Construction and Loss of Bacterial Flagellar Filaments
Abstract
1. Introduction
2. Bacterial Flagellin Transportation
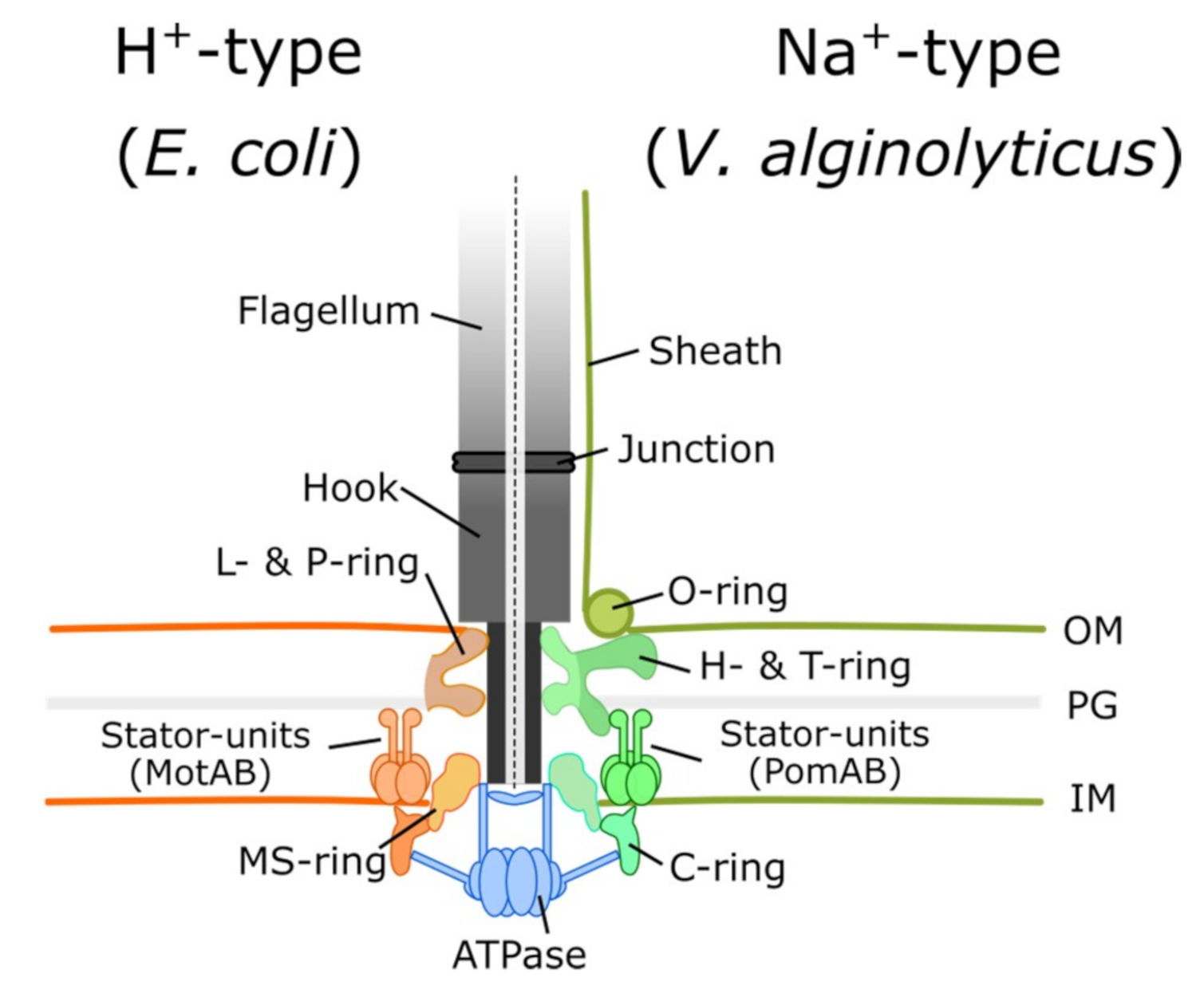
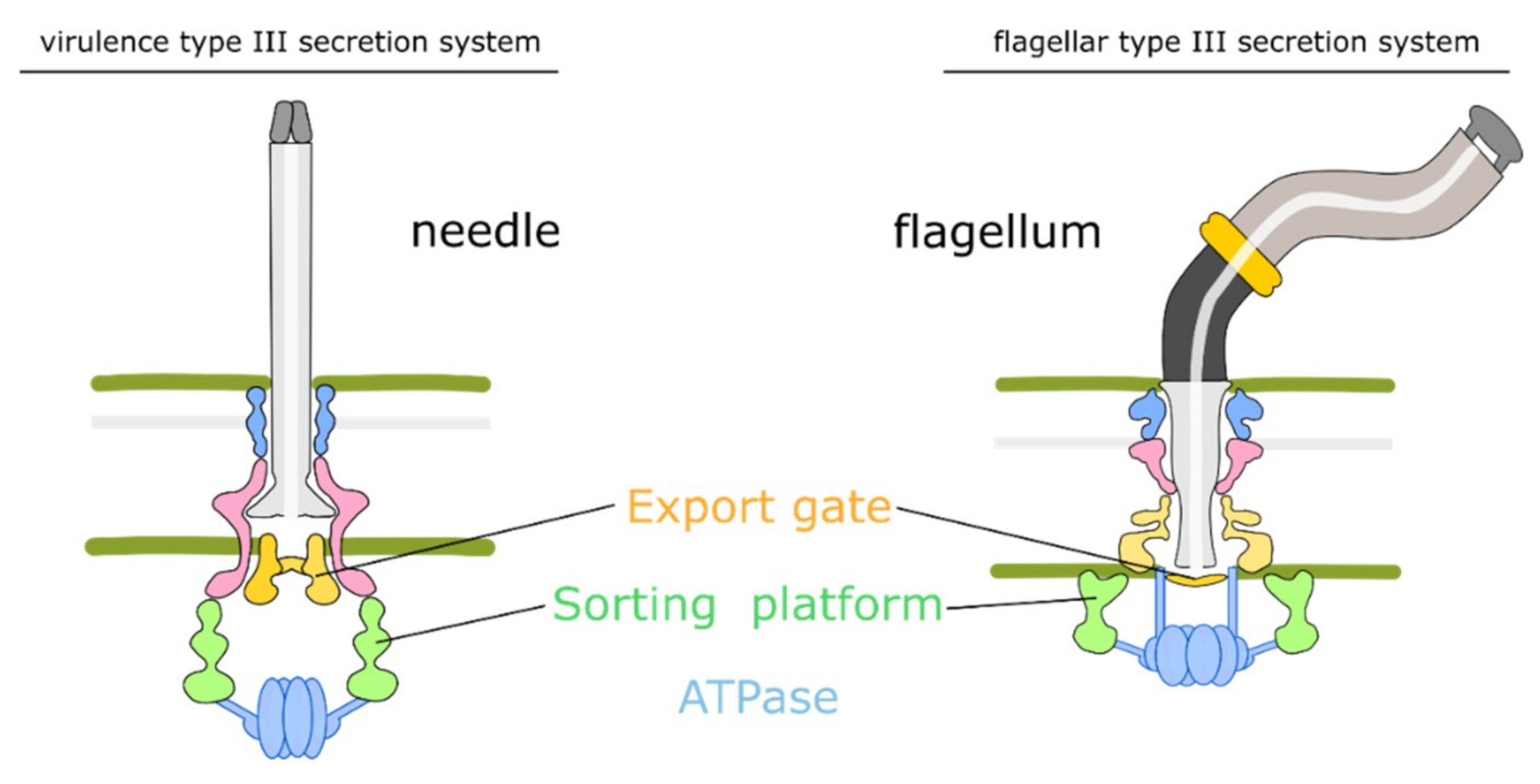
2.1. Architecture of the Type-III Secretion System
2.2. Strcture of the Flagellin
2.3. Substrates Accumulation and Delivery
3. Flagellar Filament Construction
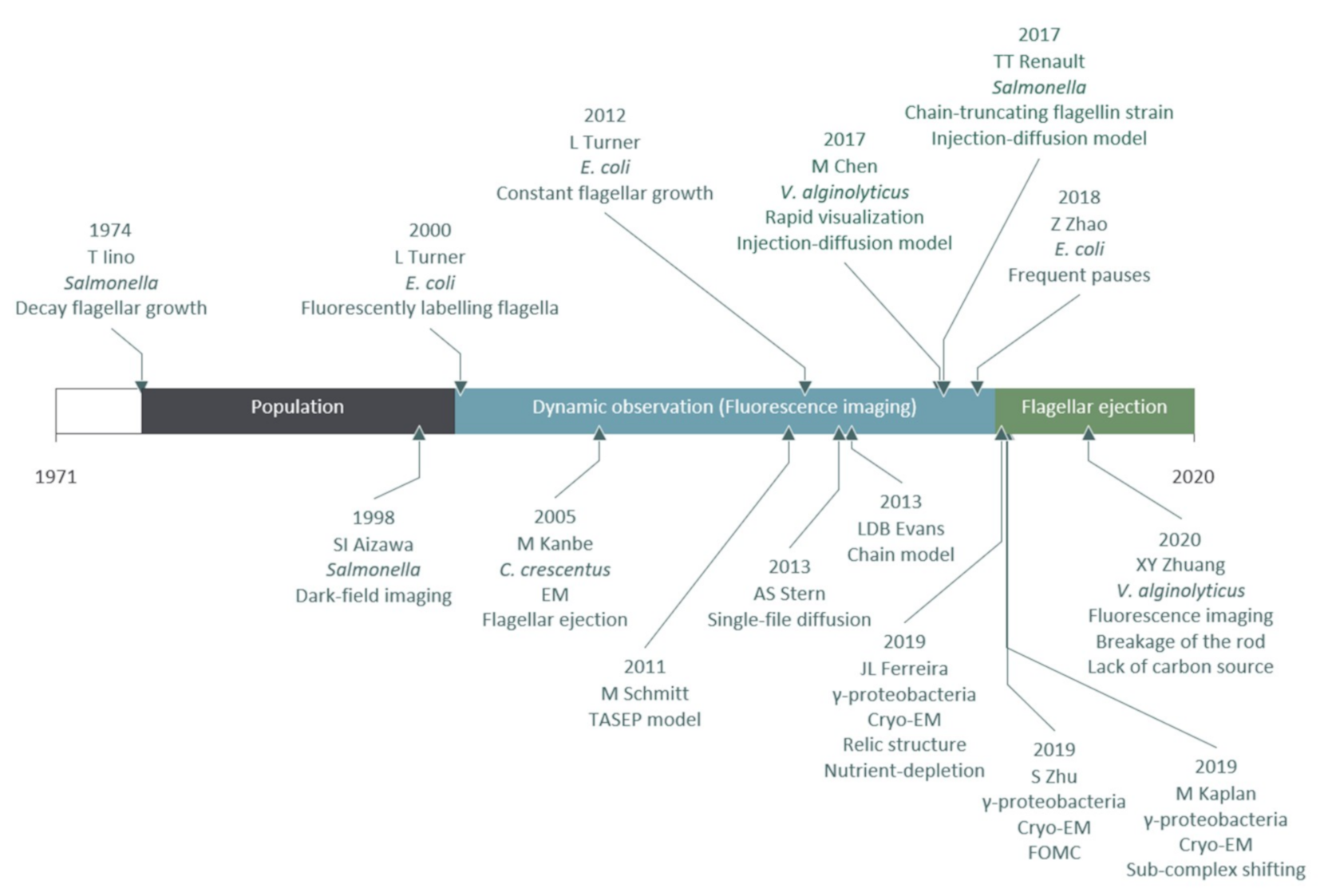
3.1. Milestones of Flagellar Filament Growth Measurements
3.2. Models for Flagellin Transport and Filament Growth
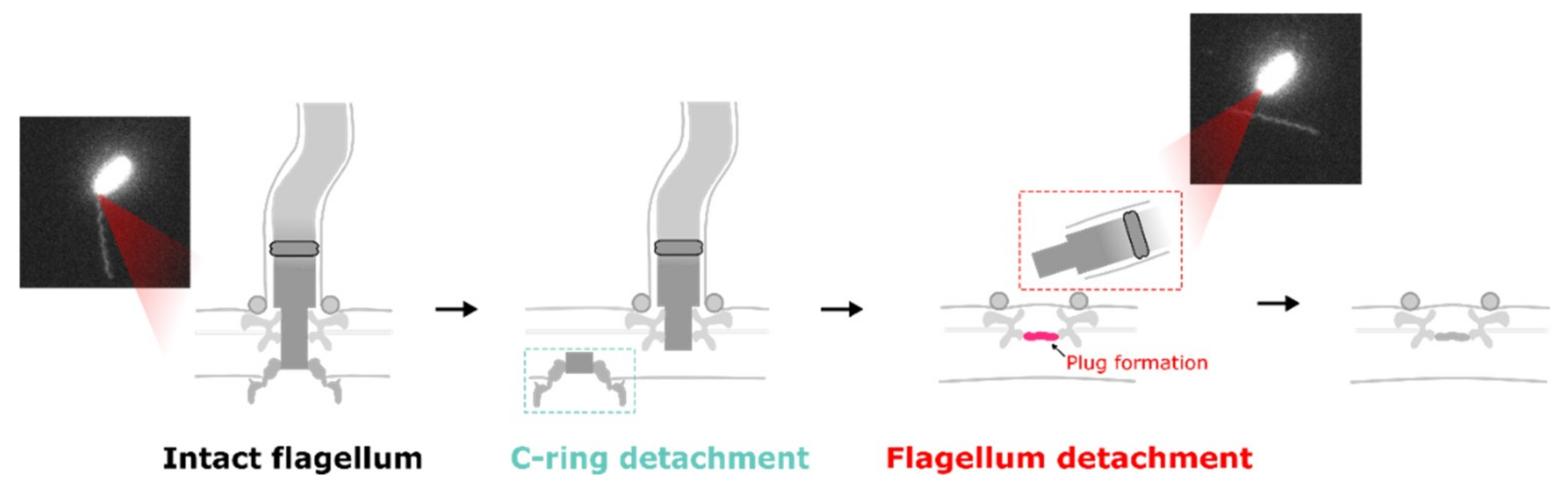
4. Loss of Flagella
5. Concluding Remarks and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, H.C.; Anderson, R.A. Bacteria swim by rotating their flagellar filaments. Nature 1973, 245, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Macnab, R.M. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003, 57, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Ryu, W.S.; Berg, H.C. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 2000, 182, 2793. [Google Scholar] [CrossRef] [PubMed]
- Seishi, K.; Imai, N.; Nishitoba, M.; Sugiyama, S.; Magariyama, Y. Asymmetric swimming pattern of Vibrio alginolyticus cells with single polar flagella. FEMS Microbiol. Lett. 2005, 242, 221–225. [Google Scholar]
- Taute, K.M.; Gude, S.; Tans, S.J.; Shimizu, T.S. High-throughput 3D tracking of bacteria on a standard phase contrast microscope. Nat. Commun. 2015, 6, 8776. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.D.; Tedesco, P.; Berg, H.C.; Harold, F.M.; Van Der Drift, C. A protonmotive force drives bacterial flagella. Proc. Natl. Acad. Sci. USA 1977, 74, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Kitada, M.; Imae, Y. Flagellar motors of alkalophilic bacillus are powered by an electrochemical potential gradient of Na+. FEBS Lett. 1981, 132, 278–280. [Google Scholar] [CrossRef]
- Hennell-James, R.; Deme, J.; Alcock, F.; Silale, A.; Lauber, F.; Berks, B.C.; Lea, S.M.; Kjaer, A. Structure of a proton-powered molecular motor that drives protein transport and gliding motility. bioRxiv 2020. [Google Scholar] [CrossRef]
- Deme, J.C.; Johnson, S.; Vickery, O.; Muellbauer, A.; Monkhouse, H.; Griffiths, T.; James, R.H.; Berks, B.C.; Coulton, J.W.; Stansfeld, P.J.; et al. Structures of the stator complex that drives rotation of the bacterial flagellum. Nat. Microbiol. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santiveri, M.; Roa-Eguiara, A.; Kühne, C.; Wadhwa, N.; Berg, H.C.; Erhardt, M.; Taylor, N.M.I. Structure and function of stator units of the bacterial flagellar motor. Cell 2020, 183, 244–257. [Google Scholar] [CrossRef]
- Furuno, M.; Atsumi, T.; Yamada, T.; Kojima, S.; Nishioka, N.; Kawagishi, I.; Homma, M. Characterization of polar-flagellar-length mutants in Vibrio alginolyticus. Microbiology 1997, 143, 1615–1621. [Google Scholar] [CrossRef]
- Xue, R.; Ma, Q.; Baker, M.A.; Bai, F. A delicate nanoscale motor made by nature—The bacterial flagellar motor. Adv. Sci. 2015, 2, 1500129. [Google Scholar] [CrossRef]
- Gabel, C.V.; Berg, H.C. The speed of the flagellar rotary motor of Escherichia coli varies linearly with protonmotive force. Proc. Natl. Acad. Sci. USA 2003, 100, 8748–8751. [Google Scholar] [CrossRef]
- Chevance, F.F.; Hughes, K.T. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 2008, 6, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.E.; Leadbetter, J.R.; Jensen, G.J. In situ structure of the complete Treponema primitia flagellar motor. Nature 2006, 442, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Francis, N.R.; Xu, C.; DeRosier, D.J. The three-dimensional structure of the flagellar rotor from a clockwise-locked mutant of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2006, 188, 7039–7048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lloyd, S.A.; Blair, D.F. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA 1998, 95, 6436–6441. [Google Scholar] [CrossRef]
- Blair, D.F. Flagellar movement driven by proton translocation. FEBS Lett. 2003, 544, 86–95. [Google Scholar] [CrossRef]
- Takekawa, N.; Kojima, S.; Homma, M. Contribution of many charged residues at the stator-rotor interface of the Na+-driven flagellar motor to torque generation in Vibrio alginolyticus. J. Bacteriol. 2014, 196, 1377–1385. [Google Scholar] [CrossRef]
- Lo, C.J.; Sowa, Y.; Pilizota, T.; Berry, R.M. Mechanism and kinetics of a sodium-driven bacterial flagellar motor. Proc. Natl. Acad. Sci. USA 2013, 110, E2544–E2551. [Google Scholar] [CrossRef]
- Yonekura, K.; Maki-Yonekura, S.; Namba, K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 2003, 424, 643–650. [Google Scholar] [CrossRef]
- Minamino, T.; Imada, K.; Namba, K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol. Biosyst. 2008, 4, 1105–1115. [Google Scholar] [CrossRef]
- Minamino, T.; Namba, K. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature 2008, 451, 485–488. [Google Scholar] [CrossRef]
- Paul, K.; Erhardt, M.; Hirano, T.; Blair, D.F.; Hughes, K.T. Energy source of flagellar type III secretion. Nature 2008, 451, 489–492. [Google Scholar] [CrossRef]
- Lee, P.C.; Rietsch, A. Fueling type III secretion. Trends Microbiol. 2015, 23, 296–300. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Gao, F.Z.; Rossmann, F.M.; Nans, A.; Brenzinger, S.; Hosseini, R.; Wilson, A.; Briegel, A.; Thormann, K.M.; Rosenthal, P.B.; et al. γ-proteobacteria eject their polar flagella under nutrient depletion, retaining flagellar motor relic structures. PLoS Biol. 2019, 17, e3000165. [Google Scholar] [CrossRef]
- Kaplan, M.; Subramanian, P.; Ghosal, D.; Oikonomou, C.M.; Pirbadian, S.; Starwalt-Lee, R.; Mageswaran, S.K.; Ortega, D.R.; Gralnick, J.A.; El-Naggar, M.Y.; et al. In situ imaging of the bacterial flagellar motor disassembly and assembly processes. EMBO J. 2019, 38, e100957. [Google Scholar] [CrossRef]
- Zhu, S.; Schniederberend, M.; Zhitnitsky, D.; Jain, R.; Galán, J.E.; Kazmierczak, B.I.; Liu, J. In situ structures of polar and lateral flagella revealed by cryo-electron tomography. J. Bacteriol. 2019, 201, e00117–e00119. [Google Scholar] [CrossRef]
- Zhuang, X.; Guo, S.; Li, Z.; Zhao, Z.; Kojima, S.; Homma, M.; Wang, P.; Lo, C.; Bai, F. Live-cell fluorescence imaging reveals dynamic production and loss of bacterial flagella. Mol. Microbiol. 2020, 114, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kanbe, M.; Shibata, S.; Umino, Y.; Jenal, U.; Aizawa, S.I. Protease susceptibility of the Caulobacter crescentus flagellar hook-basal body: A possible mechanism of flagellar ejection during cell differentiation. Microbiology 2005, 151, 433–438. [Google Scholar] [CrossRef][Green Version]
- Johnson, S.; Fong, Y.H.; Deme, J.; Furlong, E.; Kuhlen, L.; Lea, S.M. Structure of the bacterial flagellar rotor MS-ring: A minimum inventory/maximum diversity system. bioRxiv 2019, 718072. [Google Scholar] [CrossRef]
- Fabiani, F.D.; Renault, T.T.; Peters, B.; Dietsche, T.; Gálvez, E.J.C.; Guse, A.; Freier, K.; Charpentier, E.; Strowig, T.; Franz-Wachtel, M.; et al. A flagellum-specific chaperone facilitates assembly of the core type III export apparatus of the bacterial flagellum. PLoS Biol. 2017, 15, e2002267. [Google Scholar] [CrossRef]
- Fukumura, T.; Makino, F.; Dietsche, T.; Kinoshita, M.; Kato, T.; Wagner, S.; Namba, K.; Imada, K.; Minamino, T. Assembly and stoichiometry of the core structure of the bacterial flagellar type III export gate complex. PLoS Biol. 2017, 15, e2002281. [Google Scholar] [CrossRef]
- Kuhlen, L.; Abrusci, P.; Johnson, S.; Gault, J.; Deme, J.; Caesar, J.; Dietsche, T.; Mebrhatu, M.T.; Ganief, T.; Macek, B.; et al. Structure of the core of the type III secretion system export apparatus. Nat. Struct. Mol. Biol. 2018, 25, 583–590. [Google Scholar] [CrossRef]
- Suzuki, H.; Yonekura, K.; Namba, K. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J. Mol. Biol. 2004, 337, 105–113. [Google Scholar] [CrossRef]
- Lele, P.P.; Branch, R.W.; Nathan, V.S.; Berg, H.C. Mechanism for adaptive remodeling of the bacterial flagellar switch. Proc. Natl. Acad. Sci. USA 2012, 109, 20018–20022. [Google Scholar] [CrossRef]
- Zhao, R.; Pathak, N.; Jaffe, H.; Reese, T.S.; Khan, S. FliN is a major structural protein of the C-ring in the Salmonella typhimurium flagellar basal body. J. Mol. Biol. 1996, 261, 195–208. [Google Scholar] [CrossRef]
- Macnab, R.M. Type III flagellar protein export and flagellar assembly. Biochim. Biophys. Acta 2004, 1694, 207–217. [Google Scholar] [CrossRef]
- Cornelis, G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006, 4, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Dibrov, P.A.; Glagolev, A.N. ΔμH+ is required for flagellar growth in Escherichia coli. FEBS Lett. 1982, 143, 319–322. [Google Scholar] [CrossRef]
- Wilharm, G.; Lehmann, V.; Neumayer, W.; Trcek, J.; Heesemann, J. Yersinia enterocolitica type III secretion: Evidence for the ability to transport proteins that are folded prior to secretion. BMC Microbiol. 2004, 4, 27. [Google Scholar]
- Lee, P.C.; Stopford, C.M.; Svenson, A.G.; Rietsch, A. Control of effector export by the Pseudomonas aeruginosa type III secretion proteins PcrG and PcrV. Mol. Microbiol. 2010, 75, 924–941. [Google Scholar]
- Ogawa, R.; Abe-Yoshizumi, R.; Kishi, T.; Homma, M.; Kojima, S. Interaction of the C-terminal tail of FliF with FliG from the Na+-driven flagellar motor of Vibrio alginolyticus. J. Bacteriol. 2015, 197, 63. [Google Scholar]
- Lynch, M.J.; Levenson, R.; Kim, E.A.; Sircar, R.; Blair, D.F.; Dahlquist, F.W.; Crane, B.R. Co-folding of a FliF-FliG split domain forms the basis of the MS:C ring interface within the bacterial flagellar motor. Structure 2017, 25, 317–328. [Google Scholar] [PubMed]
- Erhardt, M.; Namba, K.; Hughes, K.T. Bacterial nanomachines: The flagellum and type III injectisome. Cold Spring Harb. Perspect. Biol. 2010, 2, a000299. [Google Scholar]
- Galan, J.E.; Lara-Tejero, M.; Marlovits, T.C.; Wagner, S. Bacterial type III secretion systems: Specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 2014, 68, 415–438. [Google Scholar]
- Miletic, S.; Goessweiner-Mohr, N.; Marlovits, T.C. The structure of the type III secretion system needle complex. In Bacterial Type III Protein Secretion Systems; Wagner, S., Galan, J.E., Eds.; Springer: Cham, Switzerland, 2020; pp. 67–90. [Google Scholar]
- Minamino, T.; Kawamoto, A.; Kinoshita, M.; Namba, K. Molecular organization and assembly of the export apparatus of flagellar type III secretion systems. In Bacterial Type III Protein Secretion Systems; Wagner, S., Galan, J.E., Eds.; Springer: Cham, Switzerland, 2020; pp. 91–107. [Google Scholar]
- Marlovits, T.C.; Kubori, T.; Sukhan, A.; Thomas, D.R.; Galán, J.E.; Unger, V.M. Structural insights into the assembly of the type III secretion needle complex. Science 2004, 306, 1040–1042. [Google Scholar]
- Marlovits, T.C.; Kubori, T.; Lara-Tejero, M.; Thomas, D.; Unger, V.M.; Galán, J.E. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 2006, 441, 637–640. [Google Scholar]
- Hu, J.; Worrall, L.J.; Hong, C.; Vuckovic, M.; Atkinson, C.E.; Caveney, N.; Yu, Z.; Strynadka, N.C.J. Cryo-EM analysis of the T3S injectisome reveals the structure of the needle and open secretin. Nat. Commun. 2018, 9, 3840. [Google Scholar]
- Torres-Vargas, C.E.; Kronenberger, T.; Roos, N.; Dietsche, T.; Poso, A.; Wagner, S. The inner rod of virulence-associated type III secretion systems constitutes a needle adapter of one helical turn that is deeply integrated into the system’s export apparatus. Mol. Microbiol. 2019, 112, 918–931. [Google Scholar]
- Kubori, T.; Sukhan, A.; Aizawa, S.I.; Galán, J.E. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl. Acad. Sci. USA 2000, 97, 10225–10230. [Google Scholar] [CrossRef]
- Lara-Tejero, M.; Kato, J.; Wagner, S.; Liu, X.; Galán, J.E. A sorting platform determines the order of protein secretion in bacterial type III systems. Science 2011, 331, 1188–1191. [Google Scholar] [CrossRef]
- Francis, N.R.; Sosinsky, G.E.; Thomas, D.; DeRosier, D.J. Isolation, characterization and structure of bacterial flagellar motors containing the switch complex. J. Mol. Biol. 1994, 235, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, R.; Asakura, S.; Wakabayashi, K.; Namba, K. Transition of bacterial flagella from helical to straight forms with different subunit arrangements. J. Mol. Biol. 1979, 131, 725–742. [Google Scholar] [CrossRef]
- Yamashita, l.; Hasegawa, K.; Suzuki, H.; Vonderviszt, F.; Mimori-Kiyosue, Y.; Namba, K. Structure and switching of bacterial flagellar filaments studied by X-ray fiber diffraction. Nat. Struct. Biol. 1998, 5, 125–132. [Google Scholar]
- Renault, T.T.; Guse, A.; Erhardt, M. Export mechanisms and energy transduction in type-III secretion machines. In Bacterial Type III Protein Secretion Systems; Wagner, S., Galan, J.E., Eds.; Springer: Cham, Switzerland, 2020; pp. 143–159. [Google Scholar]
- Minamino, T.; Macnab, R.M. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 1999, 181, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; MacNab, R.M. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol. Microbiol. 2000, 37, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, T.; Imada, K.; Minamino, T.; Kato, T.; Miyata, T.; Namba, K. Common architecture of the flagellar type III protein export apparatus and F- and V-type ATPases. Nat. Struct. Mol. Biol. 2011, 18, 277–282. [Google Scholar] [CrossRef]
- Bai, F.; Morimoto, Y.V.; Yoshimura, S.D.; Hara, N.; Kami-Ike, N.; Namba, K.; Minamino, T. Assembly dynamics and the roles of FliI ATPase of the bacterial flagellar export apparatus. Sci. Rep. 2014, 4, 6528. [Google Scholar] [CrossRef]
- González-Pedrajo, B.; Minamino, T.; Kihara, M.; Namba, K. Interactions between C ring proteins and export apparatus components: A possible mechanism for facilitating type III protein export. Mol. Microbiol. 2006, 60, 984–998. [Google Scholar] [CrossRef]
- Erhardt, M.; Mertens, M.E.; Fabiani, F.D.; Hughes, K.T. ATPase-independent type-III protein secretion in Salmonella enterica. PLoS Genet. 2014, 10, e1004800. [Google Scholar] [CrossRef]
- Lee, P.C.; Zmina, S.E.; Stopford, C.M.; Toska, J.; Rietsch, A. Control of type III secretion activity and substrate specificity by the cytoplasmic regulator PcrG. Proc. Natl. Acad. Sci. USA 2014, 111, E2027–E2036. [Google Scholar] [CrossRef]
- Blair, D.F.; Berg, H.C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell 1990, 60, 439–449. [Google Scholar] [CrossRef]
- Minamino, T.; Morimoto, Y.V.; Hara, N.; Namba, K. An energy transduction mechanism used in bacterial flagellar type III protein export. Nat. Commun. 2011, 2, 475. [Google Scholar] [CrossRef]
- Bange, G.; Kümmerer, N.; Engel, C.; Bozkurt, G.; Wild, K.; Sinning, I. FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system. Proc. Natl. Acad. Sci. USA 2010, 107, 11295. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hara, N.; Imada, K.; Namba, K.; Minamino, T. Interactions of bacterial flagellar chaperone–substrate complexes with FlhA contribute to co-ordinating assembly of the flagellar filament. Mol. Microbiol. 2013, 90, 1249–1261. [Google Scholar] [CrossRef]
- Abrusci, P.; Vergara-Irigaray, M.; Johnson, S.; Beeby, M.D.; Hendrixson, D.R.; Roversi, P.; Friede, M.E.; Deane, J.E.; Jensen, G.J.; Tang, C.M.; et al. Architecture of the major component of the type III secretion system export apparatus. Nat. Struct. Mol. Biol. 2013, 20, 99–104. [Google Scholar] [CrossRef]
- Iino, T. Assembly of Salmonella flagellin in vitro and in vivo. J. Supramol. Struct. 1974, 2, 372–384. [Google Scholar]
- Aizawa, S.I.; Kubori, T. Bacterial flagellation and cell division. Genes Cells 1998, 3, 625–634. [Google Scholar] [CrossRef]
- Turner, L.; Stern, A.S.; Berg, H.C. Growth of flagellar filaments of Escherichia coli is independent of filament length. J. Bacteriol. 2012, 194, 2437. [Google Scholar] [CrossRef]
- Renault, T.T.; Abraham, A.O.; Bergmiller, T.; Paradis, G.; Rainville, S.; Charpentier, E.; Guet, C.C.; Tu, Y.; Namba, K.; Keener, J.P.; et al. Bacterial flagella grow through an injection-diffusion mechanism. eLife 2017, 6, e23136. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Z.; Yang, J.; Peng, K.; Baker, M.A.B.; Bai, F.; Lo, C.J. Length-dependent flagellar growth of Vibrio alginolyticus revealed by real time fluorescent imaging. eLife 2017, 6, e22140. [Google Scholar] [CrossRef] [PubMed]
- Glauert, A.M.; Kerridge, D.; Horne, R.W. The fine structure and mode of attachment of the sheathed flagellum of Vibrio metchnikovii. J. Cell. Biol. 1963, 18, 327–336. [Google Scholar] [CrossRef]
- Allen, R.D.; Baumann, P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J. Bacteriol. 1971, 107, 295–302. [Google Scholar] [CrossRef]
- McCarter, L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001, 65, 445–462. [Google Scholar] [CrossRef]
- Grossart, H.P.; Steward, G.F.; Martinez, J.; Azam, F. A simple, rapid method for demonstrating bacterial flagella. Appl. Environ. Microbiol. 2000, 66, 3632. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Yeh, F.L.; Mao, F.; Chapman, E.R. Biophysical characterization of styryl dye-membrane interactions. Biophys. J. 2009, 97, 101–109. [Google Scholar] [CrossRef]
- Copeland, M.F.; Flickinger, S.T.; Tuson, H.H.; Weibel, D.B. Studying the dynamics of flagella in multicellular communities of Escherichia coli by using biarsenical dyes. Appl. Environ. Microbiol. 2010, 76, 1241–1250. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Y.; Zhuang, X.Y.; Lo, W.C.; Baker, M.A.B.; Lo, C.J.; Bai, F. Frequent pauses in Escherichia coli flagella elongation revealed by single cell real-time fluorescence imaging. Nat. Commun. 2018, 9, 1885. [Google Scholar] [CrossRef]
- Schmitt, M.; Stark, H. Modelling bacterial flagellar growth. Europhys. Lett. 2011, 96, 28001. [Google Scholar] [CrossRef][Green Version]
- Stern, A.S.; Berg, H.C. Single-file diffusion of flagellin in flagellar filaments. Biophys. J. 2013, 105, 182–184. [Google Scholar] [CrossRef]
- Evans, L.D.B.; Poulter, S.; Terentjev, E.M.; Hughes, C.; Fraser, G.M. A chain mechanism for flagellum growth. Nature 2013, 504, 287–290. [Google Scholar] [CrossRef]
- Grunenfelder, B.; Tawfilis, S.; Gehrig, S.; Østerås, M.; Eglin, D.; Jenal, U. Identification of the protease and the turnover signal responsible for cell cycle-dependent degradation of the Caulobacter FliF motor protein. J. Bacteriol. 2004, 186, 4960–4971. [Google Scholar] [CrossRef][Green Version]
- Ruby, E.G.; Asato, L.M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 1993, 159, 160–167. [Google Scholar] [CrossRef]
- Doerges, L.; Kutschera, U. Assembly and loss of the polar flagellum in plant-associated methylobacteria. Naturwissenschaften 2014, 101, 339–346. [Google Scholar] [CrossRef]
- Boehm, A.; Kaiser, M.; Li, H.; Spangler, C.; Kasper, C.A.; Ackermann, M.; Kaever, V.; Sourjik, V.; Roth, V.; Jenal, U. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 2010, 141, 107–116. [Google Scholar] [CrossRef]
- Paul, K.; Nieto, V.; Carlquist, W.C.; Blair, D.F.; Harshey, R.M. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol. Cell. 2010, 38, 128–139. [Google Scholar] [CrossRef]
- Fukuoka, H.; Wada, T.; Kojima, S.; Ishijima, A.; Homma, M. Sodium-dependent dynamic assembly of membrane complexes in sodium-driven flagellar motors. Mol. Microbiol. 2009, 71, 825–835. [Google Scholar] [CrossRef]
- Honko, A.N.; Mizel, S.B. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 2005, 33, 83–101. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, R.M.; McSorley, S.J. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol. Lett. 2005, 101, 117–122. [Google Scholar] [CrossRef]
- Miao, E.A.; Andersen-Nissen, E.; Warren, S.E.; Aderem, A. TLR5 and Ipaf: Dual sensors of bacterial flagellin in the innate immune system. Semin. Immunopathol. 2007, 29, 275–288. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Ozinsky, A. Toll-like receptor-5 and the innate immune response to bacterial flagellin. Curr. Top. Microbiol. Immunol. 2002, 270, 93–108. [Google Scholar]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.D.; Ozoren, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef]
- Vogler, A.P.; Homma, M.; Irikura, V.M.; Macnab, R.M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of FliI to F0F1, vacuolar, and archaebacterial ATPase subunits. J. Bacteriol. 1991, 173, 3564–3572. [Google Scholar] [CrossRef]
- Rosu, V.; Hughes, K.T. Sigma28-dependent transcription in Salmonella enterica is independent of flagellar shearing. J. Bacteriol. 2006, 188, 5196–5203. [Google Scholar] [CrossRef][Green Version]
- Zhu, S.; Gao, B. Bacterial flagella loss under starvation. Trends Microbiol. 2020, 28, 785–788. [Google Scholar] [CrossRef]

| Species | V0 (nm/min) | Secretion Rate (#/sec) | Microscopy | Reference |
|---|---|---|---|---|
| Salmonella | 550 (Decay) | 18.33 | Electron microscopy | [71] |
| Decay | Dark-field imaging | [72] | ||
| 100 (Decay) | 3.33 | Fluorescence imaging | [74] | |
| E. coli | 24 (Constant) | 0.80 | Fluorescence imaging | [73] |
| 27 (Decay, pauses) | 0.90 | Fluorescence imaging | [82] | |
| V. alginolyticus | 50 (Decay) | 1.67 | Fluorescence imaging | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.-Y.; Lo, C.-J. Construction and Loss of Bacterial Flagellar Filaments. Biomolecules 2020, 10, 1528. https://doi.org/10.3390/biom10111528
Zhuang X-Y, Lo C-J. Construction and Loss of Bacterial Flagellar Filaments. Biomolecules. 2020; 10(11):1528. https://doi.org/10.3390/biom10111528
Chicago/Turabian StyleZhuang, Xiang-Yu, and Chien-Jung Lo. 2020. "Construction and Loss of Bacterial Flagellar Filaments" Biomolecules 10, no. 11: 1528. https://doi.org/10.3390/biom10111528
APA StyleZhuang, X.-Y., & Lo, C.-J. (2020). Construction and Loss of Bacterial Flagellar Filaments. Biomolecules, 10(11), 1528. https://doi.org/10.3390/biom10111528





