Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology
Abstract
1. Introduction
1.1. Chronic Obstructive Pulmonary Disease
1.2. Naringenin and its Glycoside Naringin
2. Potential Pharmacological Effects of Naringenin in COPD
2.1. Anti-Inflammatory Activity
2.2. Antioxidative Activity
2.3. Anti-Airway Remodeling Activity
2.4. Anti-Pulmonary Fibrosis Activity
2.5. Expectorant
2.6. Antitussive
3. Network Pharmacology
3.1. Data Preparation
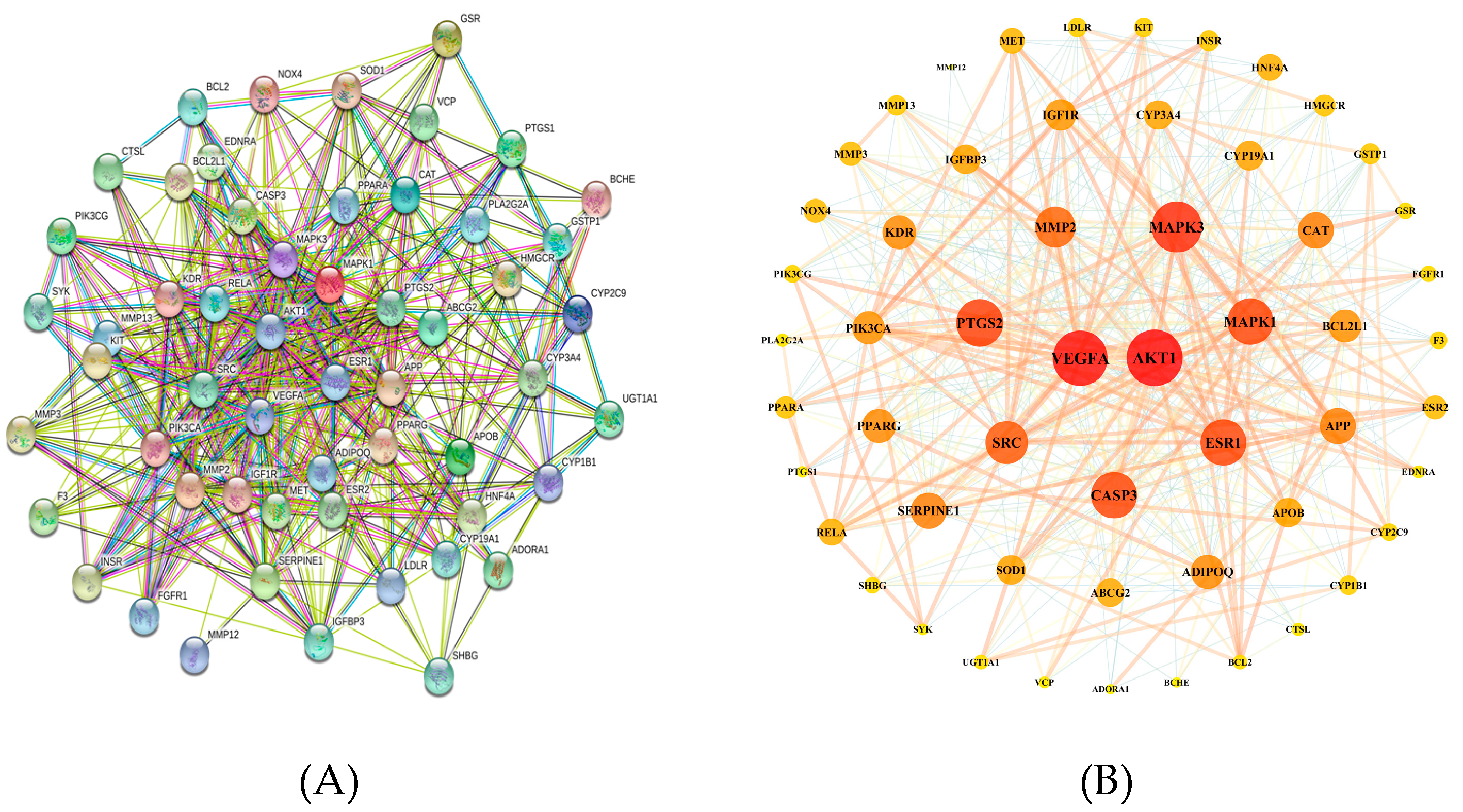
3.2. Protein–Protein Interaction (PPI) Network Construction
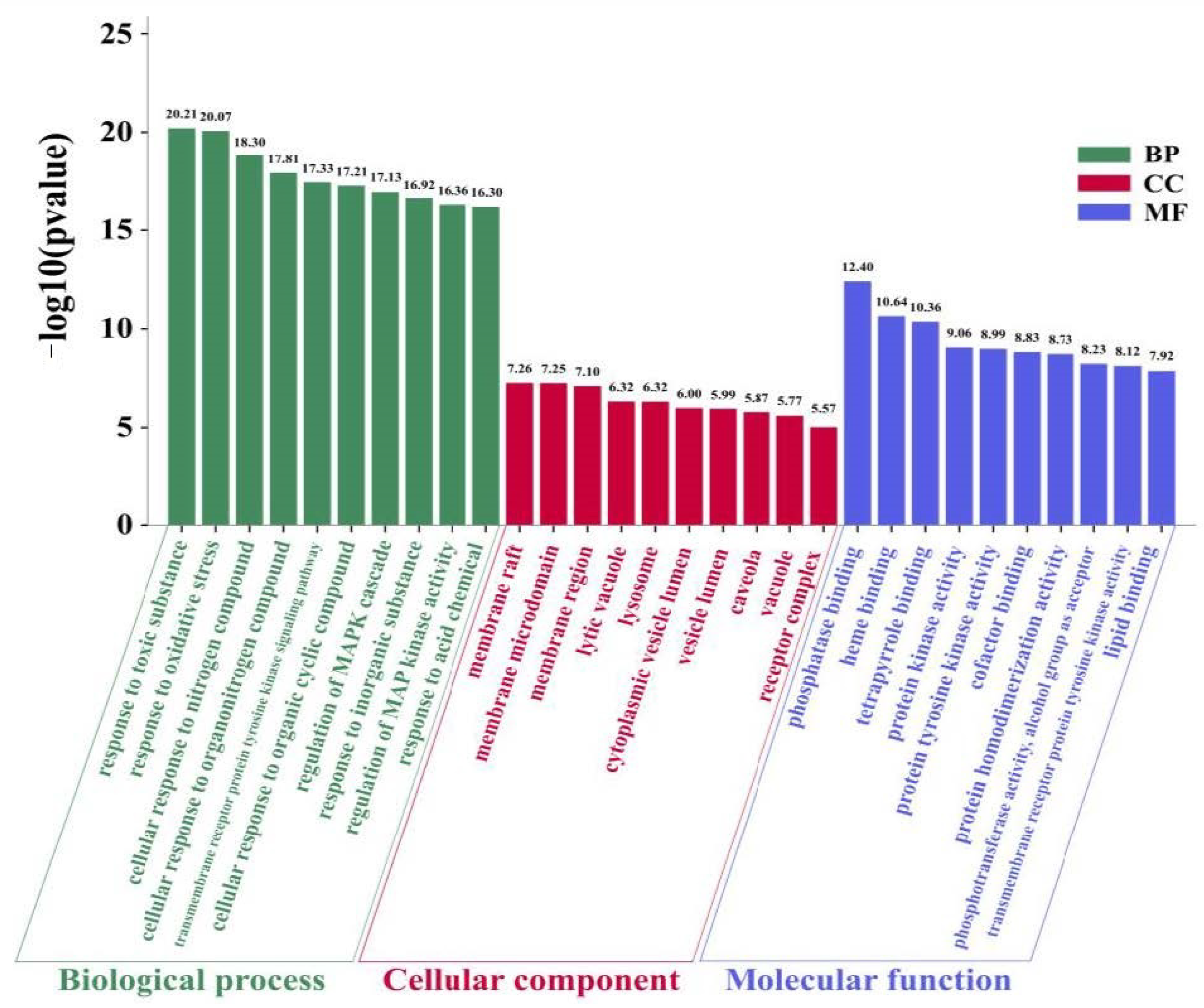
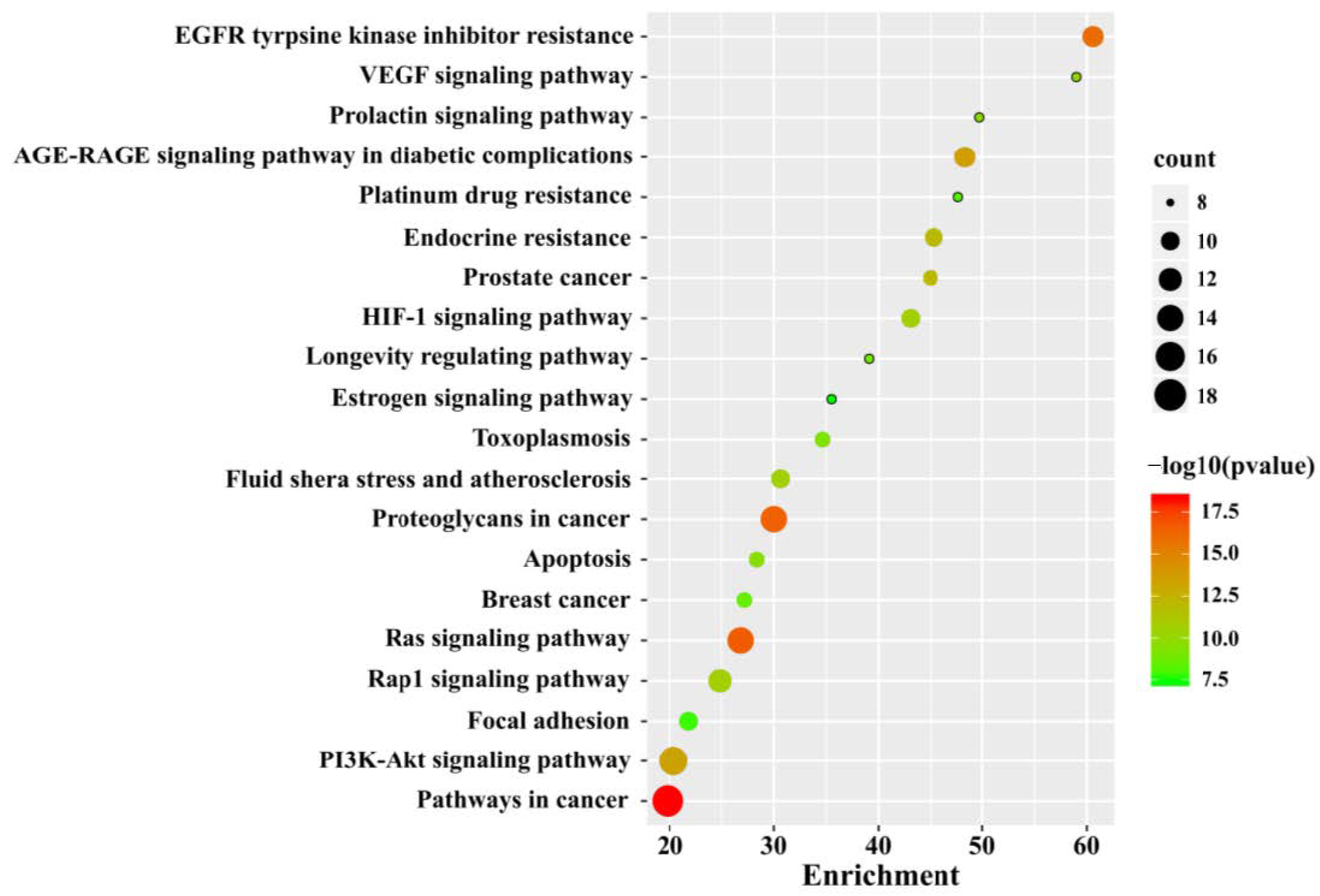
3.3. GO and KEGG Pathway Enrichment Analysis
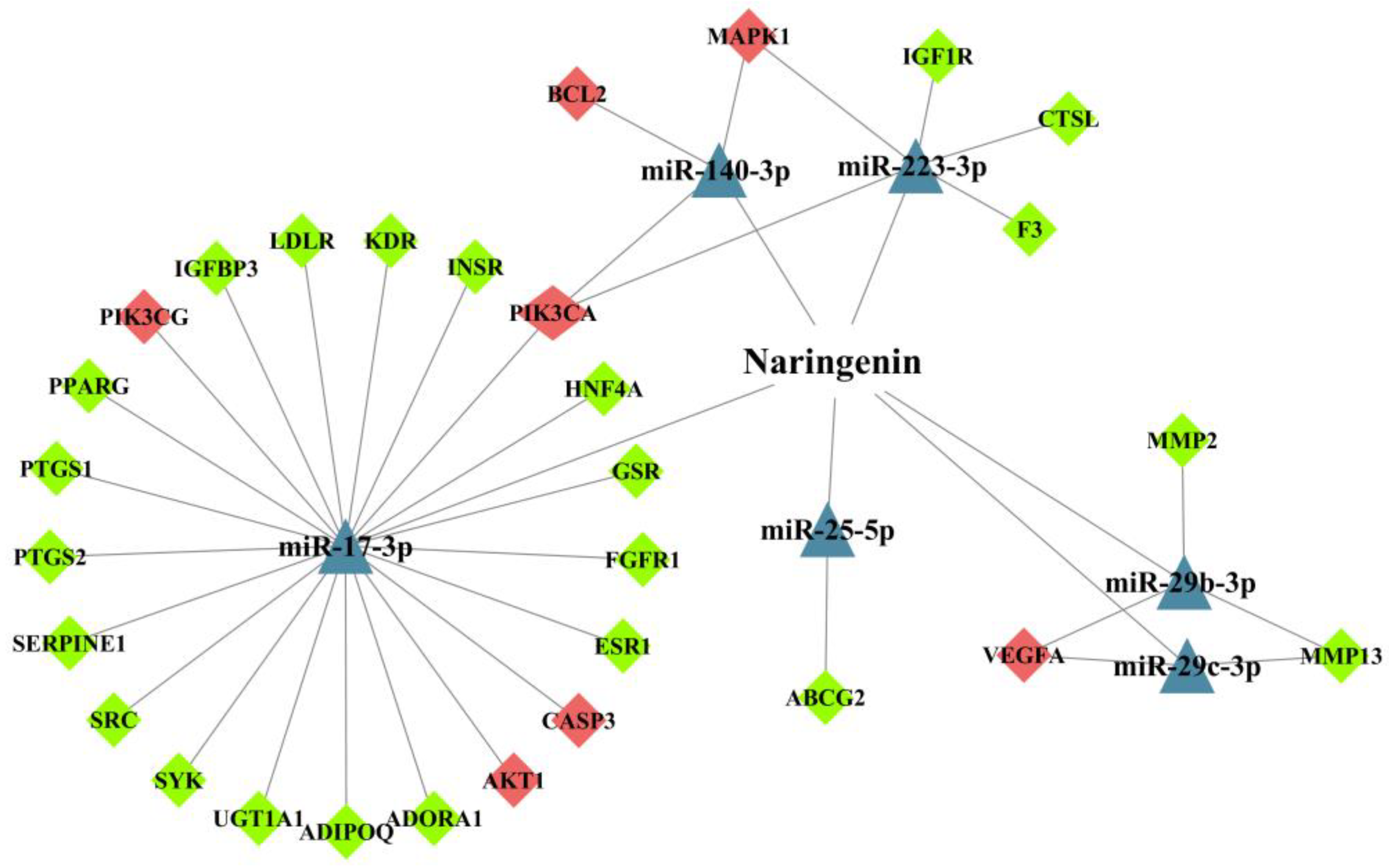
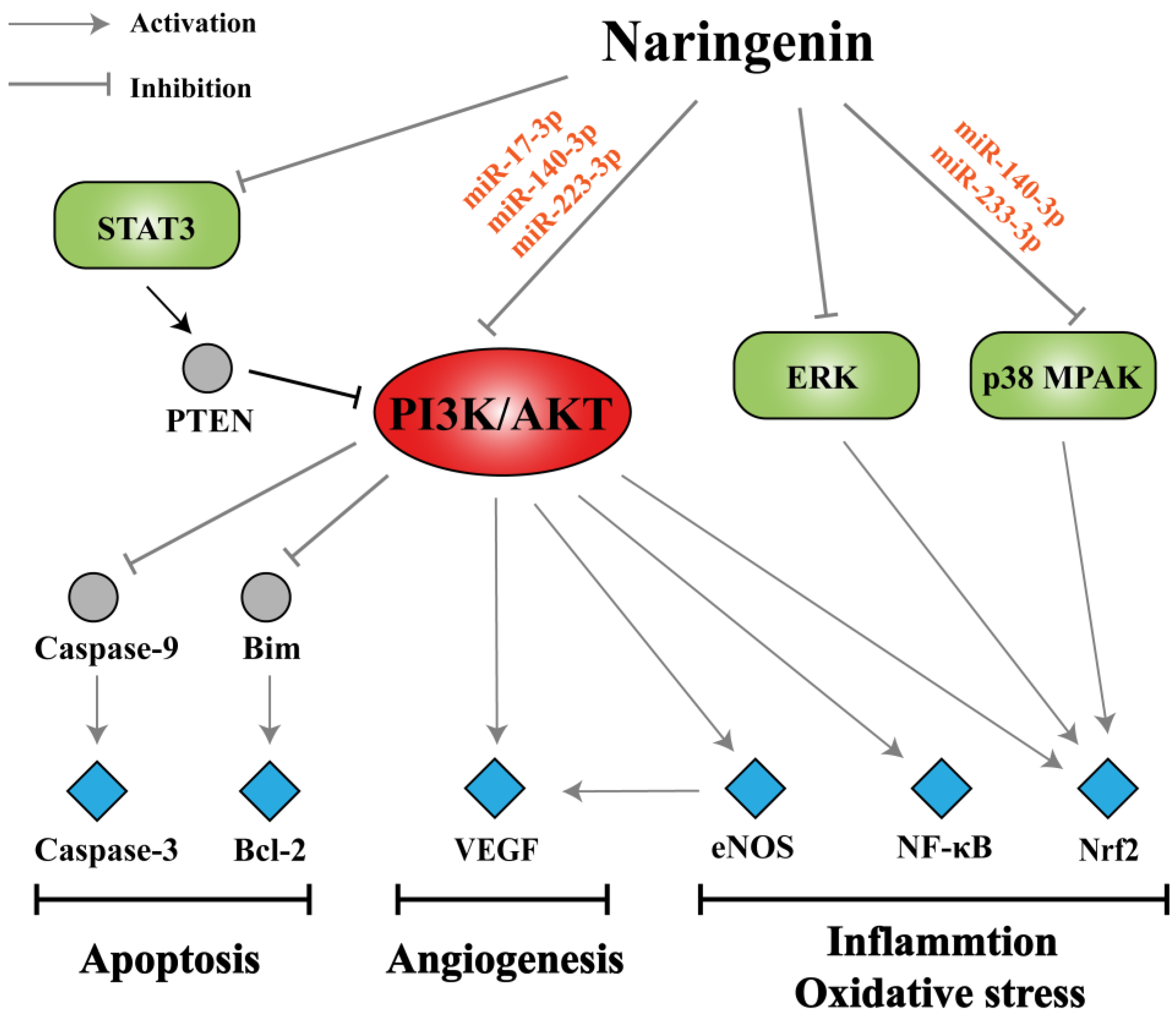
3.4. Analysis of miRNA-Mediated Naringenin in the Treatment of COPD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| CS | cigarette smoke |
| LPS | lipopolysaccharide |
| CLP | cecum ligation and puncture |
| MMP | matrix metalloproteinase |
| BALF | bronchoalveolar lavage fluid |
| ECM | extracellular matrix |
| PI3K | phosphatidylinositol 3-kinase |
| AKT | protein kinase B |
| MCP-1 | monocyte chemoattractant protein-1 |
| MIP-1α | macrophage inflammatory protein-1α |
| TSLP | thymic stromal lymphopoietin |
| RIP-2 | receptor-interacting protein-2 |
| PVP | polyvinyl pyrrolidone |
| NPs | nanoparticles |
| MAPK | P38 mitogen-activated protein kinase |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutases |
| CAT | catalases |
| XO | xanthine oxidase |
| GPx | glutathione peroxidases |
| GSH | glutathione |
| GST | glutathione s-transferase |
| GR | glutathione reductase |
| COX-2 | cyclooxygenase-2 |
| iNOS | inducible nitric oxide synthase |
| eNOS | endothelial nitric oxide synthase |
| TIMP-1 | tissue inhibitor of metalloproteinase-1 |
| HYP | hydroxyproline |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| SP | substance P |
| NK-1 | neurokinin-1 |
| NEP | neutral endopeptidase |
| PPI | protein-protein interaction |
| NT-CTs | naringenin targets-COPD targets |
| GO | Gene Ontology |
| BP | biological progress |
| CC | cellular component |
| MF | molecular function |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PTEN | phosphatase and tensin homolog deleted from chromosome ten |
| VEGF | vascular endothelial growth factor |
References
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.; Guimaraes, M.J.; van Zeller, M.; Menezes, F.; Moita, J.; Simao, P. Clinical and molecular markers in COPD. Pulmonology 2018, 24, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.B. The roles of endothelin and its receptors in cigarette smoke-associated pulmonary hypertension with chronic lung disease. Pathol. Res. Pract. 2020, 216, 153083. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.; Han, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Belchamber, K.; Donnelly, L.E. Targeting defective pulmonary innate immunity—A new therapeutic option? Pharmacol. Ther. 2020, 209, 107500. [Google Scholar] [CrossRef]
- Castaldi, P.J.; Dy, J.; Ross, J.; Chang, Y.; Washko, G.R.; Curran-Everett, D.; Williams, A.; Lynch, D.A.; Make, B.J.; Crapo, J.D.; et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax 2014, 69, 415–422. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Stolz, D.; Matera, M.G. Pharmacological treatment and current controversies in COPD. F1000Res. 2019, 8, 1533. [Google Scholar] [CrossRef]
- Hendershott, C.H.; Walker, D.R. Identification of a growth inhibitor from extracts of dormant peach flower buds. Science 1959, 130, 798–800. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Liu, B.; Chai, L.; Shi, R.; Yao, H. A Review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases. Mini-Rev. Med. Chem. 2020, 20, 286–293. [Google Scholar] [CrossRef]
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef]
- Chin, L.H.; Hon, C.M.; Chellappan, D.K.; Chellian, J.; Madheswaran, T.; Zeeshan, F.; Awasthi, R.; Aljabali, A.A.; Tambuwala, M.M.; Dureja, H.; et al. Molecular mechanisms of action of naringenin in chronic airway diseases. Eur. J. Pharmacol. 2020, 879, 173139. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Jresat, I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology 2016, 97, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019, 12, 11. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based approaches in pharmacology. Mol. Inform. 2017, 36, 36. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology, and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.S.; Sohal, S.S. Update on the pathogenesis of COPD. N. Engl. J. Med. 2019, 381, 2483–2484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Nie, Y.C.; Chen, J.L.; Su, W.W.; Li, P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-kappaB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wu, H.; Li, P.; Luo, Y.; Long, K.; Xie, L.; Shen, J.; Su, W. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J. Med. Food 2012, 15, 894–900. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.F.; Dong, J.; Wei, J.Y.; Wang, Y.N.; Dai, X.H.; Wang, X.; Luo, M.J.; Tan, W.; Deng, X.M.; et al. Inhibition of alpha-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia 2013, 86, 92–99. [Google Scholar] [CrossRef]
- Gil, M.; Kim, Y.K.; Hong, S.B.; Lee, K.J. Naringin decreases TNF-alpha and HMGB1 release from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. PLoS ONE 2016, 11, e0164186. [Google Scholar] [CrossRef]
- Huang, S.; Ding, Z.; Xiang, H.; Fu, L.; Fei, J. Association between serum S100A8/S100A9 heterodimer and pulmonary function in patients with acute exacerbation of chronic obstructive pulmonary disease. Lung 2020, 198, 645–652. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, L.; Xie, Y.; Xu, Y.; Jiao, W.; Wu, J.; Deng, X.; Fang, G.; Xue, Q.; Zheng, Y.; et al. Th1/Th17 cytokine profiles are associated with disease severity and exacerbation frequency in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1287–1299. [Google Scholar] [CrossRef]
- Falfan-Valencia, R.; Ramirez-Venegas, A.; Perez, L.J.; Ramirez-Rodriguez, S.L.; Marquez-Garcia, J.E.; Buendia-Roldan, I.; Gayosso-Gomez, L.V.; Perez-Padilla, R.; Ortiz-Quintero, B. Smoke exposure from chronic biomass burning induces distinct accumulative systemic inflammatory cytokine alterations compared to tobacco smoking in healthy women. Cytokine 2020, 131, 155089. [Google Scholar] [CrossRef]
- Garth, J.; Barnes, J.W.; Krick, S. Targeting cytokines as evolving treatment strategies in chronic inflammatory airway diseases. Int. J. Mol. Sci. 2018, 19, 3402. [Google Scholar] [CrossRef]
- Mahler, D.A.; Huang, S.; Tabrizi, M.; Bell, G.M. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: A pilot study. Chest 2004, 126, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Ora, J.; Matera, M.G. Canakinumab for the treatment of chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2015, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, J.; Zhang, J. Naringenin attenuates inflammation in chronic obstructive pulmonary disease in cigarette smoke induced mouse model and involves suppression of NF-κB. J. Microbiol. Biotechnol. 2018, 30609878. [Google Scholar] [CrossRef]
- Luo, Y.L.; Zhang, C.C.; Li, P.B.; Nie, Y.C.; Wu, H.; Shen, J.G.; Su, W.W. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int. Immunopharmacol. 2012, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 2009, 41, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, C.; Shen, F.; Wang, M.; Jia, N.; Wang, C. Naringenin ameliorates LPS-induced acute lung injury through its anti-oxidative and anti-inflammatory activity and by inhibition of the PI3K/AKT pathway. Exp. Ther. Med. 2017, 14, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, W.; Yao, Y.; Xu, B.; Wei, X.; Wang, L.; Yin, X.; Barman, A.K.; Zhang, F.; Zhang, C.; et al. Naringenin ameliorates radiation-induced lung injury by lowering IL-1beta level. J. Pharmacol. Exp. Ther. 2018, 366, 341–348. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Agarwal, A.R.; Kadam, S.; Brahme, A.; Agrawal, M.; Apte, K.; Narke, G.; Kekan, K.; Madas, S.; Salvi, S. Systemic Immuno-metabolic alterations in chronic obstructive pulmonary disease (COPD). Respir. Res. 2019, 20, 171. [Google Scholar] [CrossRef]
- Silva, B.; Lira, F.S.; Ramos, D.; Uzeloto, J.S.; Rossi, F.E.; Freire, A.; Silva, R.N.; Trevisan, I.B.; Gobbo, L.A.; Ramos, E. Severity of COPD and its relationship with IL-10. Cytokine 2018, 106, 95–100. [Google Scholar] [CrossRef]
- De Llano, L.P.; Cosio, B.G.; Iglesias, A.; de Las, C.N.; Soler-Cataluna, J.J.; Izquierdo, J.L.; Lopez-Campos, J.L.; Calero, C.; Plaza, V.; Miravitlles, M.; et al. Mixed Th2 and non-Th2 inflammatory pattern in the asthma—COPD overlap: A network approach. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.X.; Lu, L.W.; Liu, W.J.; Huang, M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) Overlap Syndrome. Med. Sci. Monit. 2016, 22, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Bleecker, E.R.; Panettieri, R.A.; Bafadhel, M.; She, D.; Ward, C.K.; Xu, X.; Birrell, C.; van der Merwe, R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir. Med. 2014, 2, 891–901. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Hanania, N.A.; Noonan, M.; Corren, J.; Korenblat, P.; Zheng, Y.; Fischer, S.K.; Cheu, M.; Putnam, W.S.; Murray, E.; Scheerens, H.; et al. Lebrikizumab in moderate-to-severe asthma: Pooled data from two randomised placebo-controlled studies. Thorax 2015, 70, 748–756. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Zoheir, K.M.; Ansari, M.A.; Korashy, H.M.; Abdel-Hamied, H.E.; Ashour, A.E.; Abd-Allah, A.R. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-kappab, STAT3 and pro-inflammatory mediators and enhancement of IkappaBalpha and anti-inflammatory cytokines. Inflammation 2015, 38, 846–857. [Google Scholar] [CrossRef]
- Bradford, E.; Jacobson, S.; Varasteh, J.; Comellas, A.P.; Woodruff, P.; O’Neal, W.; DeMeo, D.L.; Li, X.; Kim, V.; Cho, M.; et al. The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 2017, 18, 1–11. [Google Scholar] [CrossRef]
- Henrot, P.; Prevel, R.; Berger, P.; Dupin, I. Chemokines in COPD: From implication to therapeutic Use. Int. J. Mol. Sci. 2019, 20, 2785. [Google Scholar] [CrossRef]
- Liu, Y.; Su, W.W.; Wang, S.; Li, P.B. Naringin inhibits chemokine production in an LPS-induced RAW 264.7 macrophage cell line. Mol. Med. Rep. 2012, 6, 1343–1350. [Google Scholar] [CrossRef]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef]
- Redhu, N.S.; Gounni, A.S. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin. Exp. Allergy 2012, 42, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Fang, C.; Cousins, D.; Zhang, G.; Gu, S.; Gao, Z.; Shamji, B.; et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J. Immunol. 2008, 181, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Choi, I.H.; Kim, H.M. Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur. J. Pharmacol. 2011, 671, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in hepatic fibrosis. Biomed. Microdevices 2020, 22, 68. [Google Scholar] [CrossRef]
- Kumar, R.P.; Abraham, A. Inhibition of LPS induced pro-inflammatory responses in RAW 264.7 macrophage cells by PVP-coated naringenin nanoparticle via down regulation of NF-kappaB/P38MAPK mediated stress signaling. Pharmacol. Rep. 2017, 69, 908–915. [Google Scholar] [CrossRef]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar] [CrossRef]
- Moitra, S. N-acetylcysteine (NAC) in COPD: Benefits often lost in trials. QJM Int. J. Med. 2019, 112, 387–388. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Murata, K.; Fujimoto, K.; Kitaguchi, Y.; Horiuchi, T.; Kubo, K.; Honda, T. Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. J. Chronic Obstr. Pulm. Dis. 2014, 11, 81–87. [Google Scholar] [CrossRef]
- Kostikas, K.; Papatheodorou, G.; Psathakis, K.; Panagou, P.; Loukides, S. Oxidative stress in expired breath condensate of patients with COPD. Chest 2003, 124, 1373–1380. [Google Scholar] [CrossRef]
- Stefanska, J.; Sarniak, A.; Wlodarczyk, A.; Sokolowska, M.; Doniec, Z.; Bialasiewicz, P.; Nowak, D.; Pawliczak, R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm. Pharmacol. Ther. 2012, 25, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Paska, C.; Simon, B.; Barta, I. Monitoring antioxidant enzyme activity during exacerbations of chronic obstructive pulmonary disease. J. Chronic Obstr. Pulm. Dis. 2018, 15, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, S.K.; Kumar, S.; Ahmad, M.K.; Nischal, A.; Singh, S.K.; Dixit, R.K. Evaluation of oxidative stress and antioxidant status in chronic obstructive pulmonary disease. Scand. J. Immunol. 2017, 85, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Busquets-Cortes, C.; Capo, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Mizumura, K.; Maruoka, S.; Shimizu, T.; Gon, Y. Role of Nrf2 in the pathogenesis of respiratory diseases. Respir. Investig. 2020, 58, 28–35. [Google Scholar] [CrossRef]
- Ali, R.; Shahid, A.; Ali, N.; Hasan, S.K.; Majed, F.; Sultana, S. Amelioration of benzo[a]pyrene-induced oxidative stress and pulmonary toxicity by naringenin in Wistar rats: A plausible role of COX-2 and NF-κB. Hum. Exp. Toxicol. 2017, 36, 349–364. [Google Scholar] [CrossRef]
- Podder, B.; Song, H.Y.; Kim, Y.S. Naringenin exerts cytoprotective effect against paraquat-induced toxicity in human bronchial epithelial BEAS-2B cells through NRF2 activation. J. Microbiol. Biotechnol. 2014, 24, 605–613. [Google Scholar] [CrossRef]
- Jiang, W.T.; Liu, X.S.; Xu, Y.J.; Ni, W.; Chen, S.X. Expression of nitric oxide synthase isoenzyme in lung tissue of smokers with and without chronic obstructive pulmonary disease. Chin. Med. J. 2015, 128, 1584–1589. [Google Scholar] [CrossRef]
- Brindicci, C.; Kharitonov, S.A.; Ito, M.; Elliott, M.W.; Hogg, J.C.; Barnes, P.J.; Ito, K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2010, 181, 21–30. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Abioye, J.B.; Ebinama, O.N. Potential protective effects of naringin on oculo-pulmonary injury induced by PM10 (wood smoke) exposure by modulation of oxidative damage and acetylcholine esterase activity in a rat model. Curr. Ther. Res. Clin. Exp. 2020, 92, 100586. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Obaid, A.A.; Zaki, H.F.; Agha, A.M. Naringenin adds to the protective effect of L-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation, and nitric oxide. Eur. J. Pharm. Sci. 2014, 62, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Quinn, A.M.; Cancado, J.; Singh, D. The pathology of small airways disease in COPD: Historical aspects and future directions. Respir. Res. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Wang, L.F.; Yin, Y.Q. How do innate immune cells contribute to airway remodeling in copd progression? Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Noble, P.B.; Elliot, J.G.; James, A.L. Airway remodelling in COPD: It’s not asthma! Respirology 2016, 21, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Martin, J.G. Mechanisms of airway remodeling. Chest 2013, 144, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Kolahian, S.; Shahbazfar, A.A.; Ansarin, K.; Pour, M.M.; Sakhinia, M.; Sakhinia, E.; Vafa, M. Effects of the flavanone combination hesperetin-naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother. Res. 2015, 29, 591–598. [Google Scholar] [CrossRef]
- Siddiqui, S.; Shikotra, A.; Richardson, M.; Doran, E.; Choy, D.; Bell, A.; Austin, C.D.; Eastham-Anderson, J.; Hargadon, B.; Arron, J.R.; et al. Airway pathological heterogeneity in asthma: Visualization of disease microclusters using topological data analysis. J. Allergy Clin. Immunol. 2018, 142, 1457–1468. [Google Scholar] [CrossRef]
- Qin, W.; Deng, T.; Cui, H.; Zhang, Q.; Liu, X.; Yang, X.; Chen, M. Exposure to diisodecyl phthalate exacerbated Th2 and Th17-mediated asthma through aggravating oxidative stress and the activation of p38 MAPK. Food Chem. Toxicol. 2018, 114, 78–87. [Google Scholar] [CrossRef]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and non-immunologic mechanisms leading to airway remodeling in asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef]
- Guihua, X.; Shuyin, L.; Jinliang, G.; Wang, S. Naringin protects ovalbumin-induced airway inflammation in a mouse model of asthma. Inflammation 2016, 39, 891–899. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, Y.; Mao, S.; Gu, W. Naringenin inhibits allergen-induced airway remodeling in a murine model of asthma. Mol. Med. Rep. 2014, 9, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kobayashi, S.; Hanagama, M.; Ishida, M.; Sato, H.; Makiguchi, T.; Yanai, M. Clinical characteristics of Japanese patients with chronic obstructive pulmonary disease (COPD) with comorbid interstitial lung abnormalities: A cross-sectional study. PLoS ONE 2020, 15, e0239764. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; McDonald, V.M.; Gibson, P.G. Comorbidity in chronic obstructive pulmonary disease. Respir. Investig. 2015, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, B.; Schoeps, B.; Kruger, A. Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell. Biol. 2019, 29, 6–19. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Y.C.; Luo, Y.L.; Lin, F.; Zheng, Y.F.; Cheng, G.H.; Wu, H.; Zhang, K.J.; Su, W.W.; Shen, J.G.; et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 2013, 58, 133–140. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Turgut, N.H.; Kara, H.; Elagoz, S.; Deveci, K.; Gungor, H.; Arslanbas, E. The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar Rats. Pulm. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Stewart, A.G.; Thomas, B.; Koff, J. TGF-β: Master regulator of inflammation and fibrosis. Respirology 2018, 23, 1096–1097. [Google Scholar] [CrossRef]
- Lin, Y.; Tan, D.; Kan, Q.; Xiao, Z.; Jiang, Z. The protective effect of naringenin on airway remodeling after Mycoplasma pneumoniae infection by inhibiting autophagy-mediated lung inflammation and fibrosis. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Zhou-Suckow, Z.; Duerr, J.; Hagner, M.; Agrawal, R.; Mall, M.A. Airway mucus, inflammation, and remodeling: Emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017, 367, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, F.; Lee, H.J.; Lee, C.J. Recent advances in the development of novel drug candidates for regulating the secretion of pulmonary mucus. Biomol. Ther. 2020, 28, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.F.; Ieni, A.; Hansbro, P.M.; Ruggeri, P.; Di Stefano, A.; Nucera, F.; Coppolino, I.; Monaco, F.; Tuccari, G.; Adcock, I.M.; et al. Role of the mucins in pathogenesis of COPD: Implications for therapy. Expert. Rev. Respir. Med. 2020, 14, 465–483. [Google Scholar] [CrossRef]
- Samsuzzaman, M.; Uddin, M.S.; Shah, M.A.; Mathew, B. Natural inhibitors on airway mucin: Molecular insight into the therapeutic potential targeting MUC5AC expression and production. Life Sci. 2019, 231, 116485. [Google Scholar] [CrossRef]
- Lin, B.Q.; Li, P.B.; Wang, Y.G.; Peng, W.; Wu, Z.; Su, W.W.; Ji, H. The expectorant activity of naringenin. Pulm. Pharmacol. Ther. 2008, 21, 259–263. [Google Scholar] [CrossRef]
- Padra, M.; Andersson, A.; Levanen, B.; Premaratne, P.; Asgeirsdottir, H.; Tengvall, S.; Christenson, K.; Stockfelt, M.; Bozinovski, S.; Yoshihara, S.; et al. Increased MUC1 plus a larger quantity and complex size for MUC5AC in the peripheral airway lumen of long-term tobacco smokers. Clin. Sci. 2020, 134, 1107–1125. [Google Scholar] [CrossRef]
- Li, J.; Ye, Z. The potential role and regulatory mechanisms of MUC5AC in chronic obstructive pulmonary disease. Molecules 2020, 25, 4437. [Google Scholar] [CrossRef]
- Nie, Y.C.; Wu, H.; Li, P.B.; Xie, L.M.; Luo, Y.L.; Shen, J.G.; Su, W.W. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IkappaB-NF-kappaB signaling pathways. Eur. J. Pharmacol. 2012, 690, 207–213. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-kappaB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol. Cell. Biochem. 2011, 351, 29–40. [Google Scholar] [CrossRef]
- Reid, A.T.; Veerati, P.C.; Gosens, R.; Bartlett, N.W.; Wark, P.A.; Grainge, C.L.; Stick, S.M.; Kicic, A.; Moheimani, F.; Hansbro, P.M.; et al. Persistent induction of goblet cell differentiation in the airways: Therapeutic approaches. Pharmacol. Ther. 2018, 185, 155–169. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Nie, Y.C.; Li, P.B.; Shen, J.G.; Su, W.W. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ. Toxicol. Pharmacol. 2014, 38, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ehre, C.; Ridley, C.; Thornton, D.J. Cystic fibrosis: An inherited disease affecting mucin-producing organs. Int. J. Biochem. Cell. Biol. 2014, 52, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xiao, Z.T.; Zheng, Y.J.; Zhang, Y.L.; Xu, J.W.; Huang, J.H.; Zhou, W.L.; Li, P.B.; Su, W.W. Naringenin regulates CFTR activation and expression in airway epithelial cells. Cell. Physiol. Biochem. 2017, 44, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Su, W.W.; Zhu, Z.T.; Guan, M.Y.; Cheng, K.L.; Fan, W.Y.; Wei, G.Y.; Li, P.B.; Yang, Z.Y.; Yao, H.L. Regulation effects of naringin on diesel particulate matter-induced abnormal airway surface liquid secretion. Phytomedicine 2019, 63, 153004. [Google Scholar] [CrossRef]
- Crooks, M.G.; Brown, T.; Morice, A.H. Is cough important in acute exacerbations of COPD? Respir. Physiol. Neurobiol. 2018, 257, 30–35. [Google Scholar] [CrossRef]
- Song, W.J.; Chung, K.F. Pharmacotherapeutic options for chronic refractory cough. Expert Opin. Pharmacother. 2020, 21, 1345–1358. [Google Scholar] [CrossRef]
- Luo, Y.L.; Li, P.B.; Zhang, C.C.; Zheng, Y.F.; Wang, S.; Nie, Y.C.; Zhang, K.J.; Su, W.W. Effects of four antitussives on airway neurogenic inflammation in a guinea pig model of chronic cough induced by cigarette smoke exposure. Inflamm. Res. 2013, 62, 1053–1061. [Google Scholar] [CrossRef]
- Gao, S.; Li, P.; Yang, H.; Fang, S.; Su, W. Antitussive effect of naringin on experimentally induced cough in Guinea pigs. Planta Med. 2011, 77, 16–21. [Google Scholar] [CrossRef]
- Smith, J.A.; Badri, H. Cough: New pharmacology. J. Allergy Clin. Immunol. Pract. 2019, 7, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Bidan, C.M.; Brook, B.S.; Zuidhof, A.B.; Elzinga, C.; Smit, M.; Oldenburger, A.; Gosens, R.; Timens, W.; Meurs, H. Small airway hyperresponsiveness in COPD: Relationship between structure and function in lung slices. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2019, 316, L537–L546. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.Y.; Su, W.W.; Li, P.B.; Liao, Y.; Zhou, Q.; Zhu, N.; He, L.L. Therapeutic effects of naringin in a guinea pig model of ovalbumin-induced cough-variant asthma. Pulm. Pharmacol. Ther. 2015, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Pirozzi, F.; Ren, K.; Murabito, A.; Ghigo, A. PI3K signaling in chronic obstructive pulmonary disease: Mechanisms, targets, and therapy. Curr. Med. Chem. 2019, 26, 2791–2800. [Google Scholar] [CrossRef]
- Hosgood, H.D., 3rd; Menashe, I.; He, X.; Chanock, S.; Lan, Q. PTEN identified as important risk factor of chronic obstructive pulmonary disease. Respir. Med. 2009, 103, 1866–1870. [Google Scholar] [CrossRef]
- Sun, X.; Chen, L.; He, Z. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr. Drug Metab. 2019, 20, 301–304. [Google Scholar] [CrossRef]
- Lu, J.; Xie, L.; Liu, C.; Zhang, Q.; Sun, S. PTEN/PI3k/AKT regulates macrophage polarization in emphysematous mice. Scand. J. Immunol. 2017, 85, 395–405. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Baker, J.R.; Vuppusetty, C.; Fenwick, P.; Donnelly, L.E.; Ito, K.; Barnes, P.J. Decreased phosphatase PTEN amplifies PI3K signaling and enhances proinflammatory cytokine release in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L230–L239. [Google Scholar] [CrossRef]
- Xu, F.; Lin, J.; Cui, W.; Kong, Q.; Li, Q.; Li, L.; Wei, Y.; Dong, J. Scutellaria baicalensis attenuates airway remodeling via PI3K/Akt/NF-kappaB pathway in cigarette smoke mediated-COPD rats model. Evid. Based Complement Alternat. Med. 2018, 2018, 1281420. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, H.; Wang, Z.L.; Li, W.H.; Liu, H.; Zhao, Y.X. The PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J. Int. Med. Res. 2020, 48, 1220727471. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Du, J.; Meng, Y.; Guo, F.; Feng, C. Louqin Zhisou decoction inhibits mucus hypersecretion for acute exacerbation of chronic obstructive pulmonary disease rats by suppressing EGFR-PI3K-AKT signaling pathway and restoring Th17/Treg balance. Evid. Based Complement Alternat. Med. 2019, 2019, 6471815. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Oiso, Y.; Sakai, H.; Motomura, T.; Yamashita, C. Pulmonary administration of phosphoinositide 3-kinase inhibitor is a curative treatment for chronic obstructive pulmonary disease by alveolar regeneration. J. Control. Release 2015, 213, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Marwick, J.A.; Caramori, G.; Casolari, P.; Mazzoni, F.; Kirkham, P.A.; Adcock, I.M.; Chung, K.F.; Papi, A. A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2010, 125, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, X.; Zheng, D.; Li, A.; Li, C.; Li, S.; Zhao, Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress, and apoptosis in vitro and in vivo. Clin. Sci. 2019, 133, 1523–1536. [Google Scholar] [CrossRef]
- Sun, Y.; An, N.; Li, J.; Xia, J.; Tian, Y.; Zhao, P.; Liu, X.; Huang, H.; Gao, J.; Zhang, X. miRNA-206 regulates human pulmonary microvascular endothelial cell apoptosis via targeting in chronic obstructive pulmonary disease. J. Cell. Biochem. 2019, 120, 6223–6236. [Google Scholar] [CrossRef]
- Csoma, B.; Bikov, A.; Nagy, L.; Toth, B.; Tabi, T.; Szucs, G.; Komlosi, Z.I.; Muller, V.; Losonczy, G.; Lazar, Z. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir. Res. 2019, 20, 156. [Google Scholar] [CrossRef]
- Arif, E.; Ahsan, A.; Vibhuti, A.; Rajput, C.; Deepak, D.; Athar, M.; Singh, B.; Pasha, M.A. Endothelial nitric oxide synthase gene variants contribute to oxidative stress in COPD. Biochem. Biophys. Res. Commun. 2007, 361, 182–188. [Google Scholar] [CrossRef]
- Schuliga, M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. longev. 2019, 7090534. [Google Scholar] [CrossRef]
- Tan, B.; Sim, W.L.; Cheong, J.K.; Kuan, W.S.; Tran, T.; Lim, H.F. MicroRNAs in chronic airway diseases: Clinical correlation and translational applications. Pharmacol. Res. 2020, 160, 105045. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Di Lorenzo, A.; Rossi, D.; Martino, E.; Capelli, E.; Collina, S.; Daglia, M. Enantioselective modulatory effects of naringenin enantiomers on the expression levels of miR-17-3p involved in endogenous antioxidant defenses. Nutrients 2017, 9, 215. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.W.; Yang, W.; Wan, L.J.; Yan, H.L.; Li, J.C.; Tang, S.Y.; Wang, Y.Q. Naringenin induces neuroprotection against homocysteine-induced PC12 cells via the upregulation of superoxide dismutase 1 expression by decreasing miR-224-3p expression. J. Biol. Regul. Homeost. Agents. 2020, 34, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.N.; Zou, X.; Fang, X.H.; Xu, J.D.; Xiao, Z.; Zhu, J.N.; Li, H.; Yang, J.; Zeng, N.; Yuan, S.J.; et al. The Smad3-miR-29b/miR-29c axis mediates the protective effect of macrophage migration inhibitory factor against cardiac fibrosis. Biochim. Biophy. Acta Mol. Basis Dis. 2019, 1865, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.B.; Tang, P.F.; Zhang, W.; Zhao, Y.P.; Zhang, L.C.; Zhang, H. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene 2016, 592, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Wen, L.; Peng, R.; Li, H.; Liu, H.; Peng, H.; Sun, Y.; Wu, T.; Chen, L.; Duan, Q.; et al. Naringenin ameliorated kidney injury through Let-7a/TGFBR1 signaling in diabetic nephropathy. J. Diabetes Res. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, C.; Zhao, H. Defective insulin receptor signaling in patients with gestational diabetes is related to dysregulated miR-140 which can be improved by naringenin. Int. J. Biochem. Cell. Biol. 2020, 128, 105824. [Google Scholar] [CrossRef]
- Yu, Z.G.; Wang, B.Z.; Cheng, Z.Z. The association of genetic polymorphisms of hypoxia inducible factor-1 alpha and vascular endothelial growth factor with increased risk of chronic obstructive pulmonary disease: A case-control study. Kaohsiung J. Med. Sci. 2017, 33, 433–441. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Matarese, A.; Santulli, G. Angiogenesis in chronic obstructive pulmonary disease: A translational appraisal. Transl. Med. UniSa 2012, 3, 49–56. [Google Scholar] [PubMed]
- Bakakos, P.; Patentalakis, G.; Papi, A. Vascular biomarkers in asthma and COPD. Curr. Top. Med. Chem. 2016, 16, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Shi, R.; Zheng, Y.; Zeng, X.; Fan, W.; Wang, Y.; Su, W. Characterization, in vitro and in vivo evaluation of naringenin-hydroxypropyl-ß-cyclodextrin inclusion for pulmonary delivery. Molecules 2020, 25, 554. [Google Scholar] [CrossRef]
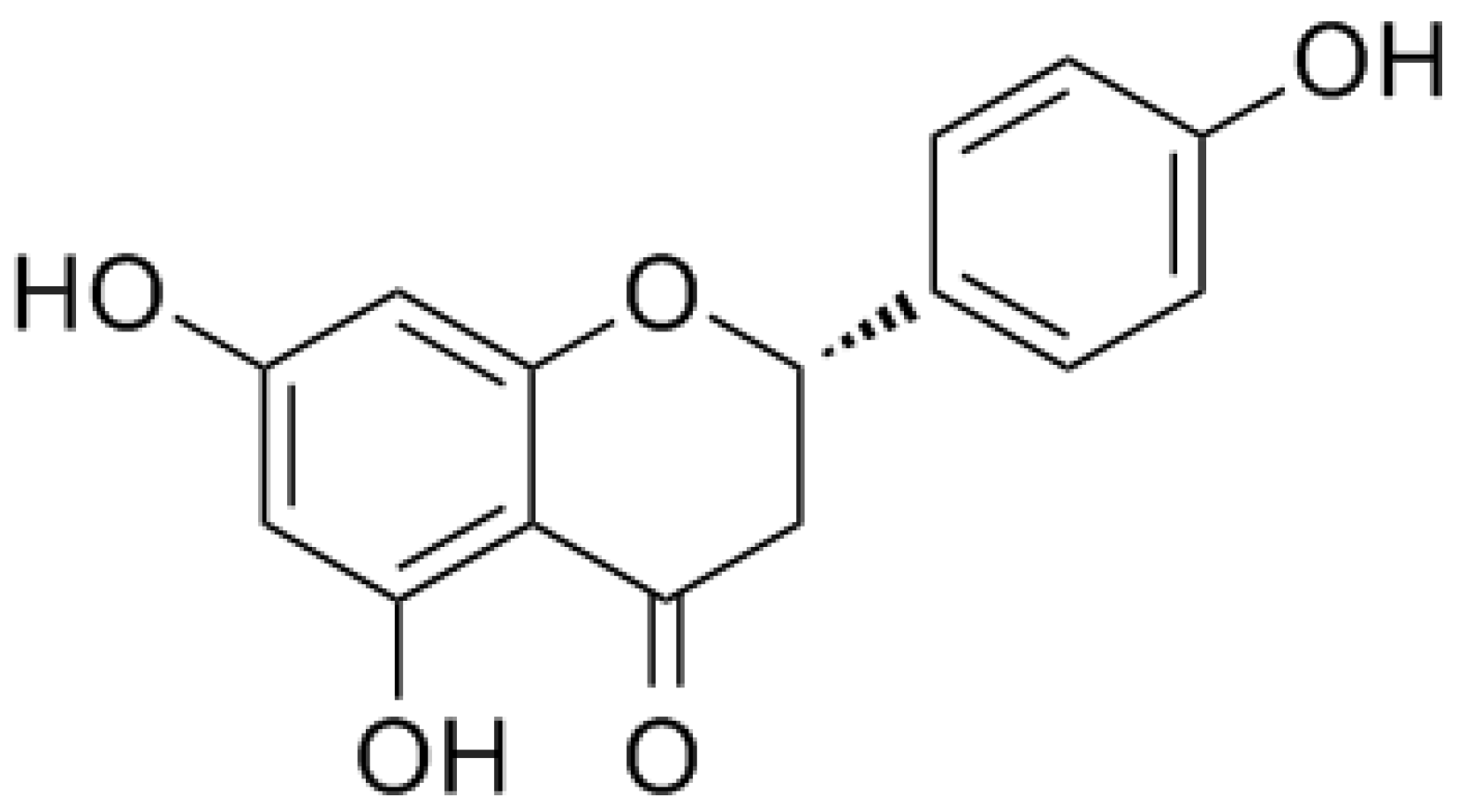
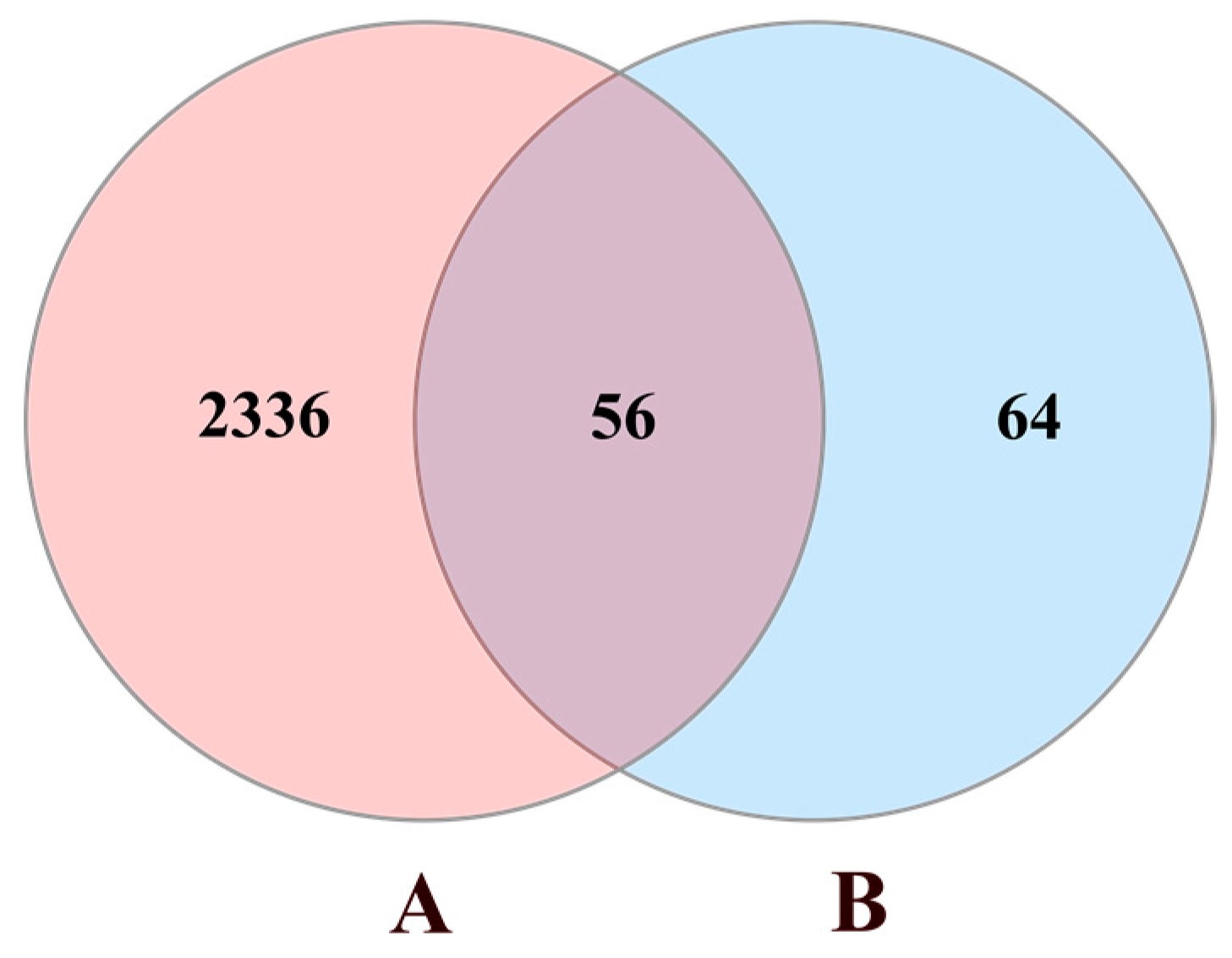
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Anti-inflammation | In vivo | LPS-induced acute lung injury mice | Naringin; 15, 30, and 60 mg/kg, p.o. | Pulmonary neutrophil infiltration and TNF-α, MPO, iNOS, and NF-κB activities ↓ | [23] |
| In vivo | CS-exposed rats | Naringin; 20, 40, and 80 mg/kg, p.o. | Infiltration of neutrophils and MPO, MMP-9, TNF-α, and IL-8 levels ↓; Level of IL-10 ↑ | [24] | |
| In vivo | Staphylococcus aureus-induced pneumonia mice | Naringenin; 100 mg/kg, i.h. | Pulmonary inflammation and inflammatory cells infiltration ↓ | [25] | |
| Both in vitro and in vivo | LPS-induced RAW 264.7 cell line; CLP-induced mice | Naringin; 50, 100, 200 μM (in vitro) 200 mg/kg, i.p. (in vivo) | TNF-α expression and HMGB1 release ↓; HO-1 expression via the AMPK-p38-Nrf2 pathway ↓ (in vitro) Lung injury ↓; TNF-α and HMGB1 expression ↓ (in vivo) | [26] | |
| Both in vitro and in vivo | CS-exposed A549 cell line and mice | Naringenin; 2, 20, 50 mM (in vitro) 20, 40, and 80 mg/kg, p.o. (in vivo) | NF-κB activity ↓; Levels of GR mRNA and protein ↑ (in vitro) Inflammatory cells and the production of IL-8, TNF-α, and MMP-9 ↓ (in vivo) | [33] | |
| In vivo | CS-exposed chronic bronchitis guinea pigs | Naringin; 9.2, 18.4 and 36.8 mg/kg, p.o. | Levels of IL-8 and TNF-α and MPO ↓ | [34] | |
| In vivo | LPS-induced acute lung injury mice | Naringenin; 100 mg/kg, p.o. | Pulmonary edema, neutrophil infiltration and the levels of TNF-α, IL-1β, IL-6, and MIP-2 ↓; The activities of PI3K and AKT ↓ | [36] | |
| In vivo | Radiation-induced lung injury mice | Naringenin; 100 and 200 mg/kg, p.o. | Level of IL-1β ↓ | [37] | |
| In vivo | LPS-induced acute lung injury rats | Naringenin; 50 and 100 mg/kg, p.o. | Levels of IL-6, MPO, TNF-α, and caspase-3 ↓; HSP70 expression ↑ | [12] | |
| In vivo | Carrageenan-induced pleurisy mice | Naringin; 40 and 80 mg/kg, p.o. | Th1 cytokines (TNF-α, IL-2, IL-6, and IL-17) ↓; NF-κB and STAT3 activities↓; Th2 cytokines (IL-4 and IL-10) ↑ | [46] | |
| In vitro | LPS-induced RAW 264.7 cell line | Naringin; 50, 100, and 200 μM | Secretion of IL-8, MCP-1 and MIP-1α ↓; NF-κB and MAPK activities ↓ | [49] | |
| In vivo | Allergen-induced asthma mice | Naringenin; 25, 50, and 100 mg/kg, i.p. | Levels of CCL5 and CCL11 and NF-κB activity ↓ | [50] | |
| In vitro | LPS-induced acute lung injury mice | Naringenin; 100 μM | TSLP production and levels of RIP-2 and caspase-1 ↓ | [53] | |
| In vitro | LPS-induced RAW 264.7 cell line | Naringenin NPs; 25μg/mL | NF-κB and MAPK activities ↓; Levels of TNF-α, IL-6, MCP-1, and IL-1β ↓ | [55] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Antioxidation | In vivo | LPS-induced acute lung injury mice | Naringenin; 100 mg/kg, p.o. | Levels of H2O2 and MDA ↓ | [36] |
| In vivo | Benzo[a]pyrene-induced rats | Naringenin; 100 mg/kg, p.o. | Levels of GSH, GPx, GST, GR, SOD, CAT, and XO ↑; Expression of COX-2 through blockage of NF-κB ↓ | [66] | |
| In vitro | Paraquat-induced BEAS-2B cell line | Naringenin; 100 μM | Generation of ROS ↓; Antioxidant-related genes including GPX2, GPX3, GPX5, and GPX7 and Nrf2 activity ↑ | [67] | |
| In vivo | Wood smoke-exposed rats | Naringin; 80 mg/kg, p.o. | The activities of SOD and CAT ↑; Levels of NO ↓ | [70] | |
| In vivo | Monocrotaline-induced pulmonary hypertension rats | Naringenin; 50 mg/kg, p.o. | GSH content and eNOS protein expression ↑; Expression of iNOS ↓ | [71] | |
| In vitro | LPS-induced RAW 264.7 cell line | Naringenin NPs; 25 μg/mL | Expression of iNOS and COX-2 and the production of NO ↓ | [55] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Anti-Airway Remodeling | In vivo | House dust mite-induced asthma mice | Naringenin; 9 mg/mL, p.o. | Subepithelial fibrosis and smooth muscle hypertrophy ↓ | [76] |
| In vivo | Ovalbumin-induced asthma mice | Naringin; 5 and 10 mg/kg, p.o. | Mean airway resistance and the level of IgE ↓ Percentage of Th1/Th2 cells ↑ | [80] | |
| In vivo | Ovalbumin-induced asthma mice | Naringenin; 50 mg/kg, i.p. | Area of airway fibrosis and the levels of Th2 cytokines ↓ | [81] | |
| In vivo | CS-exposed rats | Naringin; 20, 40, and 80 mg/kg, p.o. | Thickening of the bronchial wall ↓ | [24] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Anti-Pulmonary Fibrosis | In vivo | Paraquat-induced pulmonary fibrosis mice | Naringin; 60 and 120 mg/kg, p.o. | Expression of TNF-α, MMP-9, and TIMP-1 and the pulmonary fibrosis deposition ↓ | [86] |
| In vivo | Bleomycin-induced fibrosis rats | 80 mg/kg, p.o. | Levels of HYP and lung collagen content ↓ | [88] | |
| Both in virto and in vivo | Mycoplasma pneumoniae-induced BEAS-2B cell line and pneumonia mice | Naringenin; 100 μM (in vitro) 100 mg/kg, p.o. (in vivo) | Fibrosis-related proteins (TGF-β, α-SMA, collagen I and collagen III) expression and autophagy ↓ (in vitro) Level of TGF-β and autophagy relative protein LC3 and Beclin-1 expression ↓ (in vivo) | [90] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Expectorant | In vivo | Several animal models | Naringenin; 30–67 mg/kg, p.o. | Volume of airway secretions ↑ (mice); Mucociliary clearability and tracheal mucociliary velocity ↑ (pigeons); Mucin secretion ↓ (rats) | [95] |
| In vitro | EGF-induced A549 cell line | Naringenin; 30 and 100 μM | Expression of MUC5AC and phosphorylation of EGF receptor, MAPK, ERK1/2, JNK, NF-κB p65, and AP1 ↓ | [98] | |
| In vitro | Human neutrophil elastase induced-human airway epithelial cell line | Naringenin; 100 μM | MUC5AC expression, production of ROS and NF-κB activity ↓ | [99] | |
| In vivo | LPS-induced mice and beagle dogs | Naringin; 15 and 60 mg/kg, p.o. (mice); 12.4 mg/kg, p.o. (beagle dogs) | Expression of MUC5AC and goblet cell hyperplasia ↓ (mice); Sputum volume ↓ and elasticity and viscosity of sputum ↑ (beagle dogs) | [102] | |
| In vitro | LPS-induced airway epithelial cell and Calu-3 cell line | Naringenin; 100 μM | CFTR expression ↑ by Na+-K+-2Cl− co-transporters and K+ channels and regulated by intracellular cAMP | [103] | |
| Both in vitro and in vivo | DPM-induced Calu-3 cell line and mice | Naringenin; 25, 50, 100 μM (in vitro); Naringin; 30, 60, and 120 mg/kg, p.o. (in vivo) | Liquid viscosity, MUC5AC and total protein secretion ↓; CFTR, AQP1, and AQP5 expression and intracellular cAMP ↑ | [104] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Antitussive | In vivo | CS-exposed guinea pigs | Naringin; 18.4 mg/kg, p.o. | Airway hyperresponsiveness, chronic cough and expression of SP content, NK-1 receptor and NEP activity ↓ | [107] |
| In vivo | Different cough guinea pig models | Naringin; 15, 30, and 60 mg/kg, i.v. 0.5, 1.0, and 2.0 µM, i.c.v. | Exerted peripheral antitussive effects | [108] | |
| In vivo | Capsaicin-induced cough-variant asthma guinea pigs | Naringin; 18.4 mg/kg, p.o. | Airway hyperresponsiveness and cough ↓ | [111] |
| NO | Gene Name | Protein Name | Degree | NO | Gene Name | Protein Name | Degree |
|---|---|---|---|---|---|---|---|
| 1 | AKT1 | RAC-alpha serine/threonine-protein kinase | 42 | 29 | MMP3 | Stromelysin-1 | 14 |
| 2 | VEGFA | Vascular endothelial growth factor A | 41 | 30 | NOX4 | NADPH oxidase 4 | 14 |
| 3 | MAPK3 | Mitogen-activated protein kinase 3 | 37 | 31 | PPARA | Peroxisome proliferator-activated receptor alpha | 13 |
| 4 | PTGS2 | Prostaglandin G/H synthase 2 | 34 | 32 | HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | 13 |
| 5 | ESR1 | Estrogen receptor | 33 | 33 | INSR | Insulin receptor | 12 |
| 6 | MAPK1 | Mitogen-activated protein kinase 1 | 33 | 34 | MMP13 | Collagenase 3 | 12 |
| 7 | CASP3 | Caspase-3 | 33 | 35 | GSTP1 | Glutathione S transferase P | 12 |
| 8 | SRC | Proto-oncogene tyrosine-protein kinase Src | 30 | 36 | LDLR | Low-density lipoprotein receptor | 11 |
| 9 | MMP2 | 72 kDa type IV collagenase | 28 | 37 | KIT | Mast/stem cell growth factor receptor Kit | 11 |
| 10 | CAT | Catalase | 24 | 38 | CYP1B1 | Cytochrome P450 1B1 | 11 |
| 11 | SERPINE1 | Plasminogen activator inhibitor 1 | 24 | 39 | PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | 10 |
| 12 | APP | Amyloid-beta precursor protein | 24 | 40 | CYP2C9 | Cytochrome P450 2C9 | 10 |
| 13 | KDR | Vascular endothelial growth factor receptor 2 | 22 | 41 | F3 | Tissue factor | 10 |
| 14 | ADIPOQ | Adiponectin | 22 | 42 | GSR | Glutathione reductase, mitochondrial | 9 |
| 15 | PPARG | Peroxisome proliferator-activated receptor gamma | 22 | 43 | FGFR1 | Fibroblast growth factor receptor 1 | 9 |
| 16 | PIK3CA | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | 21 | 44 | SHBG | Sex hormone-binding globulin | 9 |
| 17 | BCL2L1 | Bcl-2-like protein 1 | 21 | 45 | BCL2 | Apoptosis regulator Bcl-2 | 8 |
| 18 | IGF1R | Insulin-like growth factor 1 receptor | 20 | 46 | UGT1A1 | UDP-glucuronosyltransferase 1A1 | 8 |
| 19 | SOD1 | Superoxide dismutase | 18 | 47 | EDNRA | Endothelin-1 receptor | 7 |
| 20 | APOB | Apolipoprotein B-100 | 18 | 48 | PLA2G2A | Phospholipase A2 | 7 |
| 21 | CYP3A4 | Cytochrome P450 3A4 | 18 | 49 | CTSL | Procathepsin L | 7 |
| 22 | IGFBP3 | Insulin-like growth factor-binding protein 3 | 18 | 50 | SYK | Tyrosine-protein kinase SYK | 6 |
| 23 | CYP19A1 | Aromatase | 18 | 51 | VCP | Transitional endoplasmic reticulum ATPase | 6 |
| 24 | ABCG2 | Broad substrate specificity ATP-binding cassette transporter ABCG2 | 17 | 52 | PTGS1 | Prostaglandin G/H synthase 1 | 6 |
| 25 | RELA | Transcription factor p65 | 16 | 53 | ADORA1 | Adenosine receptor A1 | 5 |
| 26 | HNF4A | Hepatocyte nuclear factor 4-alpha | 16 | 54 | BCHE | Cholinesterase | 5 |
| 27 | MET | Hepatocyte growth factor receptor | 15 | 55 | MMP12 | Macrophage metalloelastase | 2 |
| 28 | ESR2 | Estrogen receptor beta | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Chen, P.; Wu, H.; Shi, R.; Su, W.; Wang, Y.; Li, P. Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules 2020, 10, 1644. https://doi.org/10.3390/biom10121644
Chen Z, Chen P, Wu H, Shi R, Su W, Wang Y, Li P. Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules. 2020; 10(12):1644. https://doi.org/10.3390/biom10121644
Chicago/Turabian StyleChen, Zhen, Pan Chen, Hao Wu, Rui Shi, Weiwei Su, Yonggang Wang, and Peibo Li. 2020. "Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology" Biomolecules 10, no. 12: 1644. https://doi.org/10.3390/biom10121644
APA StyleChen, Z., Chen, P., Wu, H., Shi, R., Su, W., Wang, Y., & Li, P. (2020). Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules, 10(12), 1644. https://doi.org/10.3390/biom10121644





