carrigan, D.R.; Marino, I.R. Encephalopathy associated with human herpesvirus 6 in a liver transplant recipient. Liver Transplant. Surg. 1999, 5, 454–455. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.; Olszewski, J.; Lloyd-Smith, D. Focal seizures due to chronic localized encephalitis. Neurology 1958, 8, 435. [Google Scholar] [CrossRef]
- Ito, M.; Baker, J.V.; Mock, D.J.; Goodman, A.D.; Blumberg, B.M.; Shrier, D.A.; Powers, J.M. Human herpesvirus 6-meningoencephalitis in an HIV patient with progressive multifocal leukoencephalopathy. Acta Neuropathol. 2000, 100, 337–341. [Google Scholar] [CrossRef]
- Mock, D.J.; Powers, J.M.; Goodman, A.D.; Blumenthal, S.R.; Ergin, N.; Baker, J.V.; Mattson, D.H.; Assouline, J.G.; Bergey, E.J.; Chen, B.; et al. Association of human herpesvirus 6 with the demyelinative lesions of progressive multifocal leukoencephalopathy. J. Neurovirol. 1999, 5, 363–373. [Google Scholar] [CrossRef]
- Epstein, L.G.; Shinnar, S.; Hesdorffer, D.C.; Nordli, D.R.; Hamidullah, A.; Benn, E.K.T.; Pellock, J.M.; Frank, L.M.; Lewis, D.V.; Moshe, S.L.; et al. Human herpesvirus 6 and 7 in febrile status epilepticus: The FEBSTAT study. Epilepsia 2012, 53, 1481–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehusmann, P.; Mittelstaedt, T.; Bien, C.G.; Drexler, J.F.; Grote, A.; Schoch, S.; Becker, A.J. Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia 2010, 51, 2478–2483. [Google Scholar] [CrossRef]
- Fotheringham, J.; Donati, D.; Akhyani, N.; Fogdell-Hahn, A.; Vortmeyer, A.; Heiss, J.D.; Williams, E.; Weinstein, S.; Bruce, D.A.; Gaillard, W.D.; et al. Association of Human Herpesvirus-6B with Mesial Temporal Lobe Epilepsy. PLoS Med. 2007, 4, e180. [Google Scholar] [CrossRef] [Green Version]
- Esposito, L.; Drexler, J.F.; Braganza, O.; Doberentz, E.; Grote, A.; Widman, G.; Drosten, C.; Eis-Hübinger, A.M.; Schoch, S.; Elger, C.E.; et al. Large-scale analysis of viral nucleic acid spectrum in temporal lobe epilepsy biopsies. Epilepsia 2015, 56, 234–243. [Google Scholar] [CrossRef]
- Kawamura, Y.; Sugata, K.; Ihira, M.; Mihara, T.; Mutoh, T.; Asano, Y.; Yoshikawa, T. Different characteristics of human herpesvirus 6 encephalitis between primary infection and viral reactivation. J. Clin. Virol. 2011, 51, 12–19. [Google Scholar] [CrossRef]
- Li, J.M.; Lei, D.; Peng, F.; Zeng, Y.J.; Li, L.; Xia, Z.L.; Xia, X.Q.; Zhou, D. Detection of human herpes virus 6B in patients with mesial temporal lobe epilepsy in West China and the possible association with elevated NF-κB expression. Epilepsy Res. 2011, 94, 1–9. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann. Neurol. 2007, 61, 288–299. [Google Scholar] [CrossRef]
- Leibovitch, E.C.; Jacobson, S. Evidence linking HHV-6 with Multiple Sclerosis: An Update Introduction: Pathogens in Multiple Sclerosis. Curr. Opin. Virol. 2014, 9, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Voumvourakis, K.I.; Kitsos, D.K.; Tsiodras, S.; Petrikkos, G.; Stamboulis, E. Human herpesvirus 6 infection as a trigger of multiple sclerosis. Mayo Clin. Proc. 2010, 85, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S.; Taylor, B.; Dwyer, D.E.; Taylor, J.; Blizzard, L.; Ponsonby, A.-L.; Pittas, F.; Dwyer, T.; van der Mei, I. Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult. Scler. J. 2012, 18, 799–806. [Google Scholar] [CrossRef]
- Ortega-Madueño, I.; Garcia-Montojo, M.; Dominguez-Mozo, M.I.; Garcia-Martinez, A.; Arias-Leal, A.M.; Casanova, I.; Arroyo, R.; Alvarez-Lafuente, R. Anti-human herpesvirus 6A/B IgG correlates with relapses and progression in multiple sclerosis. PLoS ONE 2014, 9, e104836. [Google Scholar] [CrossRef]
- Soldan, S.S.; Berti, R.; Salem, N.; Secchiero, P.; Flamand, L.; Calabresi, P.A.; Brennan, M.B.; Maloni, H.W.; Mcfarland, H.F.; Lin, H.C.; et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: Increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 1997, 3, 1394–1397. [Google Scholar] [CrossRef]
- Engdahl, E.; Gustafsson, R.; Huang, J.; Biström, M.; Lima Bomfim, I.; Stridh, P.; Khademi, M.; Brenner, N.; Butt, J.; Michel, A.; et al. Increased Serological Response Against Human Herpesvirus 6A Is Associated With Risk for Multiple Sclerosis. Front. Immunol. 2019, 10, 2715. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.R.M.; Bell, J. HHV-6 and multiple sclerosis. Nat. Med. 1998, 4, 537–538. [Google Scholar] [CrossRef]
- Reynaud, J.M.; Horvat, B. Human Herpesvirus 6 and Neuroinflammation. Artic. ID 2013, 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H.; Wekerle, H. The pathology of multiple sclerosis. In McAlpine’s Multiple Sclerosis: Fourth Edition; Elsevier Inc.: Philadelphia, PA, USA, 2005; pp. 557–599. ISBN 9780443072710. [Google Scholar]
- Challoner, P.B.; Smith, K.T.; Parker, J.D.; Macleod, D.L.; Coulter, S.N.; Rose, T.M.; Schultz, E.R.; Bennett, J.L.; Garber, R.L.; Chang, M.; et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 7440–7444. [Google Scholar] [CrossRef] [Green Version]
- Carrigan, D.R.; Harrington, D.; Knox, K.K. Subacute leukoencephalitis caused by CNS infection with human herpesvirus-6 manifesting as acute multiple sclerosis. Neurology 1996, 47, 145–148. [Google Scholar] [CrossRef]
- Chan, P.K.S.; Ng, H.-K.; Hui, M.; Cheng, A.F. Prevalence and distribution of human herpesvirus 6 variants A and B in adult human brain. J. Med. Virol. 2001, 64, 42–46. [Google Scholar] [CrossRef]
- Akhyani, N.; Berti, R.; Brennan, M.B.; Soldan, S.S.; Eaton, J.M.; McFarland, H.F.; Jacobson, S. Tissue Distribution and Variant Characterization of Human Herpesvirus (HHV)–6: Increased Prevalence of HHV-6A in Patients with Multiple Sclerosis. J. Infect. Dis. 2000, 182, 1321–1325. [Google Scholar] [CrossRef]
- Rotola, A.; Merlotti, I.; Caniatti, L.; Caselli, E.; Granieri, E.; Tola, M.R.; Di Luca, D.; Cassai, E. Human herpesvirus 6 infects the central nervous system of multiple sclerosis patients in the early stages of the disease. Mult. Scler. 2004, 10, 348–354. [Google Scholar] [CrossRef]
- Soldan, S.S.; Leist, T.P.; Juhng, K.N.; McFarland, H.F.; Jacobson, S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann. Neurol. 2000, 47, 306–313. [Google Scholar] [CrossRef]
- Ahlqvist, J.; Fotheringham, J.; Akhyani, N.; Yao, K.; Fogdell-Hahn, A.; Jacobson, S. Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J. Neurovirol. 2005, 11, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M.; Cuomo, L.; Zompetta, C.; Ruggieri, S.; Frati, L.; Faggioni, A.; Ragona, G. Human herpesvirus 6 and multiple sclerosis: A study of T cell cross-reactivity to viral and myelin basic protein antigens. J. Med. Virol. 2002, 68, 268–272. [Google Scholar] [CrossRef]
- Tejada-Simon, M.V.; Zang, Y.C.Q.; Hong, J.; Rivera, V.M.; Zhang, J.Z. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann. Neurol. 2003, 53, 189–197. [Google Scholar] [CrossRef]
- Cheng, W.; Ma, Y.; Gong, F.; Hu, C.; Qian, L.; Huang, Q.; Yu, Q.; Zhang, J.; Chen, S.; Liu, Z.; et al. Cross-reactivity of autoreactive T cells with MBP and viral antigens in patients with MS. Front. Biosci. 2012, 17, 1648–1658. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.; Hogestyn, J.M.; Folts, C.J.; Lopez, B.; Pröschel, C.; Mock, D.; Mayer-Pröschel, M. Expression of the Human Herpesvirus 6A Latency-Associated Transcript U94A Disrupts Human Oligodendrocyte Progenitor Migration. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Falcão, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; Ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar] [CrossRef]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Wai Lee, K.; Knuesel, I.; Malhotra, D.; ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2012. [Google Scholar] [CrossRef]
- Lambert, J.-C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Llerena, C.; Phillips, A.; Reitboeck, P.G.; Hardy, J.; Pocock, J.M. Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr. Opin. Neurobiol. 2016, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjoneska, E.; Pfenning, A.R.; Mathys, H.; Quon, G.; Kundaje, A.; Tsai, L.-H.; Kellis, M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 2015, 518, 365–369. [Google Scholar] [CrossRef]
- Ball, M.J. Limbic Predilection in Alzheimer Dementia: Is Reactivated Herpesvirus Involved? Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 1982, 9, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Itzhaki, R.F. Corroboration of a Major Role for Herpes Simplex Virus Type 1 in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, G.A.; Maitland, N.J.; Wilcock, G.K.; Craske, J.; Itzhaki, R.F. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J. Med. Virol. 1991, 33, 224–227. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Lin, W.R.; Shang, D.; Wilcock, G.K.; Faragher, B.; Jamieson, G.A. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet 1997, 349, 241–244. [Google Scholar] [CrossRef]
- Hemling, N.; Röyttä, M.; Rinne, J.; Pöllänen, P.; Broberg, E.; Tapio, V.; Vahlberg, T.; Hukkanen, V. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann. Neurol. 2003, 54, 267–271. [Google Scholar] [CrossRef]
- Wozniak, M.; Mee, A.; Itzhaki, R. Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J. Pathol. 2009, 217, 131–138. [Google Scholar] [CrossRef]
- Fotheringham, J.; Akhyani, N.; Vortmeyer, A.; Donati, D.; Williams, E.; Oh, U.; Bishop, M.; Barrett, J.; Gea-Banacloche, J.; Jacobson, S. Detection of Active Human Herpesvirus–6 Infection in the Brain: Correlation with Polymerase Chain Reaction Detection in Cerebrospinal Fluid. J. Infect. Dis. 2007, 195, 450–454. [Google Scholar] [CrossRef]
- Lin, W.-R.; Wozniak, M.A.; Cooper, R.J.; Wilcock, G.K.; Itzhaki, R.F. Herpesviruses in brain and Alzheimer’s disease. J. Pathol. 2002, 197, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; D’Accolti, M.; Soffritti, I.; Zatelli, M.C.; Rossi, R.; Degli Uberti, E.; Di Luca, D. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol. J. 2017, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gatto, G.; Rossi, A.; Rossi, D.; Kroening, S.; Bonatti, S.; Mallardo, M. Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-iB pathway. Nucleic Acids Res. 2008, 36, 6608–6619. [Google Scholar] [CrossRef]
- Gottwein, E.; Mukherjee, N.; Sachse, C.; Frenzel, C.; Majoros, W.H.; Chi, J.T.A.; Braich, R.; Manoharan, M.; Soutschek, J.; Ohler, U.; et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature 2007, 450, 1096–1099. [Google Scholar] [CrossRef]
- Rampelli, S.; Soverini, M.; Turroni, S.; Quercia, S.; Biagi, E.; Brigidi, P.; Candela, M. ViromeScan: A new tool for metagenomic viral community profiling. BMC Genomics 2016, 17, 165. [Google Scholar] [CrossRef] [Green Version]
- Brister, J.R.; Ako-Adjei, D.; Bao, Y.; Blinkova, O. NCBI Viral Genomes Resource. Nucleic Acids Res. 2014, 43, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, R.; Soffritti, I.; D’Accolti, M.; Bortolotti, D.; Di Luca, D.; Caselli, E. HHV-6A/6B Infection of NK Cells Modulates the Expression of miRNAs and Transcription Factors Potentially Associated to Impaired NK Activity. Front. Microbiol. 2017, 8, 2143. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.A.; Pedamallu, C.S.; Ojesina, A.I.; Bullman, S.; Sharpe, T.; Whelan, C.W.; Meyerson, M. GATK PathSeq: A customizable computational tool for the discovery and identification of microbial sequences in libraries from eukaryotic hosts. Bioinformatics 2018, 34, 4287–4289. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, M.A.; Itzhaki, R.F.; Shipley, S.J.; Dobson, C.B. Herpes simplex virus infection causes cellular β-amyloid accumulation and secretase upregulation. Neurosci. Lett. 2007, 429, 95–100. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Frost, A.L.; Itzhaki, R.F. Alzheimer’s disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J. Alzheimer’s Dis. 2009, 16, 341–350. [Google Scholar] [CrossRef]
- Zambrano, Á.; Solis, L.; Salvadores, N.; Cortés, M.; Lerchundi, R.; Otth, C. Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1. J. Alzheimer’s Dis. 2008, 14, 259–269. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Frost, A.L.; Preston, C.M.; Itzhaki, R.F. Antivirals reduce the formation of key Alzheimer’s disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS ONE 2011, 6, e25152. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Piacentini, R.; Li Puma, D.D.; Ripoli, C.; Marcocci, M.E.; De Chiara, G.; Garaci, E.; Teresa Palamara, A.; Grassi, C. Herpes Simplex Virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-β protein accumulation. Sci. Rep. 2015, 5, 15444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristen, H.; Sastre, I.; Muñoz-Galdeano, T.; Recuero, M.; Aldudo, J.; Bullido, M.J. The lysosome system is severely impaired in a cellular model of neurodegeneration induced by HSV-1 and oxidative stress. Neurobiol. Aging 2018, 68, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Itzhaki, R.F.; Golde, T.E.; Heneka, M.T.; Readhead, B. Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 2020. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Cheng, W.-L.; Sheu, J.-J.; Huang, C.-C.; Shia, B.-C.; Kao, L.-T.; Lin, H.-C. Increased risk of dementia following herpes zoster ophthalmicus. PLoS ONE 2017, 12, e0188490. [Google Scholar] [CrossRef] [Green Version]
- Ounanian, A.; Seigneurin, J.-M.; Guilbert, B.; Avrameas, S.; Renverez, J.-C. Antibodies to viral antigens, xenoantigens, and autoantigens in alzheimer’s disease. J. Clin. Lab. Anal. 1990, 4, 367–375. [Google Scholar] [CrossRef]



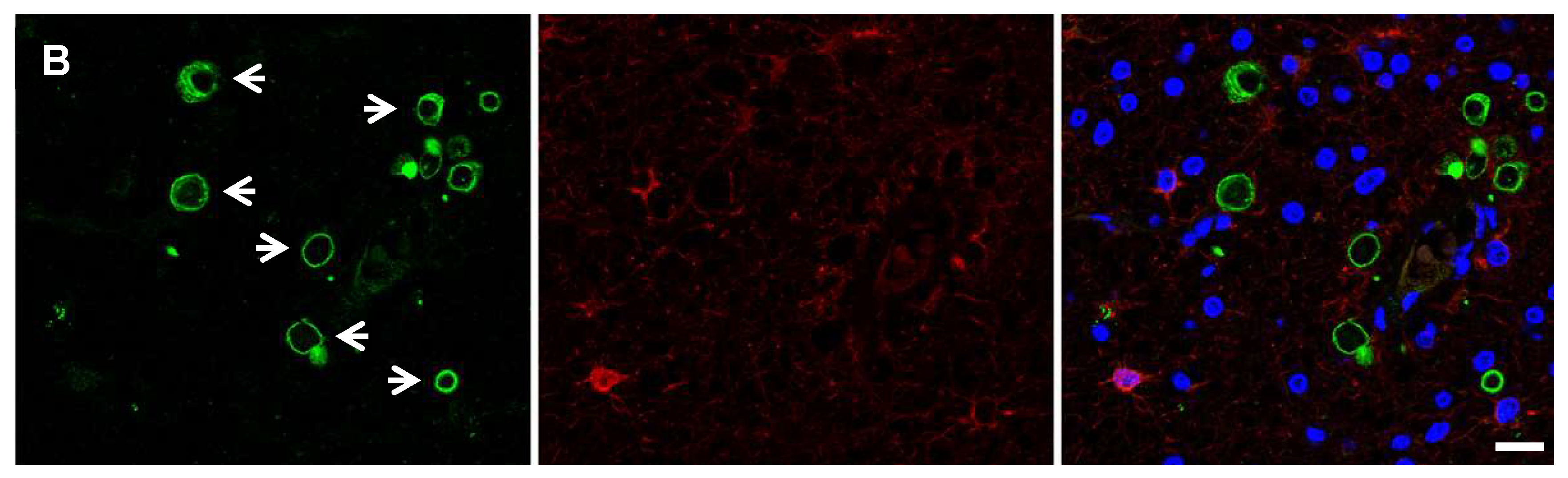
| Protein Target | Stage | Localization | Antibody | Type | Species | Source | Ref | Cell Type |
|---|---|---|---|---|---|---|---|---|
| p101 | Late | Tegument | C3108-103 | Mouse monoclonal | B | Dr. Philip Pellet (Pellet et al. 1993) | Drobyski et al. 1994 Knox et al. 1995 Challoner et al. 1995 Saito et al. 1995 Friedman et al. 1999 | Astrocytes Neurons Oligodendrocytes Macrophague Microglia |
| Chemicon | Challoner et al. 1995 Friedman et al. 1999 Mock et al. 1999 Ito et al. 2000 Blumberg et al. 2000 Wainwright et al. 2001 Goodman et al. 2003 | Astrocytes Neurons Oligodendrocytes Microglia Endothelial Lymphocytes | ||||||
| USBiological Cat# H2034-17A | Opsahl et al. 2005 | Oligodendrocytes | ||||||
| Abcam Cat# ab64536 | Li et al. 2011 | Astrocytes Microglia | ||||||
| Abcam Cat# ab128404 | Liu et al. 2018 | Astrocytes Neurons | ||||||
| Unk | Unk | Biometria | Novoa et al. 1997 | Astrocytes Oligodendrocytes Lymphocytes | ||||
| Unk | Unk | Virotech | Wang et al. 1999 | Microglia Macrophages Endothelial Lymphocytes | ||||
| Unk | Unk | Unk | #30-HSB | Rabbit antiserum | Unk | Dr. Donald Carrigan (Russler et al. 1991) | Drobyski et al. 1994 Knox et al. 1995 Mckenzie et al. 1995 Carrigan et al. 1996 | Astrocytes Neurons Oligodendrocytes Microglia |
| p41 | Early | Nuclear | C5 | Mouse monoclonal | A and B | Biodesign (Agulnick et al. 1993) | Challoner et al. 1995 | Astrocytes Neurons Oligodendrocytes Macrophague |
| Unk | Unk | Unk | Virotech | Mock et al. 1999 Ito et al. 2000 Blumberg et al. 2000 Wainwright et al. 2001 Goodman et al. 2003 | Neurons Oligodendrocytes Microglia Endothelial Lymphocytes | |||
| 9A5D12 | Mouse monoclonal | A and B | Dr. Bala Chandran (Balachandran et al. 1989) | Saito et al. 1995 Wagner et al. 1997 | N/A | |||
| Unk | Mouse monoclonal | Unk | Autogen-Bioclear | Opsahl et al. 2005 | Oligodendrocytes | |||
| Unk | Unk | Unk | Unk | Cuomo et al. 2001 | Glial cells (Schwann) | |||
| gp116/gp64/gp54 | Late | Core | 6A5D5 | Mouse monoclonal | A and B | Dr. Bala Chandran | Saito et al. 1995 | N/A |
| 6A6G3 | Mouse monoclonal | A and B | Advanced Biotechnologies Cat# 13-219-001 or 13-218-100 (Balachandran et al. 1989) | Goodman et al. 2003 Donati et al. 2003 Fotheringham et al. 2007a Fotheringham et al. 2007b Niehusmann et al. 2010 Esposito et al. 2015 | Astrocytes Neurons Oligodendrocytes Macrophages Lymphocytes | |||
| HHV-6 Foundation | Huang et al. 2015 | Neurons | ||||||
| Unk | Mouse monoclonal | A and B | Autogen-Bioclear | Opsahl et al. 2005 | Oligodendrocytes | |||
| gp82/gp105 | Late | Envelope | UK82 | Rabbit antiserum | Unk | Dr. Bala Chandran | Saito et al. 1995 | N/A |
| 2D6 | Mouse monoclonal | A | Dr. Bala Chandran (Balachandran et al. 1989) | Knox et al. 2000 | N/A | |||
| gp110/60 | Late | Envelope | H-AR3 | Mouse monoclonal | Unk | Dr. Luca | Wagner et al. 1997 | Astrocytes Neurons Oligodendrocytes |
| Unk | Unk | Unk | Unk | Le Guennec et al. 2017 | Glial cells | |||
| gH | Late | Envelope | OHV-3 | Mouse monoclonal | B | Advanced Biotechnologies (Okuno et al. 1990) | Knox et al. 2000 | N/A |
| HHV-6 Foundation | Huang et al. 2015 | Neurons | ||||||
| 37KDa early antigen | Early | Unk | 1.B.367 | Mouse monoclonal | A | USBiologicalCat# H2034-01 | Opsahl et al. 2005 | Oligodendrocytes |
| U94 | Latency | Nuclear | MORI | Mouse monoclonal | B | HHV-6 Foundation | Huang et al. 2015 | Neurons |
| Unk | Unk | Unk | sc-65463 | Mouse monoclonal | A and B | Santa Cruz | Chapenko et al. 2016 Skuja et al. 2017 | Astrocytes Oligodendrocytes Microglia Endothelial Lymphocytes Fibroblasts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santpere, G.; Telford, M.; Andrés-Benito, P.; Navarro, A.; Ferrer, I. The Presence of Human Herpesvirus 6 in the Brain in Health and Disease. Biomolecules 2020, 10, 1520. https://doi.org/10.3390/biom10111520
Santpere G, Telford M, Andrés-Benito P, Navarro A, Ferrer I. The Presence of Human Herpesvirus 6 in the Brain in Health and Disease. Biomolecules. 2020; 10(11):1520. https://doi.org/10.3390/biom10111520
Chicago/Turabian StyleSantpere, Gabriel, Marco Telford, Pol Andrés-Benito, Arcadi Navarro, and Isidre Ferrer. 2020. "The Presence of Human Herpesvirus 6 in the Brain in Health and Disease" Biomolecules 10, no. 11: 1520. https://doi.org/10.3390/biom10111520








