Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants
Abstract
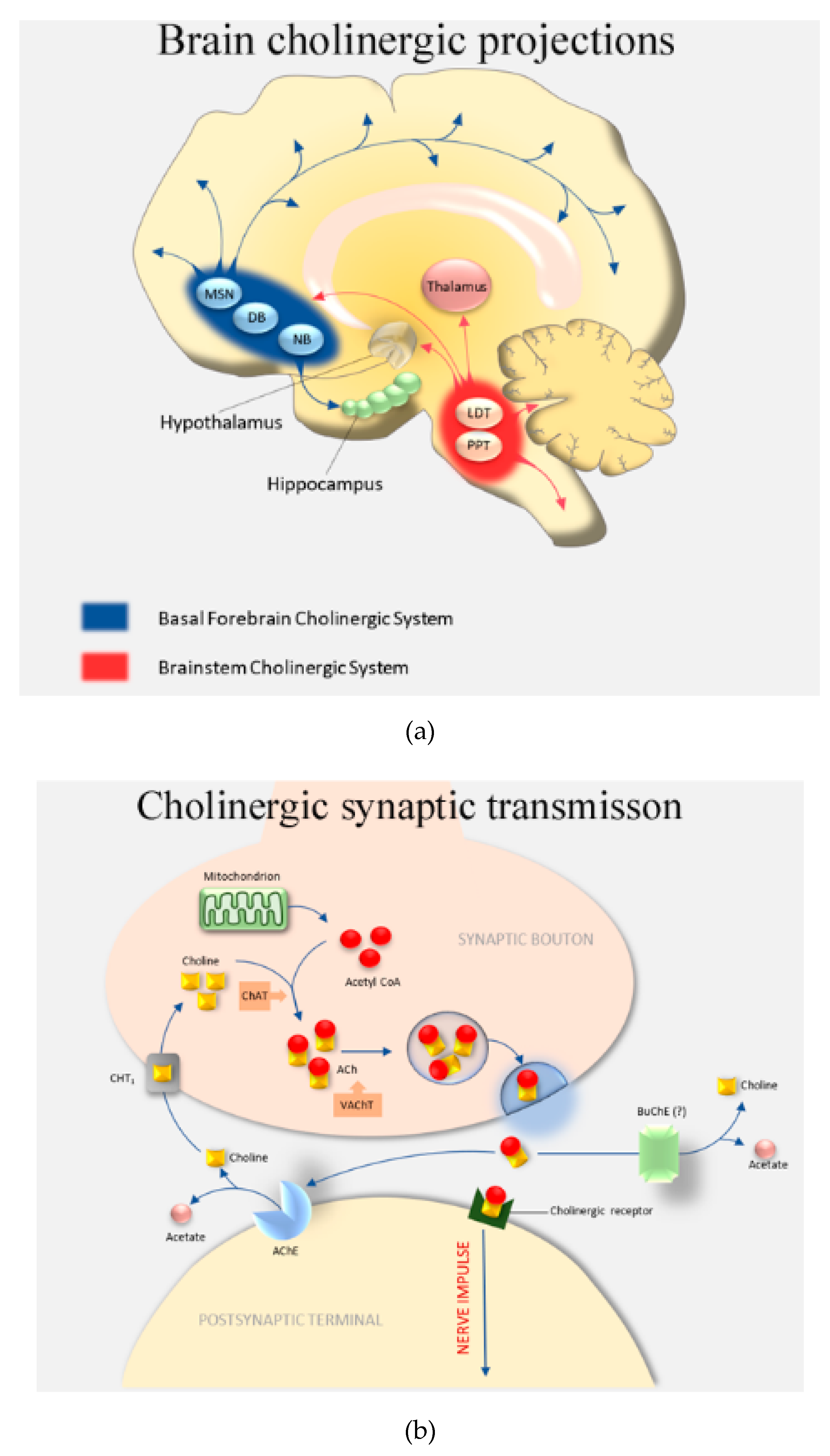
:1. Introduction
2. Materials and Methods
2.1. Plant Samples and Chemicals
2.2. Preparation of Plant Extracts
2.3. AChE and BuChE Inhibitory Activity
2.4. Antioxidant Activity
2.5. Cytotoxicity Assays
2.6. Liquid-Chromatography Mass-Spectrometry (LC-MS)
2.7. In Silico Molecular Docking Studies
2.8. Statistical Analysis
3. Results
3.1. Assessment of Plant Extract AChE and BuChE Inhibitory Activity
3.2. Assessment of Plant Extract Antioxidant Activity
3.3. Assessment of Plant Extract Neuronal Toxicity
3.4. Assessment of Associations between Cholinesterase Inhibition, Radical Scavenging, and Cell Toxicity
3.5. Liquid-Chromatography Mass-Spectrometry (LC-MS)
3.6. Molecular Docking of Potential ChEIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2017—Highlights (ST/ESA/SER.A/397). Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed on 14 November 2020).
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- WHO. 10 Facts on Dementia. WHO Media Centre, 2016. Available online: https://www.who.int/features/factfiles/dementia/en/ (accessed on 28 September 2020).
- Launer, L.J.; Andersen, K.; Dewey, M.E.; Letenneur, L.; Ott, A.; Amaducci, L.A.; Brayne, C.E.; Copeland, J.R.M.; Dartigues, J.F.; Kragh-Sorensen, P.; et al. Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. Neurology 1999, 52, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulson, H.; Igo, I. Genetics of Dementia. Semin. Neurol. 2011, 31, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Killin, L.O.J.; Starr, J.M.; Shiue, I.J.; Russ, T.C. Environmental risk factors for dementia: A systematic review. BMC Geriatr. 2016, 16, 175. [Google Scholar] [CrossRef] [Green Version]
- Sabia, S.; Elbaz, A.; Britton, A.; Bell, S.; Dugravot, A.; Shipley, M.; Kivimaki, M.; Singh-Manoux, A. Alcohol consumption and cognitive decline in early old age. Neurology 2014, 82, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Tangney, C.C. Dietary fat composition and dementia risk. Neurobiol. Aging 2014, 35, S59–S64. [Google Scholar] [CrossRef] [Green Version]
- Anstey, K.J.; Mack, H.A.; Cherbuin, N. Alcohol Consumption as a Risk Factor for Dementia and Cognitive Decline: Meta-Analysis of Prospective Studies. Am. J. Geriatr. Psychiatry 2009, 17, 542–555. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; Julián, B.S.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Hardman, R.J.; Kennedy, G.; MacPherson, H.; Scholey, A.B.; Pipingas, A. Adherence to a Mediterranean-Style Diet and Effects on Cognition in Adults: A Qualitative Evaluation and Systematic Review of Longitudinal and Prospective Trials. Front. Nutr. 2016, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Loughrey, D.G.; Lavecchia, S.; Brennan, S.; Lawlor, B.A.; Kelly, M.E. The Impact of the Mediterranean Diet on the Cognitive Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 571–586. [Google Scholar] [CrossRef]
- Rehm, J.; Gmel, G.E., Sr.; Gmel, G.; Hasan, O.S.M.; Imtiaz, S.; Popova, S.; Probst, C.; Roerecke, M.; Room, R.; Samokhvalov, A.V.; et al. The relationship between different dimensions of alcohol use and the burden of disease—An update. Addiction 2017, 112, 968–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardener, H.; Caunca, M.R. Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Dadhania, V.P.; Trivedi, P.P.; Vikram, A.; Tripathi, D.N. Nutraceuticals against Neurodegeneration: A Mechanistic Insight. Curr. Neuropharmacol. 2016, 14, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Mahalanobish, S.; Sil, P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 100–114. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Alikwe, P.C.N.; Elmorsy, E.; Carter, W.G. An Investigation of Potential Sources of Nutraceuticals from the Niger Delta Areas, Nigeria for Attenuating Oxidative Stress. Medicines 2019, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Perl, D.P. Neuropathology of Alzheimer’s Disease. Mt. Sinai J. Med. J. Transl. Pers. Med. 2010, 77, 32–42. [Google Scholar] [CrossRef]
- Schaffert, L.-N.; Carter, W.G. Do Post-Translational Modifications Influence Protein Aggregation in Neurodegenerative Diseases: A Systematic Review. Brain Sci. 2020, 10, 232. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, Oxidative Stress and the Pathogenesis of Alzheimers Disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Arendt, T.; Brückner, M.K.; Lange, M.; Bigl, V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development—A study of molecular forms. Neurochem. Int. 1992, 21, 381–396. [Google Scholar] [CrossRef]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease: Getting on and staying on. Curr. Ther. Res. 2003, 64, 216–235. [Google Scholar] [CrossRef] [Green Version]
- Colović, M.; Krstić, D.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s Disease. Prim. Care Companion CNS Disord. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Río, J.A.; Fuster, M.D.; Gómez, P.; Porras, I.; García-Lidón, A.; Ortuño, A. Citrus limon: A source of flavonoids of pharmaceutical interest. Food Chem. 2004, 84, 457–461. [Google Scholar] [CrossRef]
- Jain, V.; Verma, S.K.; Katewa, S.S. Myths, traditions and fate of multipurpose Bombax ceiba L.—An appraisal. Indian J. Tradit. Knowl. 2009, 8, 638–644. [Google Scholar]
- Rameshwar, V.; Kishor, D.; Tushar, G.; Siddharth, G.; Sudarshan, G. A pharmacognostic and pharmacological overview on Bombax ceiba. Sch. Acad. J. Pharm. 2014, 3, 100–107. [Google Scholar]
- Chaudhary, G.; Goyal, S.; Poonia, P. Lawsonia inermis Linnaeus: A phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 91–98. [Google Scholar]
- Borade, A.S.; Kale, B.N.; Shete, R.V. A phytopharmacological review on Lawsonia inermis (Linn.). Int. J. Pharm. Life Sci. 2011, 2, 536–541. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Cartwright-Jones, C.; Viljoen, A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 2014, 155, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.M.; Patel, P.R. Review on Lawsonia inermis Linn.: An Update. Asian J. Pharm. Technol. 2017, 7, 237. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiate essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Pan, M.; Lei, Q.; Zang, N.; Zhang, H. A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves. Int. J. Mol. Sci. 2019, 20, 3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical Characterization of Basil (Ocimum basilicumL.) Found in Local Accessions and Used in Traditional Medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorný, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Karousou, R.; Balta, M.; Hanlidou, E.; Kokkini, S. “Mints”, smells and traditional uses in Thessaloniki (Greece) and other Mediterranean countries. J. Ethnopharmacol. 2007, 109, 248–257. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Elmorsy, E.; Thornton, J.; Wijamunige, B.; Wijesekara, A.; Tarbox, R.; Warren, A.; Carter, W.G. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol. 2017, 55, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Nwidu, L.L.; Elmorsy, E.; Aprioku, J.S.; Siminialayi, I.; Carter, W.G. In Vitro Anti-Cholinesterase and Antioxidant Activity of Extracts of Moringa oleifera Plants from Rivers State, Niger Delta, Nigeria. Medicines 2018, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.-B.; Chen, G.-L.; Guo, M. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentón-Rol, G.; Cervantes-Llanos, M. Report on the Symposium “Molecular Mechanisms Involved in Neurodegeneration”. Behav. Sci. 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiem, A.; Smail, A.; Wissem, M.; Faleiro, M.L.; Miguel, M. Antioxidant, anti-inflammatory and anti-acetylcholinesterase activities of leaf, flower and seed aqueous extracts of Lawsonia inermis from Tunisia. Int. J. Pharm. Pharm. Sci. 2014, 6, 445–452. [Google Scholar]
- Gholamhoseinian, A.; Moradi, M.; Sharifi-Far, F. Screening the methanol extracts of some Iranian plants for acetylcholinesterase inhibitory activity. Res. Pharm. Sci. 2009, 4, 105–112. [Google Scholar] [PubMed]
- Lynch, T.J.; Mattes, C.E.; Singh, A.; Bradley, R.M.; Brady, R.O.; Dretchen, K.L. Cocaine Detoxification by Human Plasma Butyrylcholinesterase. Toxicol. Appl. Pharmacol. 1997, 145, 363–371. [Google Scholar] [CrossRef]
- Chen, V.P.; Gao, Y.; Geng, L.; Brimijoin, S. Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int. J. Obes. 2017, 41, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Dorling, J.L.; Clayton, D.J.; Jones, J.; Carter, W.G.; Thackray, A.E.; King, J.A.; Pucci, A.; Batterham, R.L.; Stensel, D.J. A randomized crossover trial assessing the effects of acute exercise on appetite, circulating ghrelin concentrations, and butyrylcholinesterase activity in normal-weight males with variants of the obesity-linked FTO rs9939609 polymorphism. Am. J. Clin. Nutr. 2019, 110, 1055–1066. [Google Scholar] [CrossRef]
- Manoharan, I.; Boopathy, R.; Darvesh, S.; Lockridge, O. A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India. Clin. Chim. Acta 2007, 378, 128–135. [Google Scholar] [CrossRef]
- Lockridge, O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef]
- Mesulam, M.; Guillozet, A.; Shaw, P.; Quinn, B. Widely Spread Butyrylcholinesterase Can Hydrolyze Acetylcholine in the Normal and Alzheimer Brain. Neurobiol. Dis. 2002, 9, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Reinikainen, K.J.; Riekkinen, P.J.; Paljärvi, L.; Soininen, H.; Helkala, E.L.; Jolkkonen, J.; Laakso, M. Cholinergic deficit in Alzheimer’s disease: A study based on CSF and autopsy data. Neurochem. Res. 1988, 13, 135–146. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Selective Inhibitors of Butyrylcholinesterase: A valid alternative for therapy of Alzheimer’s disease? Drugs Aging 2001, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.R.; Pal, S.C.; Kasture, V.S.; Kasture, S.B. Effect of Lawsonia inermis on memory and behaviour mediated via monoamine neurotransmitters. Indian J. Pharmacol. 1998, 30, 181–185. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Trigui, M.; Culioli, G.; Blache, Y.; Jaoua, S. Antioxidant constituents from Lawsonia inermis leaves: Isolation, structure elucidation and antioxidative capacity. Food Chem. 2011, 125, 193–200. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and Antiacetylcholinesterase Activities of Some Commercial Essential Oils and Their Major Compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Jindal, D.K.; Parle, M.; Kumar, A.; Dhingra, S. Targeting oxidative stress, acetylcholinesterase, proinflammatory cytokine, dopamine and GABA by eucalyptus oil (Eucalyptus globulus) to alleviate ketamine-induced psychosis in rats. Inflammopharmacology 2018, 27, 301–311. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Costa, P.C.; Bahia, M. Oxygen and Nitrogen Reactive Species Are Effectively Scavenged by Eucalyptus globulus Leaf Water Extract. J. Med. Food 2009, 12, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Ito, H.; Hatano, T.; Yoshida, T.; Tonogai, Y. Constituents and their antioxidative effects in eucalyptus leaf extract used as a natural food additive. Food Chem. 2002, 77, 47–56. [Google Scholar] [CrossRef]
- Dessí, M.A.; Deiana, M.; Rosa, A.; Piredda, M.; Cottiglia, F.; Bonsignore, L.; Deidda, D.; Pompei, R.; Corongiu, F.P. Antioxidant activity of extracts from plants growing in Sardinia. Phytotherapy Res. 2001, 15, 511–518. [Google Scholar] [CrossRef]
- Castro, V.T.N.D.A.E.; Sobrinho, T.J.D.S.P.; Corrêa, A.J.C.; Araújo, T.A.D.S.; Da Silva, T.G.; De Amorim, E.L.C. The anticholinesterase properties of plants from the northeast of brazil selected by an ethnopharmacological study for disorders relating to the nervous system. Pharmacogn. Mag. 2016, 12, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef] [Green Version]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential Oil from Lemon Peels Inhibit Key Enzymes Linked to Neurodegenerative Conditions and Pro-oxidant Induced Lipid Peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Kou, J.; Li, F.; Huo, D.; Xu, J.; Zhou, X.; Meng, D.; Ghulam, M.; Artyom, B.; Gao, X.; et al. Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging 2020, 12, 8622–8639. [Google Scholar] [CrossRef]
- Kamal, M.; Ashraf, M.Y.; Hussain, A.U.; Shahzadi, A.; Chughtai, M.I. Antioxidant potential of peel essential oils of three Pakistani citrus species: Citrus recticulata, citrus sinensis and Citrus paradisii. Pak. J. Bot. 2013, 45, 1449–1454. [Google Scholar]
- Tan, S.J.; Ismail, I.S. Potency of Selected Berries, Grapes, and Citrus Fruit as Neuroprotective Agents. Evid. Based Complement. Altern. Med. 2020, 2020, 3582947. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G. Anti-acetylcholinesterase potential and metabolome classification of 4 Ocimum species as determined via UPLC/qTOF/MS and chemometric tools. J. Pharm. Biomed. Anal. 2016, 125, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.M.; Niemeyer, E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarahroodi, S.; Esmaeili, S.; Hemmati, Z.; Mikaili, P.; Saberi, Y. The effects of green Ocimum basilicum hydroalcoholic extract on retention and retrieval of memory in mice. Anc. Sci. Life 2012, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.O.; Said, A.; Aboutabl, E.; Azzam, M.; Creczynski-Pasa, T.B. Antioxidant activity of methanolic extract of Bombax ceiba. Redox Rep. 2009, 14, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Kumar, B.; Singh, D.K.; Luqman, S.; Singh, M.; Singh, A. Antioxidant and Choline Esterase Inhibitory Activity of Phenolic Rich Extracts from Bombax ceiba L. Flowers. Free Radic. Antioxid. 2018, 8, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, N.M. β-Amyrin Rich Bombax ceiba Leaf Extract with Potential Neuroprotective Activity against Scopolamine-Induced Memory Impairment in Rats. Rec. Nat. Prod. 2018, 12, 480–492. [Google Scholar] [CrossRef]
- Sinha, S.; Kumar, B.; Luqman, S.; Singh, D. Neuroprotective potential of Cucurbita maxima Duchesne ex Poir, Caeselpenia bunduc (L.) Roxb and Bombax ceiba Linn extracts. S. Afr. J. Bot. 2019, 120, 319–325. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Umemoto, K.; Kameoka, H. Inhibition of Acetylcholinesterase Activity by Essential Oils of Mentha Species. J. Agric. Food Chem. 1998, 46, 3431–3434. [Google Scholar] [CrossRef]
- Mata, A.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Bernas, T.; Dobrucki, J. Mitochondrial and nonmitochondrial reduction of MTT: Interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 2002, 47, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L.; Markossian, S.; Sittampalam, G.S.; Grossman, A.; et al. Cell viablity assays. In Assay Guidance Manual; Markosian, S., Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Eli Lilly & Company and the National Centre for Advancing Translational Sciences: Bethesda, MD, USA, 2013; updated 2016. [Google Scholar]
- Elmorsy, E.; Al-Ghafari, A.; Almutairi, F.M.; Aggour, A.M.; Carter, W.G. Antidepressants are cytotoxic to rat primary blood brain barrier endothelial cells at high therapeutic concentrations. Toxicol. Vitr. 2017, 44, 154–163. [Google Scholar] [CrossRef]
- Al-Ghafari, A.; Elmorsy, E.; Fikry, E.; Alrowaili, M.; Carter, W.G. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PLoS ONE 2019, 14, e0225341. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, B.D.; Seth, V.; Ahmed, R. Pesticide-Induced Oxidative Stress: Perspective and Trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef]
- Carter, W.G.; Tarhoni, M.; Rathbone, A.J.; Ray, D.E. Differential protein adduction by seven organophosphorus pesticides in both brain and thymus. Hum. Exp. Toxicol. 2007, 26, 347–354. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of Flavonoids toward Cultured Normal Human Cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free. Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Budryn, G.; Grzelczyk, J.; Jaśkiewicz, A.; Żyżelewicz, D.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P. Evaluation of butyrylcholinesterase inhibitory activity by chlorogenic acids and coffee extracts assed in ITC and docking simulation models. Food Res. Int. 2018, 109, 268–277. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G. Analysis of the activity of hydroxycinnamic acids from green and roasted coffee extracts as acetylcholinesterase inhibitors using an isothermal method of titration calorimetry. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2019, 349, 15–24. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Amat-Ur-Rasool, H.; Ahmed, M. Designing Second Generation Anti-Alzheimer Compounds as Inhibitors of Human Acetylcholinesterase: Computational Screening of Synthetic Molecules and Dietary Phytochemicals. PLoS ONE 2015, 10, e0136509. [Google Scholar] [CrossRef] [PubMed]






| Agent | Alternative or Common Name | IC50 (mg/mL) | ED50 (µg/mL) | IC50 (mg/mL) | |
|---|---|---|---|---|---|
| AChE | BuChE | DPPH | MTT | ||
| Citrus limon | Lemon | 2.59 ± 0.14 | 1.82 ± 0.10 | 839.40 ± 135.2 | 1.77 ± 0.03 |
| Bombax ceiba | Red silk-cotton | 6.26 ± 0.72 | 7.38 ± 0.96 | 69.69 ± 3.01 | 1.51 ± 0.08 |
| Lawsonia inermis | Henna | 0.33 ± 0.02 | 0.41 ± 0.02 | 33.68 ± 1.04 | 0.58 ± 0.02 |
| Eucalyptus globulus | Eucalyptus | 1.11 ± 0.07 | 0.99 ± 0.05 | 22.15 ± 1.23 | 0.40 ± 0.01 |
| Ocimum basilicum | Basil | 4.84 ± 0.47 | 5.90 ± 0.81 | 56.13 ± 2.01 | 0.98 ± 0.03 |
| Citrus reticulata | Mandarin | 1.56 ± 0.20 | 1.51 ± 0.09 | 359.30 ± 31.64 | 0.68 ± 0.03 |
| Mentha spicata | Spearmint | 10.49 ± 2.06 | 5.82 ± 0.77 | 28.94 ± 0.96 | 1.12 ± 0.03 |
| Galantamine | Galanthamine | 0.00019 | 0.00049 | ND | 3.53 ± 0.21 |
| Vitamin E | α-tocopherol | ND | ND | 7.73 ± 0.66 | ND |
| Peak # | [M-H] | Putative Molecular Formula | Putative Identification | M. oleifera * | C. lemon | B. ceiba | L. inermis | E. globulus | O. basilicum | C. reticulata | M. spicata |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 665 | C24H42O21 | Cellotetraose | x | 665.17 | x | x | x | x | 665.17 | x |
| 3 | 341 | C12H22O11 | Sucrose | 341.11 | 341.11 | 341.11 | 341.10 | 341.09 | 341.11 | 341.09 | x |
| 4 | 503 | C18H32O16 | Cellotriose | 503.15 | 503.17 | 503.17 | x | 503.14 | x | x | 503.14 |
| 2 | 367 | C17H20O9 | Methyl 4-caffeoylquinate | 367.10 | 367.14 | 367.16 | x | x | x | x | x |
| 5 | 278 | C14H17NO5 | Niazirin | 278.07 | x | x | x | x | x | x | x |
| 6 | 191 | C7H12O6 | Quinic acid isomer 1 | 191.06 | 191.06 | 191.04 | x | 191.05 | x | 191.05 | x |
| 10 | 191 | C7H12O6 | Quinic acid isomer 2 | 191.02 | x | 191.02 | x | x | x | x | x |
| 7 | 586 | C20H29NO15S2 | 3-Hydroxy-4-(α-l-rhamnopyranosyloxy) benzyl glucosinolate | 586.09 | x | x | x | x | x | x | x |
| 8 | 570 | C20H29NO14S2 | Glucomoringin | 570.07 | x | x | x | x | x | x | x |
| 11 | 408 | C14H19NO9S2 | Glucotropaeolin | x | x | x | x | x | x | x | 408.12 |
| 12 | 612 | C22H31NO15S2 | Acetyl-4-(α-l-rhamnopyranosyloxy) benzyl glucosinolate | 612.07 | x | x | x | x | x | x | x |
| 9 | 353 | C16H18O9 | 3-Caffeoylquinic acid | 353.08 | x | x | 353.08 | 353.07 | x | x | 353.06 |
| 13 | 609 | C27H30O16 | Rutin (quercetin-3-O-rutinoside) | 609.14 | 609.14 | 609.14 | 609.14 | 609.13 | 609.12 | 609.14 | 609.13 |
| 14 | 463 | C21H20O12 | Quercetin 3-O-glucoside | 463.08 | x | 463.08 | 463.09 | 463.07 | 463.07 | x | x |
| 15 | 505 | C23H22O13 | Quercetin-acetyl-glycoside | 505.13 | x | 505.26 | x | 505.11 | 505.11 | 505.25 | x |
| 16 | 447 | C21H20O11 | Kaempferol 3-O-glucoside | 447.08 | 447.09 | 447.09 | 447.07 | 447.07 | 447.07 | 447.06 | 447.06 |
| 17 | 489 | C23H22O12 | Kaempferol-acetyl-glycoside | 489.10 | x | x | 489.09 | 489.08 | x | 489.07 | 489.13 |
| Ligand | 2D Structures | Binding Affinity (kcal/mol) | |
|---|---|---|---|
| AChE | BuChE | ||
| Galantamine |  | −7.7 | −8.7 |
| 3-Caffeoylquinic acid |  | −9.2 | −8.6 |
| Methyl 4-caffeoylquinate |  | −8.8 | −8.9 |
| Kaempferol-acetyl-glycoside |  | −8.4 | −10.4 |
| Quercetin 3-rutinoside (Rutin) |  | −8.3 | −11.0 |
| Quercetin-acetyl-glycoside |  | −8.0 | −10.4 |
| Kaempferol 3-O-glucoside (Astragalin) |  | −7.9 | −9.7 |
| Quercetin 3-O-glucoside (Isoquercitrin) |  | −7.6 | −9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.-N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants. Biomolecules 2020, 10, 1556. https://doi.org/10.3390/biom10111556
Amat-ur-Rasool H, Symes F, Tooth D, Schaffert L-N, Elmorsy E, Ahmed M, Hasnain S, Carter WG. Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants. Biomolecules. 2020; 10(11):1556. https://doi.org/10.3390/biom10111556
Chicago/Turabian StyleAmat-ur-Rasool, Hafsa, Fenella Symes, David Tooth, Larissa-Nele Schaffert, Ekramy Elmorsy, Mehboob Ahmed, Shahida Hasnain, and Wayne G. Carter. 2020. "Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants" Biomolecules 10, no. 11: 1556. https://doi.org/10.3390/biom10111556
APA StyleAmat-ur-Rasool, H., Symes, F., Tooth, D., Schaffert, L.-N., Elmorsy, E., Ahmed, M., Hasnain, S., & Carter, W. G. (2020). Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants. Biomolecules, 10(11), 1556. https://doi.org/10.3390/biom10111556








