Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology
Abstract
:1. Introduction
1.1. Chronic Obstructive Pulmonary Disease
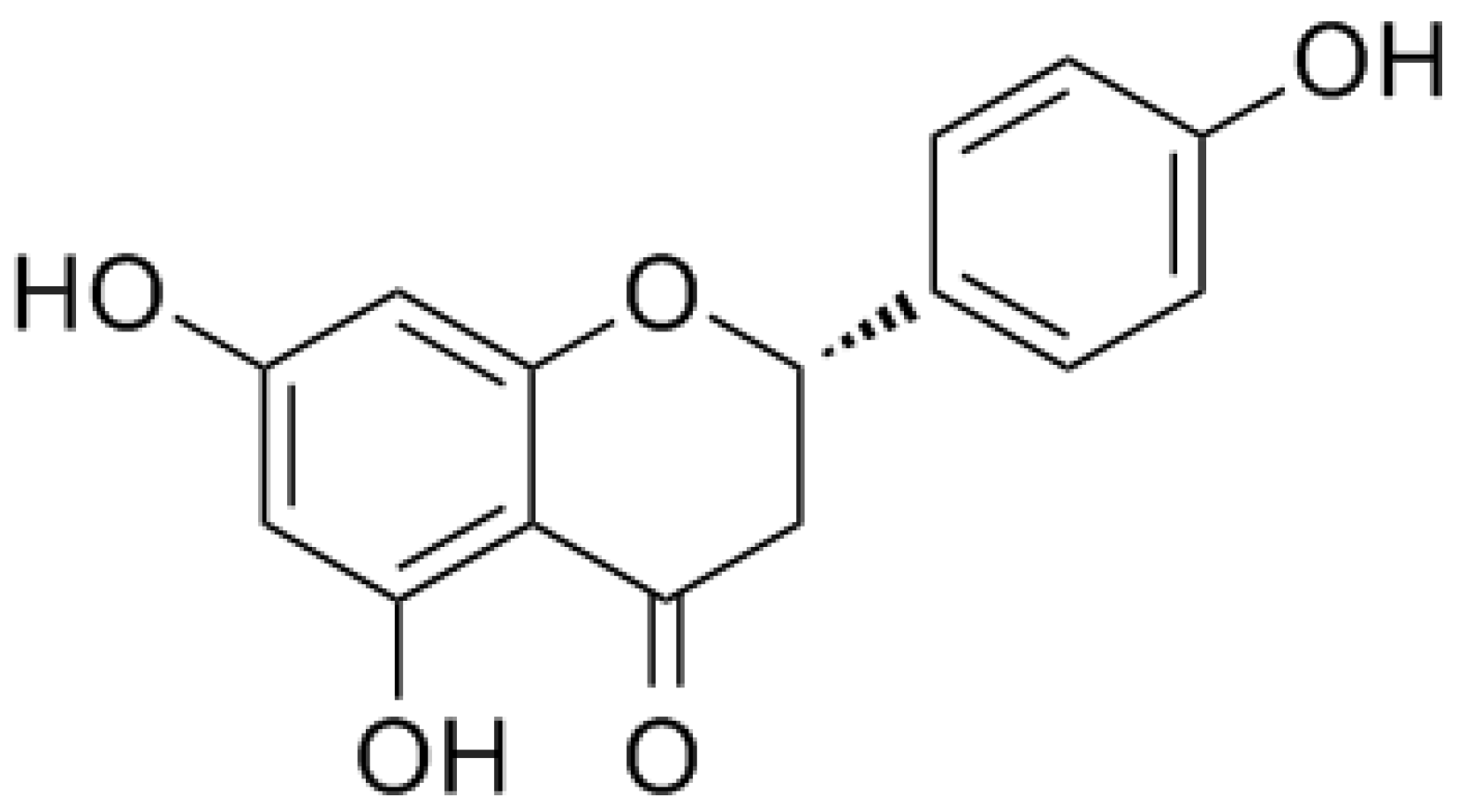
1.2. Naringenin and its Glycoside Naringin
2. Potential Pharmacological Effects of Naringenin in COPD
2.1. Anti-Inflammatory Activity
2.2. Antioxidative Activity
2.3. Anti-Airway Remodeling Activity
2.4. Anti-Pulmonary Fibrosis Activity
2.5. Expectorant
2.6. Antitussive
3. Network Pharmacology
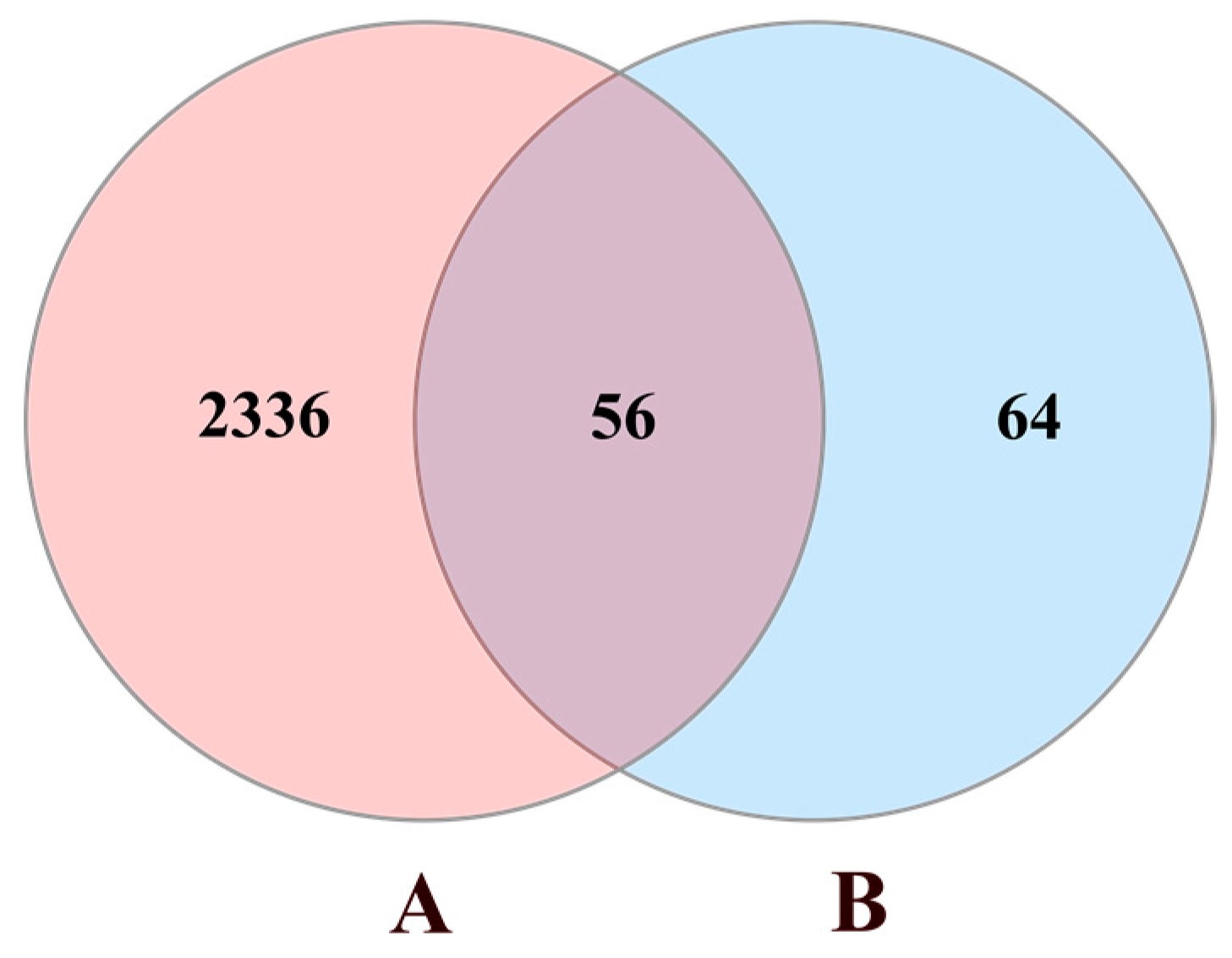
3.1. Data Preparation
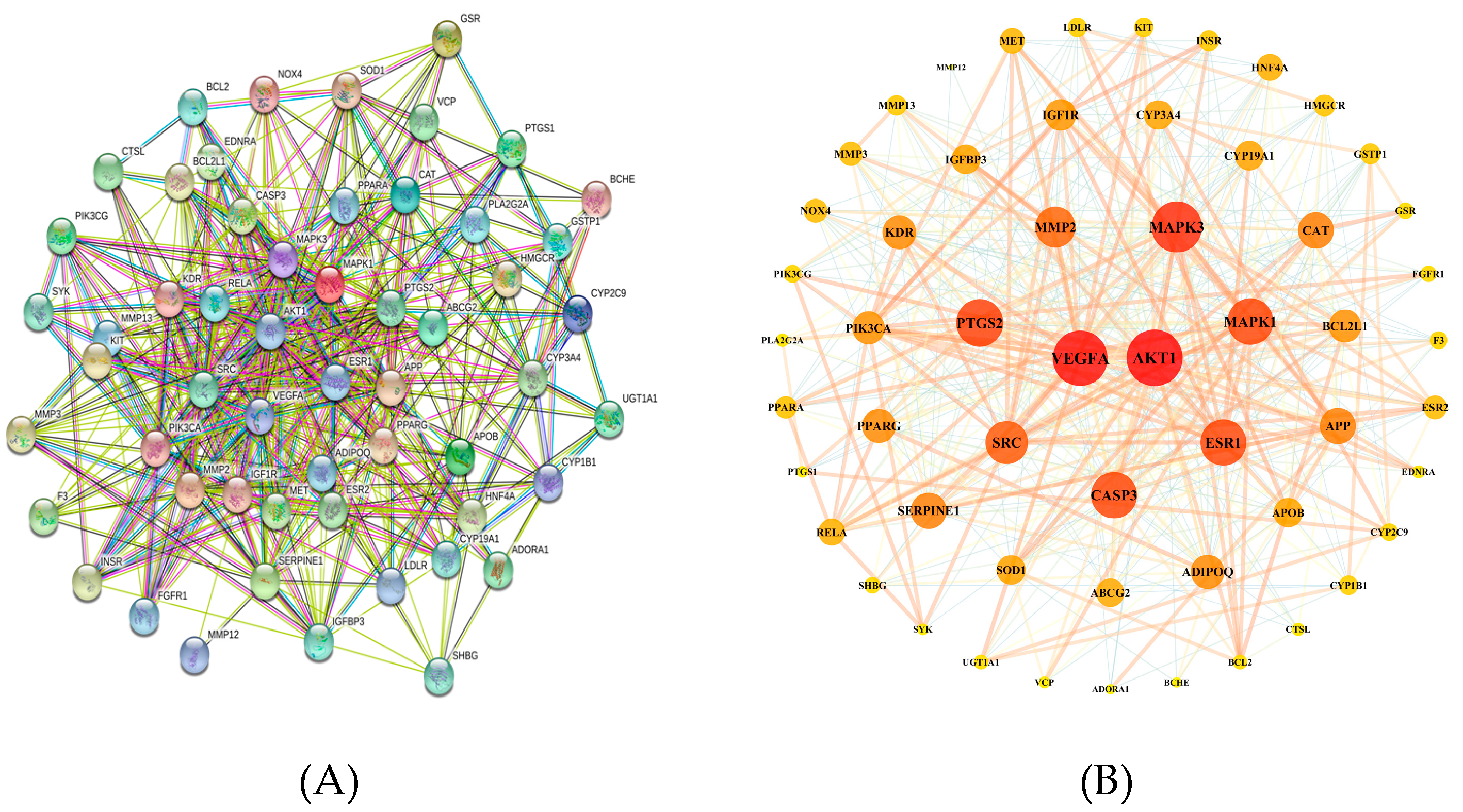
3.2. Protein–Protein Interaction (PPI) Network Construction
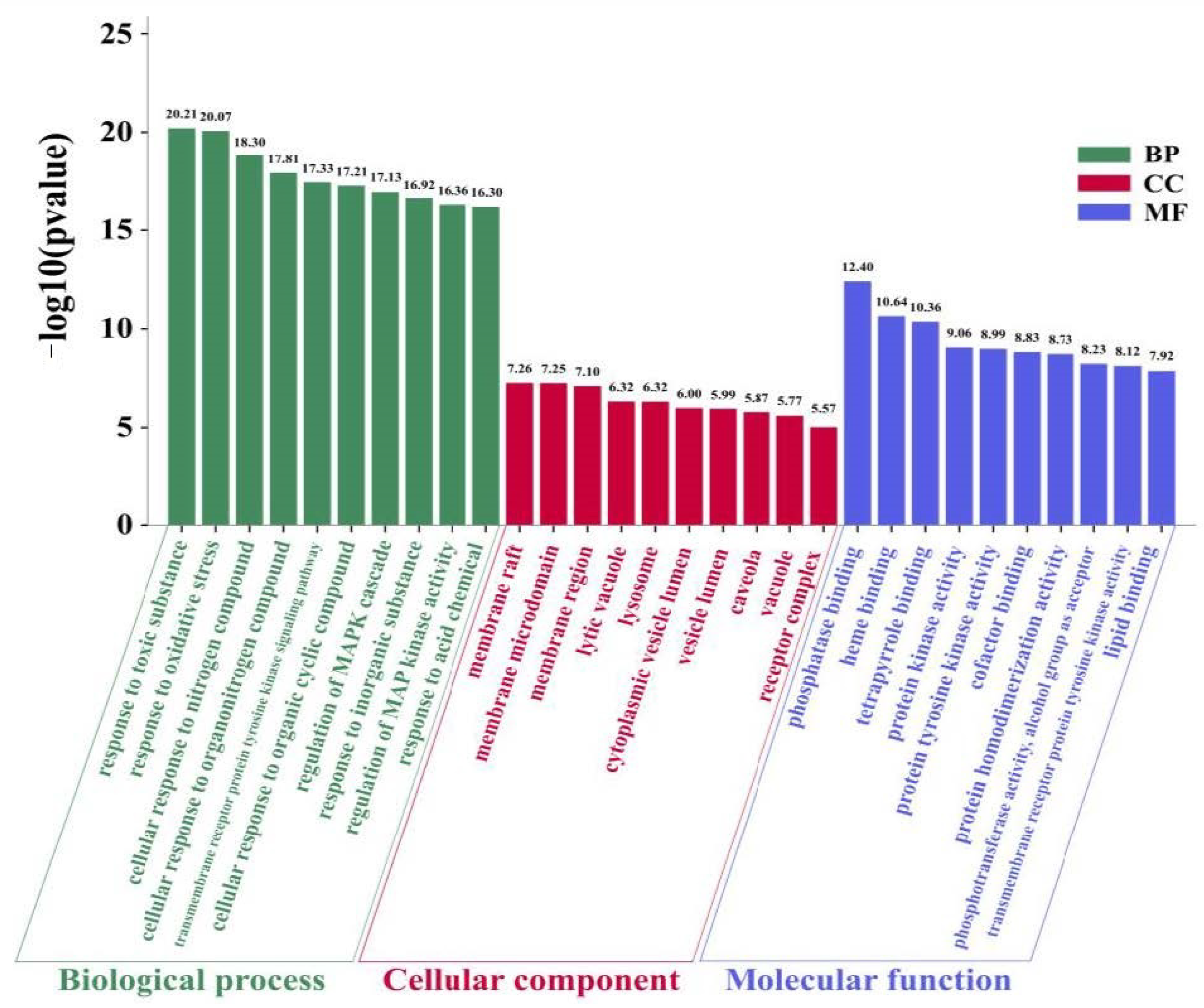
3.3. GO and KEGG Pathway Enrichment Analysis
3.4. Analysis of miRNA-Mediated Naringenin in the Treatment of COPD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| CS | cigarette smoke |
| LPS | lipopolysaccharide |
| CLP | cecum ligation and puncture |
| MMP | matrix metalloproteinase |
| BALF | bronchoalveolar lavage fluid |
| ECM | extracellular matrix |
| PI3K | phosphatidylinositol 3-kinase |
| AKT | protein kinase B |
| MCP-1 | monocyte chemoattractant protein-1 |
| MIP-1α | macrophage inflammatory protein-1α |
| TSLP | thymic stromal lymphopoietin |
| RIP-2 | receptor-interacting protein-2 |
| PVP | polyvinyl pyrrolidone |
| NPs | nanoparticles |
| MAPK | P38 mitogen-activated protein kinase |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutases |
| CAT | catalases |
| XO | xanthine oxidase |
| GPx | glutathione peroxidases |
| GSH | glutathione |
| GST | glutathione s-transferase |
| GR | glutathione reductase |
| COX-2 | cyclooxygenase-2 |
| iNOS | inducible nitric oxide synthase |
| eNOS | endothelial nitric oxide synthase |
| TIMP-1 | tissue inhibitor of metalloproteinase-1 |
| HYP | hydroxyproline |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| SP | substance P |
| NK-1 | neurokinin-1 |
| NEP | neutral endopeptidase |
| PPI | protein-protein interaction |
| NT-CTs | naringenin targets-COPD targets |
| GO | Gene Ontology |
| BP | biological progress |
| CC | cellular component |
| MF | molecular function |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PTEN | phosphatase and tensin homolog deleted from chromosome ten |
| VEGF | vascular endothelial growth factor |
References
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.; Guimaraes, M.J.; van Zeller, M.; Menezes, F.; Moita, J.; Simao, P. Clinical and molecular markers in COPD. Pulmonology 2018, 24, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.B. The roles of endothelin and its receptors in cigarette smoke-associated pulmonary hypertension with chronic lung disease. Pathol. Res. Pract. 2020, 216, 153083. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.; Han, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Belchamber, K.; Donnelly, L.E. Targeting defective pulmonary innate immunity—A new therapeutic option? Pharmacol. Ther. 2020, 209, 107500. [Google Scholar] [CrossRef]
- Castaldi, P.J.; Dy, J.; Ross, J.; Chang, Y.; Washko, G.R.; Curran-Everett, D.; Williams, A.; Lynch, D.A.; Make, B.J.; Crapo, J.D.; et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax 2014, 69, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, M.; Rogliani, P.; Stolz, D.; Matera, M.G. Pharmacological treatment and current controversies in COPD. F1000Res. 2019, 8, 1533. [Google Scholar] [CrossRef] [Green Version]
- Hendershott, C.H.; Walker, D.R. Identification of a growth inhibitor from extracts of dormant peach flower buds. Science 1959, 130, 798–800. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Liu, B.; Chai, L.; Shi, R.; Yao, H. A Review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases. Mini-Rev. Med. Chem. 2020, 20, 286–293. [Google Scholar] [CrossRef]
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.H.; Hon, C.M.; Chellappan, D.K.; Chellian, J.; Madheswaran, T.; Zeeshan, F.; Awasthi, R.; Aljabali, A.A.; Tambuwala, M.M.; Dureja, H.; et al. Molecular mechanisms of action of naringenin in chronic airway diseases. Eur. J. Pharmacol. 2020, 879, 173139. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Jresat, I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology 2016, 97, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals. 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based approaches in pharmacology. Mol. Inform. 2017, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology, and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.S.; Sohal, S.S. Update on the pathogenesis of COPD. N. Engl. J. Med. 2019, 381, 2483–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wu, H.; Nie, Y.C.; Chen, J.L.; Su, W.W.; Li, P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-kappaB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wu, H.; Li, P.; Luo, Y.; Long, K.; Xie, L.; Shen, J.; Su, W. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J. Med. Food 2012, 15, 894–900. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.F.; Dong, J.; Wei, J.Y.; Wang, Y.N.; Dai, X.H.; Wang, X.; Luo, M.J.; Tan, W.; Deng, X.M.; et al. Inhibition of alpha-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia 2013, 86, 92–99. [Google Scholar] [CrossRef]
- Gil, M.; Kim, Y.K.; Hong, S.B.; Lee, K.J. Naringin decreases TNF-alpha and HMGB1 release from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. PLoS ONE 2016, 11, e0164186. [Google Scholar] [CrossRef]
- Huang, S.; Ding, Z.; Xiang, H.; Fu, L.; Fei, J. Association between serum S100A8/S100A9 heterodimer and pulmonary function in patients with acute exacerbation of chronic obstructive pulmonary disease. Lung 2020, 198, 645–652. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, L.; Xie, Y.; Xu, Y.; Jiao, W.; Wu, J.; Deng, X.; Fang, G.; Xue, Q.; Zheng, Y.; et al. Th1/Th17 cytokine profiles are associated with disease severity and exacerbation frequency in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1287–1299. [Google Scholar] [CrossRef]
- Falfan-Valencia, R.; Ramirez-Venegas, A.; Perez, L.J.; Ramirez-Rodriguez, S.L.; Marquez-Garcia, J.E.; Buendia-Roldan, I.; Gayosso-Gomez, L.V.; Perez-Padilla, R.; Ortiz-Quintero, B. Smoke exposure from chronic biomass burning induces distinct accumulative systemic inflammatory cytokine alterations compared to tobacco smoking in healthy women. Cytokine 2020, 131, 155089. [Google Scholar] [CrossRef]
- Garth, J.; Barnes, J.W.; Krick, S. Targeting cytokines as evolving treatment strategies in chronic inflammatory airway diseases. Int. J. Mol. Sci. 2018, 19, 3402. [Google Scholar] [CrossRef] [Green Version]
- Mahler, D.A.; Huang, S.; Tabrizi, M.; Bell, G.M. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: A pilot study. Chest 2004, 126, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogliani, P.; Calzetta, L.; Ora, J.; Matera, M.G. Canakinumab for the treatment of chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2015, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, J.; Zhang, J. Naringenin attenuates inflammation in chronic obstructive pulmonary disease in cigarette smoke induced mouse model and involves suppression of NF-κB. J. Microbiol. Biotechnol. 2018, 30609878. [Google Scholar] [CrossRef]
- Luo, Y.L.; Zhang, C.C.; Li, P.B.; Nie, Y.C.; Wu, H.; Shen, J.G.; Su, W.W. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int. Immunopharmacol. 2012, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 2009, 41, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, C.; Shen, F.; Wang, M.; Jia, N.; Wang, C. Naringenin ameliorates LPS-induced acute lung injury through its anti-oxidative and anti-inflammatory activity and by inhibition of the PI3K/AKT pathway. Exp. Ther. Med. 2017, 14, 2228–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zeng, W.; Yao, Y.; Xu, B.; Wei, X.; Wang, L.; Yin, X.; Barman, A.K.; Zhang, F.; Zhang, C.; et al. Naringenin ameliorates radiation-induced lung injury by lowering IL-1beta level. J. Pharmacol. Exp. Ther. 2018, 366, 341–348. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Agarwal, A.R.; Kadam, S.; Brahme, A.; Agrawal, M.; Apte, K.; Narke, G.; Kekan, K.; Madas, S.; Salvi, S. Systemic Immuno-metabolic alterations in chronic obstructive pulmonary disease (COPD). Respir. Res. 2019, 20, 171. [Google Scholar] [CrossRef]
- Silva, B.; Lira, F.S.; Ramos, D.; Uzeloto, J.S.; Rossi, F.E.; Freire, A.; Silva, R.N.; Trevisan, I.B.; Gobbo, L.A.; Ramos, E. Severity of COPD and its relationship with IL-10. Cytokine 2018, 106, 95–100. [Google Scholar] [CrossRef] [Green Version]
- De Llano, L.P.; Cosio, B.G.; Iglesias, A.; de Las, C.N.; Soler-Cataluna, J.J.; Izquierdo, J.L.; Lopez-Campos, J.L.; Calero, C.; Plaza, V.; Miravitlles, M.; et al. Mixed Th2 and non-Th2 inflammatory pattern in the asthma—COPD overlap: A network approach. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.X.; Lu, L.W.; Liu, W.J.; Huang, M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) Overlap Syndrome. Med. Sci. Monit. 2016, 22, 2800–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brightling, C.E.; Bleecker, E.R.; Panettieri, R.A.; Bafadhel, M.; She, D.; Ward, C.K.; Xu, X.; Birrell, C.; van der Merwe, R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir. Med. 2014, 2, 891–901. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Hanania, N.A.; Noonan, M.; Corren, J.; Korenblat, P.; Zheng, Y.; Fischer, S.K.; Cheu, M.; Putnam, W.S.; Murray, E.; Scheerens, H.; et al. Lebrikizumab in moderate-to-severe asthma: Pooled data from two randomised placebo-controlled studies. Thorax 2015, 70, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Zoheir, K.M.; Ansari, M.A.; Korashy, H.M.; Abdel-Hamied, H.E.; Ashour, A.E.; Abd-Allah, A.R. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-kappab, STAT3 and pro-inflammatory mediators and enhancement of IkappaBalpha and anti-inflammatory cytokines. Inflammation 2015, 38, 846–857. [Google Scholar] [CrossRef]
- Bradford, E.; Jacobson, S.; Varasteh, J.; Comellas, A.P.; Woodruff, P.; O’Neal, W.; DeMeo, D.L.; Li, X.; Kim, V.; Cho, M.; et al. The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 2017, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Henrot, P.; Prevel, R.; Berger, P.; Dupin, I. Chemokines in COPD: From implication to therapeutic Use. Int. J. Mol. Sci. 2019, 20, 2785. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Su, W.W.; Wang, S.; Li, P.B. Naringin inhibits chemokine production in an LPS-induced RAW 264.7 macrophage cell line. Mol. Med. Rep. 2012, 6, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef]
- Redhu, N.S.; Gounni, A.S. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin. Exp. Allergy 2012, 42, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Fang, C.; Cousins, D.; Zhang, G.; Gu, S.; Gao, Z.; Shamji, B.; et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J. Immunol. 2008, 181, 2790–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, P.D.; Choi, I.H.; Kim, H.M. Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur. J. Pharmacol. 2011, 671, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in hepatic fibrosis. Biomed. Microdevices 2020, 22, 68. [Google Scholar] [CrossRef]
- Kumar, R.P.; Abraham, A. Inhibition of LPS induced pro-inflammatory responses in RAW 264.7 macrophage cells by PVP-coated naringenin nanoparticle via down regulation of NF-kappaB/P38MAPK mediated stress signaling. Pharmacol. Rep. 2017, 69, 908–915. [Google Scholar] [CrossRef]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar] [CrossRef] [Green Version]
- Moitra, S. N-acetylcysteine (NAC) in COPD: Benefits often lost in trials. QJM Int. J. Med. 2019, 112, 387–388. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Murata, K.; Fujimoto, K.; Kitaguchi, Y.; Horiuchi, T.; Kubo, K.; Honda, T. Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. J. Chronic Obstr. Pulm. Dis. 2014, 11, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Kostikas, K.; Papatheodorou, G.; Psathakis, K.; Panagou, P.; Loukides, S. Oxidative stress in expired breath condensate of patients with COPD. Chest 2003, 124, 1373–1380. [Google Scholar] [CrossRef]
- Stefanska, J.; Sarniak, A.; Wlodarczyk, A.; Sokolowska, M.; Doniec, Z.; Bialasiewicz, P.; Nowak, D.; Pawliczak, R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm. Pharmacol. Ther. 2012, 25, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Paska, C.; Simon, B.; Barta, I. Monitoring antioxidant enzyme activity during exacerbations of chronic obstructive pulmonary disease. J. Chronic Obstr. Pulm. Dis. 2018, 15, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, S.K.; Kumar, S.; Ahmad, M.K.; Nischal, A.; Singh, S.K.; Dixit, R.K. Evaluation of oxidative stress and antioxidant status in chronic obstructive pulmonary disease. Scand. J. Immunol. 2017, 85, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, M.D.; Busquets-Cortes, C.; Capo, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Mizumura, K.; Maruoka, S.; Shimizu, T.; Gon, Y. Role of Nrf2 in the pathogenesis of respiratory diseases. Respir. Investig. 2020, 58, 28–35. [Google Scholar] [CrossRef]
- Ali, R.; Shahid, A.; Ali, N.; Hasan, S.K.; Majed, F.; Sultana, S. Amelioration of benzo[a]pyrene-induced oxidative stress and pulmonary toxicity by naringenin in Wistar rats: A plausible role of COX-2 and NF-κB. Hum. Exp. Toxicol. 2017, 36, 349–364. [Google Scholar] [CrossRef]
- Podder, B.; Song, H.Y.; Kim, Y.S. Naringenin exerts cytoprotective effect against paraquat-induced toxicity in human bronchial epithelial BEAS-2B cells through NRF2 activation. J. Microbiol. Biotechnol. 2014, 24, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.T.; Liu, X.S.; Xu, Y.J.; Ni, W.; Chen, S.X. Expression of nitric oxide synthase isoenzyme in lung tissue of smokers with and without chronic obstructive pulmonary disease. Chin. Med. J. 2015, 128, 1584–1589. [Google Scholar] [CrossRef]
- Brindicci, C.; Kharitonov, S.A.; Ito, M.; Elliott, M.W.; Hogg, J.C.; Barnes, P.J.; Ito, K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2010, 181, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Akintunde, J.K.; Abioye, J.B.; Ebinama, O.N. Potential protective effects of naringin on oculo-pulmonary injury induced by PM10 (wood smoke) exposure by modulation of oxidative damage and acetylcholine esterase activity in a rat model. Curr. Ther. Res. Clin. Exp. 2020, 92, 100586. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Obaid, A.A.; Zaki, H.F.; Agha, A.M. Naringenin adds to the protective effect of L-arginine in monocrotaline-induced pulmonary hypertension in rats: Favorable modulation of oxidative stress, inflammation, and nitric oxide. Eur. J. Pharm. Sci. 2014, 62, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Quinn, A.M.; Cancado, J.; Singh, D. The pathology of small airways disease in COPD: Historical aspects and future directions. Respir. Res. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, T.; Wang, L.F.; Yin, Y.Q. How do innate immune cells contribute to airway remodeling in copd progression? Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.L.; Noble, P.B.; Elliot, J.G.; James, A.L. Airway remodelling in COPD: It’s not asthma! Respirology 2016, 21, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Martin, J.G. Mechanisms of airway remodeling. Chest 2013, 144, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Kolahian, S.; Shahbazfar, A.A.; Ansarin, K.; Pour, M.M.; Sakhinia, M.; Sakhinia, E.; Vafa, M. Effects of the flavanone combination hesperetin-naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother. Res. 2015, 29, 591–598. [Google Scholar] [CrossRef]
- Siddiqui, S.; Shikotra, A.; Richardson, M.; Doran, E.; Choy, D.; Bell, A.; Austin, C.D.; Eastham-Anderson, J.; Hargadon, B.; Arron, J.R.; et al. Airway pathological heterogeneity in asthma: Visualization of disease microclusters using topological data analysis. J. Allergy Clin. Immunol. 2018, 142, 1457–1468. [Google Scholar] [CrossRef]
- Qin, W.; Deng, T.; Cui, H.; Zhang, Q.; Liu, X.; Yang, X.; Chen, M. Exposure to diisodecyl phthalate exacerbated Th2 and Th17-mediated asthma through aggravating oxidative stress and the activation of p38 MAPK. Food Chem. Toxicol. 2018, 114, 78–87. [Google Scholar] [CrossRef]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and non-immunologic mechanisms leading to airway remodeling in asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef] [Green Version]
- Guihua, X.; Shuyin, L.; Jinliang, G.; Wang, S. Naringin protects ovalbumin-induced airway inflammation in a mouse model of asthma. Inflammation 2016, 39, 891–899. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, Y.; Mao, S.; Gu, W. Naringenin inhibits allergen-induced airway remodeling in a murine model of asthma. Mol. Med. Rep. 2014, 9, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kobayashi, S.; Hanagama, M.; Ishida, M.; Sato, H.; Makiguchi, T.; Yanai, M. Clinical characteristics of Japanese patients with chronic obstructive pulmonary disease (COPD) with comorbid interstitial lung abnormalities: A cross-sectional study. PLoS ONE 2020, 15, e0239764. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; McDonald, V.M.; Gibson, P.G. Comorbidity in chronic obstructive pulmonary disease. Respir. Investig. 2015, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunwald, B.; Schoeps, B.; Kruger, A. Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell. Biol. 2019, 29, 6–19. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Y.C.; Luo, Y.L.; Lin, F.; Zheng, Y.F.; Cheng, G.H.; Wu, H.; Zhang, K.J.; Su, W.W.; Shen, J.G.; et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 2013, 58, 133–140. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Turgut, N.H.; Kara, H.; Elagoz, S.; Deveci, K.; Gungor, H.; Arslanbas, E. The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar Rats. Pulm. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.G.; Thomas, B.; Koff, J. TGF-β: Master regulator of inflammation and fibrosis. Respirology 2018, 23, 1096–1097. [Google Scholar] [CrossRef]
- Lin, Y.; Tan, D.; Kan, Q.; Xiao, Z.; Jiang, Z. The protective effect of naringenin on airway remodeling after Mycoplasma pneumoniae infection by inhibiting autophagy-mediated lung inflammation and fibrosis. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou-Suckow, Z.; Duerr, J.; Hagner, M.; Agrawal, R.; Mall, M.A. Airway mucus, inflammation, and remodeling: Emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017, 367, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, F.; Lee, H.J.; Lee, C.J. Recent advances in the development of novel drug candidates for regulating the secretion of pulmonary mucus. Biomol. Ther. 2020, 28, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.F.; Ieni, A.; Hansbro, P.M.; Ruggeri, P.; Di Stefano, A.; Nucera, F.; Coppolino, I.; Monaco, F.; Tuccari, G.; Adcock, I.M.; et al. Role of the mucins in pathogenesis of COPD: Implications for therapy. Expert. Rev. Respir. Med. 2020, 14, 465–483. [Google Scholar] [CrossRef]
- Samsuzzaman, M.; Uddin, M.S.; Shah, M.A.; Mathew, B. Natural inhibitors on airway mucin: Molecular insight into the therapeutic potential targeting MUC5AC expression and production. Life Sci. 2019, 231, 116485. [Google Scholar] [CrossRef]
- Lin, B.Q.; Li, P.B.; Wang, Y.G.; Peng, W.; Wu, Z.; Su, W.W.; Ji, H. The expectorant activity of naringenin. Pulm. Pharmacol. Ther. 2008, 21, 259–263. [Google Scholar] [CrossRef]
- Padra, M.; Andersson, A.; Levanen, B.; Premaratne, P.; Asgeirsdottir, H.; Tengvall, S.; Christenson, K.; Stockfelt, M.; Bozinovski, S.; Yoshihara, S.; et al. Increased MUC1 plus a larger quantity and complex size for MUC5AC in the peripheral airway lumen of long-term tobacco smokers. Clin. Sci. 2020, 134, 1107–1125. [Google Scholar] [CrossRef]
- Li, J.; Ye, Z. The potential role and regulatory mechanisms of MUC5AC in chronic obstructive pulmonary disease. Molecules 2020, 25, 4437. [Google Scholar] [CrossRef]
- Nie, Y.C.; Wu, H.; Li, P.B.; Xie, L.M.; Luo, Y.L.; Shen, J.G.; Su, W.W. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IkappaB-NF-kappaB signaling pathways. Eur. J. Pharmacol. 2012, 690, 207–213. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-kappaB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol. Cell. Biochem. 2011, 351, 29–40. [Google Scholar] [CrossRef]
- Reid, A.T.; Veerati, P.C.; Gosens, R.; Bartlett, N.W.; Wark, P.A.; Grainge, C.L.; Stick, S.M.; Kicic, A.; Moheimani, F.; Hansbro, P.M.; et al. Persistent induction of goblet cell differentiation in the airways: Therapeutic approaches. Pharmacol. Ther. 2018, 185, 155–169. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Nie, Y.C.; Li, P.B.; Shen, J.G.; Su, W.W. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ. Toxicol. Pharmacol. 2014, 38, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ehre, C.; Ridley, C.; Thornton, D.J. Cystic fibrosis: An inherited disease affecting mucin-producing organs. Int. J. Biochem. Cell. Biol. 2014, 52, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Xiao, Z.T.; Zheng, Y.J.; Zhang, Y.L.; Xu, J.W.; Huang, J.H.; Zhou, W.L.; Li, P.B.; Su, W.W. Naringenin regulates CFTR activation and expression in airway epithelial cells. Cell. Physiol. Biochem. 2017, 44, 1146–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Su, W.W.; Zhu, Z.T.; Guan, M.Y.; Cheng, K.L.; Fan, W.Y.; Wei, G.Y.; Li, P.B.; Yang, Z.Y.; Yao, H.L. Regulation effects of naringin on diesel particulate matter-induced abnormal airway surface liquid secretion. Phytomedicine 2019, 63, 153004. [Google Scholar] [CrossRef]
- Crooks, M.G.; Brown, T.; Morice, A.H. Is cough important in acute exacerbations of COPD? Respir. Physiol. Neurobiol. 2018, 257, 30–35. [Google Scholar] [CrossRef]
- Song, W.J.; Chung, K.F. Pharmacotherapeutic options for chronic refractory cough. Expert Opin. Pharmacother. 2020, 21, 1345–1358. [Google Scholar] [CrossRef]
- Luo, Y.L.; Li, P.B.; Zhang, C.C.; Zheng, Y.F.; Wang, S.; Nie, Y.C.; Zhang, K.J.; Su, W.W. Effects of four antitussives on airway neurogenic inflammation in a guinea pig model of chronic cough induced by cigarette smoke exposure. Inflamm. Res. 2013, 62, 1053–1061. [Google Scholar] [CrossRef]
- Gao, S.; Li, P.; Yang, H.; Fang, S.; Su, W. Antitussive effect of naringin on experimentally induced cough in Guinea pigs. Planta Med. 2011, 77, 16–21. [Google Scholar] [CrossRef]
- Smith, J.A.; Badri, H. Cough: New pharmacology. J. Allergy Clin. Immunol. Pract. 2019, 7, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Bidan, C.M.; Brook, B.S.; Zuidhof, A.B.; Elzinga, C.; Smit, M.; Oldenburger, A.; Gosens, R.; Timens, W.; Meurs, H. Small airway hyperresponsiveness in COPD: Relationship between structure and function in lung slices. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2019, 316, L537–L546. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.Y.; Su, W.W.; Li, P.B.; Liao, Y.; Zhou, Q.; Zhu, N.; He, L.L. Therapeutic effects of naringin in a guinea pig model of ovalbumin-induced cough-variant asthma. Pulm. Pharmacol. Ther. 2015, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Pirozzi, F.; Ren, K.; Murabito, A.; Ghigo, A. PI3K signaling in chronic obstructive pulmonary disease: Mechanisms, targets, and therapy. Curr. Med. Chem. 2019, 26, 2791–2800. [Google Scholar] [CrossRef]
- Hosgood, H.D., 3rd; Menashe, I.; He, X.; Chanock, S.; Lan, Q. PTEN identified as important risk factor of chronic obstructive pulmonary disease. Respir. Med. 2009, 103, 1866–1870. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Chen, L.; He, Z. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr. Drug Metab. 2019, 20, 301–304. [Google Scholar] [CrossRef]
- Lu, J.; Xie, L.; Liu, C.; Zhang, Q.; Sun, S. PTEN/PI3k/AKT regulates macrophage polarization in emphysematous mice. Scand. J. Immunol. 2017, 85, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, S.; Baker, J.R.; Vuppusetty, C.; Fenwick, P.; Donnelly, L.E.; Ito, K.; Barnes, P.J. Decreased phosphatase PTEN amplifies PI3K signaling and enhances proinflammatory cytokine release in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L230–L239. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Lin, J.; Cui, W.; Kong, Q.; Li, Q.; Li, L.; Wei, Y.; Dong, J. Scutellaria baicalensis attenuates airway remodeling via PI3K/Akt/NF-kappaB pathway in cigarette smoke mediated-COPD rats model. Evid. Based Complement Alternat. Med. 2018, 2018, 1281420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Ma, H.; Wang, Z.L.; Li, W.H.; Liu, H.; Zhao, Y.X. The PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J. Int. Med. Res. 2020, 48, 1220727471. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Du, J.; Meng, Y.; Guo, F.; Feng, C. Louqin Zhisou decoction inhibits mucus hypersecretion for acute exacerbation of chronic obstructive pulmonary disease rats by suppressing EGFR-PI3K-AKT signaling pathway and restoring Th17/Treg balance. Evid. Based Complement Alternat. Med. 2019, 2019, 6471815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, M.; Oiso, Y.; Sakai, H.; Motomura, T.; Yamashita, C. Pulmonary administration of phosphoinositide 3-kinase inhibitor is a curative treatment for chronic obstructive pulmonary disease by alveolar regeneration. J. Control. Release 2015, 213, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Marwick, J.A.; Caramori, G.; Casolari, P.; Mazzoni, F.; Kirkham, P.A.; Adcock, I.M.; Chung, K.F.; Papi, A. A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2010, 125, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, X.; Zheng, D.; Li, A.; Li, C.; Li, S.; Zhao, Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress, and apoptosis in vitro and in vivo. Clin. Sci. 2019, 133, 1523–1536. [Google Scholar] [CrossRef]
- Sun, Y.; An, N.; Li, J.; Xia, J.; Tian, Y.; Zhao, P.; Liu, X.; Huang, H.; Gao, J.; Zhang, X. miRNA-206 regulates human pulmonary microvascular endothelial cell apoptosis via targeting in chronic obstructive pulmonary disease. J. Cell. Biochem. 2019, 120, 6223–6236. [Google Scholar] [CrossRef]
- Csoma, B.; Bikov, A.; Nagy, L.; Toth, B.; Tabi, T.; Szucs, G.; Komlosi, Z.I.; Muller, V.; Losonczy, G.; Lazar, Z. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir. Res. 2019, 20, 156. [Google Scholar] [CrossRef]
- Arif, E.; Ahsan, A.; Vibhuti, A.; Rajput, C.; Deepak, D.; Athar, M.; Singh, B.; Pasha, M.A. Endothelial nitric oxide synthase gene variants contribute to oxidative stress in COPD. Biochem. Biophys. Res. Commun. 2007, 361, 182–188. [Google Scholar] [CrossRef]
- Schuliga, M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. longev. 2019, 7090534. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Sim, W.L.; Cheong, J.K.; Kuan, W.S.; Tran, T.; Lim, H.F. MicroRNAs in chronic airway diseases: Clinical correlation and translational applications. Pharmacol. Res. 2020, 160, 105045. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Di Lorenzo, A.; Rossi, D.; Martino, E.; Capelli, E.; Collina, S.; Daglia, M. Enantioselective modulatory effects of naringenin enantiomers on the expression levels of miR-17-3p involved in endogenous antioxidant defenses. Nutrients 2017, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, M.W.; Yang, W.; Wan, L.J.; Yan, H.L.; Li, J.C.; Tang, S.Y.; Wang, Y.Q. Naringenin induces neuroprotection against homocysteine-induced PC12 cells via the upregulation of superoxide dismutase 1 expression by decreasing miR-224-3p expression. J. Biol. Regul. Homeost. Agents. 2020, 34, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.N.; Zou, X.; Fang, X.H.; Xu, J.D.; Xiao, Z.; Zhu, J.N.; Li, H.; Yang, J.; Zeng, N.; Yuan, S.J.; et al. The Smad3-miR-29b/miR-29c axis mediates the protective effect of macrophage migration inhibitory factor against cardiac fibrosis. Biochim. Biophy. Acta Mol. Basis Dis. 2019, 1865, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.B.; Tang, P.F.; Zhang, W.; Zhao, Y.P.; Zhang, L.C.; Zhang, H. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene 2016, 592, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Wen, L.; Peng, R.; Li, H.; Liu, H.; Peng, H.; Sun, Y.; Wu, T.; Chen, L.; Duan, Q.; et al. Naringenin ameliorated kidney injury through Let-7a/TGFBR1 signaling in diabetic nephropathy. J. Diabetes Res. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, C.; Zhao, H. Defective insulin receptor signaling in patients with gestational diabetes is related to dysregulated miR-140 which can be improved by naringenin. Int. J. Biochem. Cell. Biol. 2020, 128, 105824. [Google Scholar] [CrossRef]
- Yu, Z.G.; Wang, B.Z.; Cheng, Z.Z. The association of genetic polymorphisms of hypoxia inducible factor-1 alpha and vascular endothelial growth factor with increased risk of chronic obstructive pulmonary disease: A case-control study. Kaohsiung J. Med. Sci. 2017, 33, 433–441. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Matarese, A.; Santulli, G. Angiogenesis in chronic obstructive pulmonary disease: A translational appraisal. Transl. Med. UniSa 2012, 3, 49–56. [Google Scholar] [PubMed]
- Bakakos, P.; Patentalakis, G.; Papi, A. Vascular biomarkers in asthma and COPD. Curr. Top. Med. Chem. 2016, 16, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Shi, R.; Zheng, Y.; Zeng, X.; Fan, W.; Wang, Y.; Su, W. Characterization, in vitro and in vivo evaluation of naringenin-hydroxypropyl-ß-cyclodextrin inclusion for pulmonary delivery. Molecules 2020, 25, 554. [Google Scholar] [CrossRef]
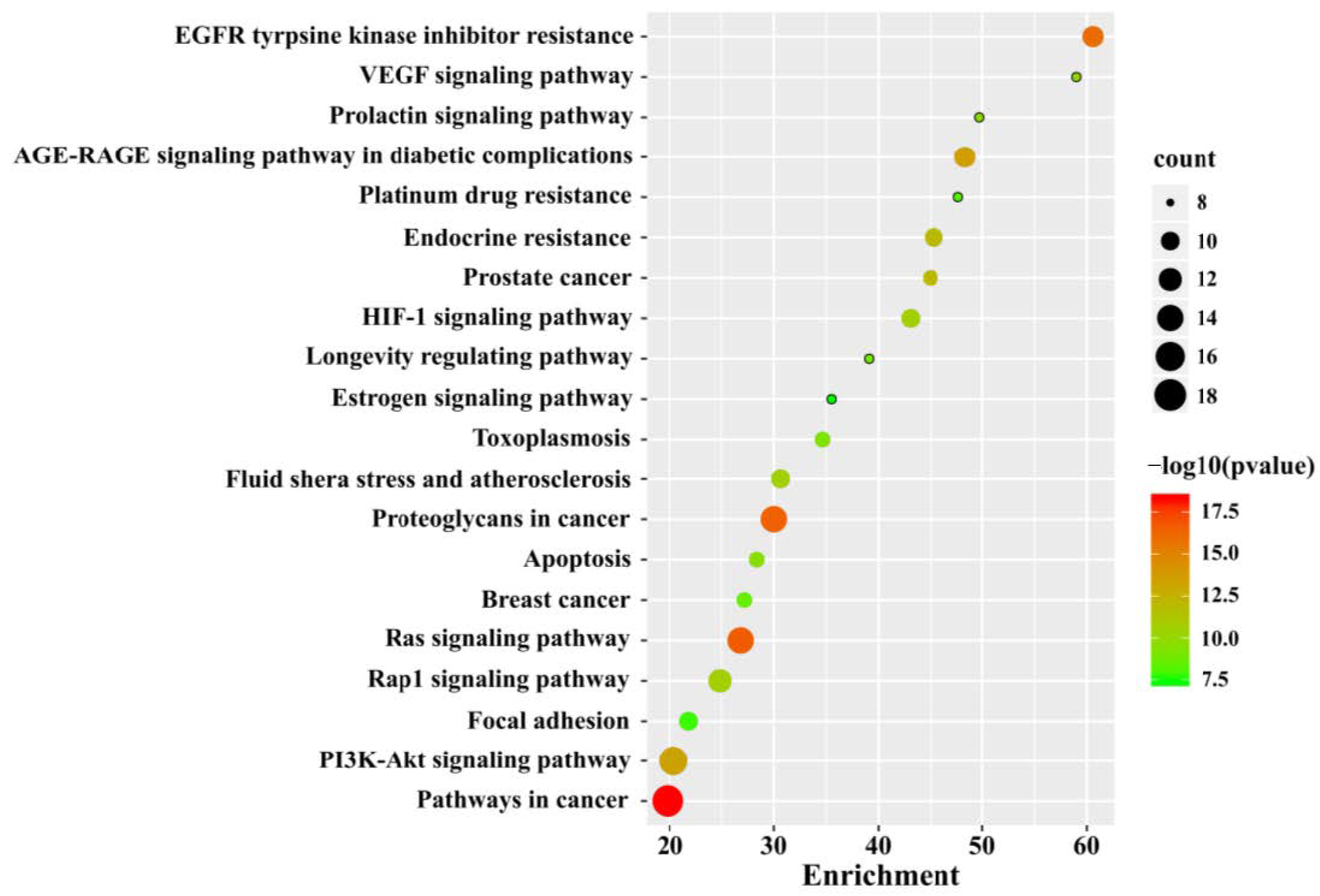
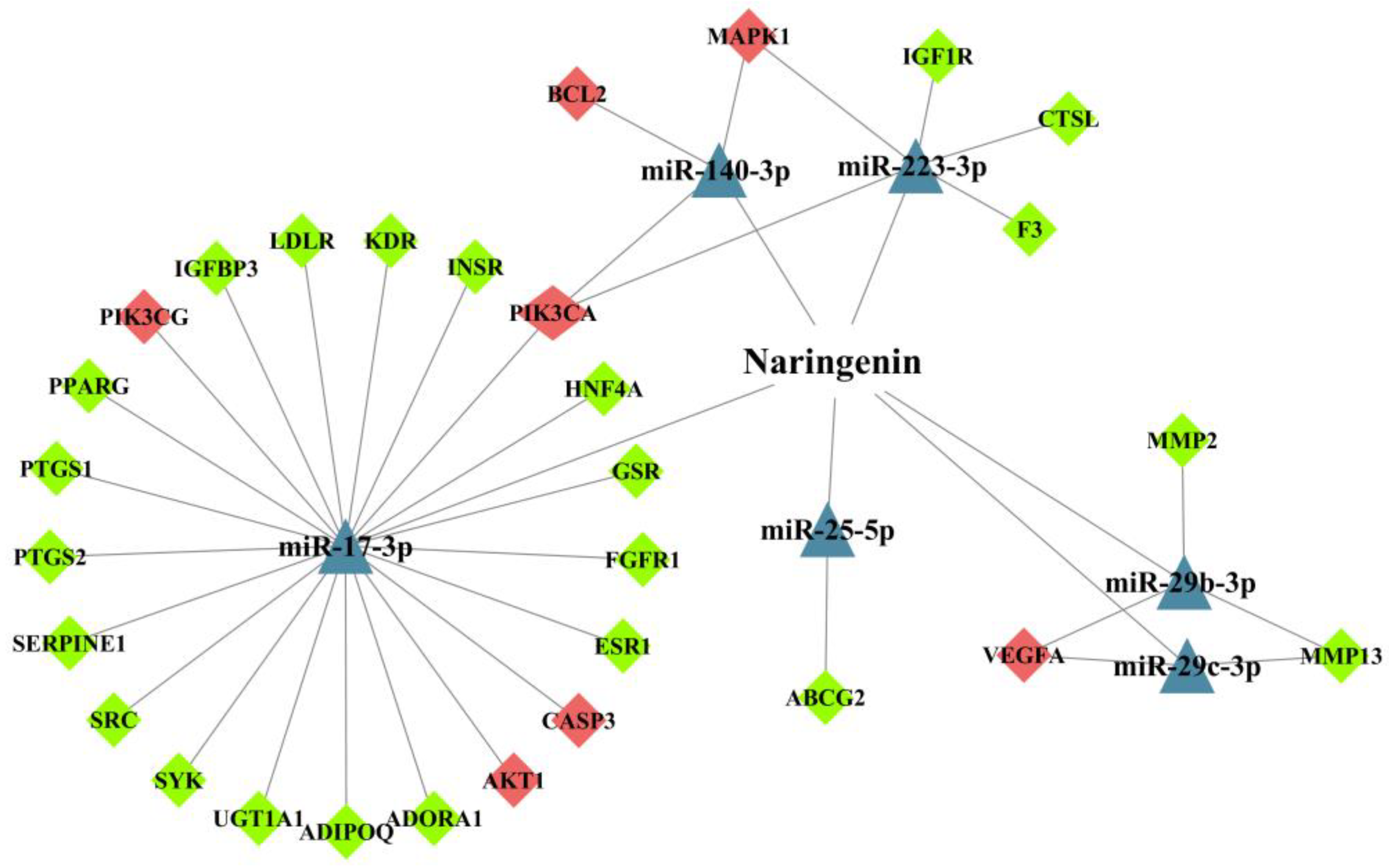
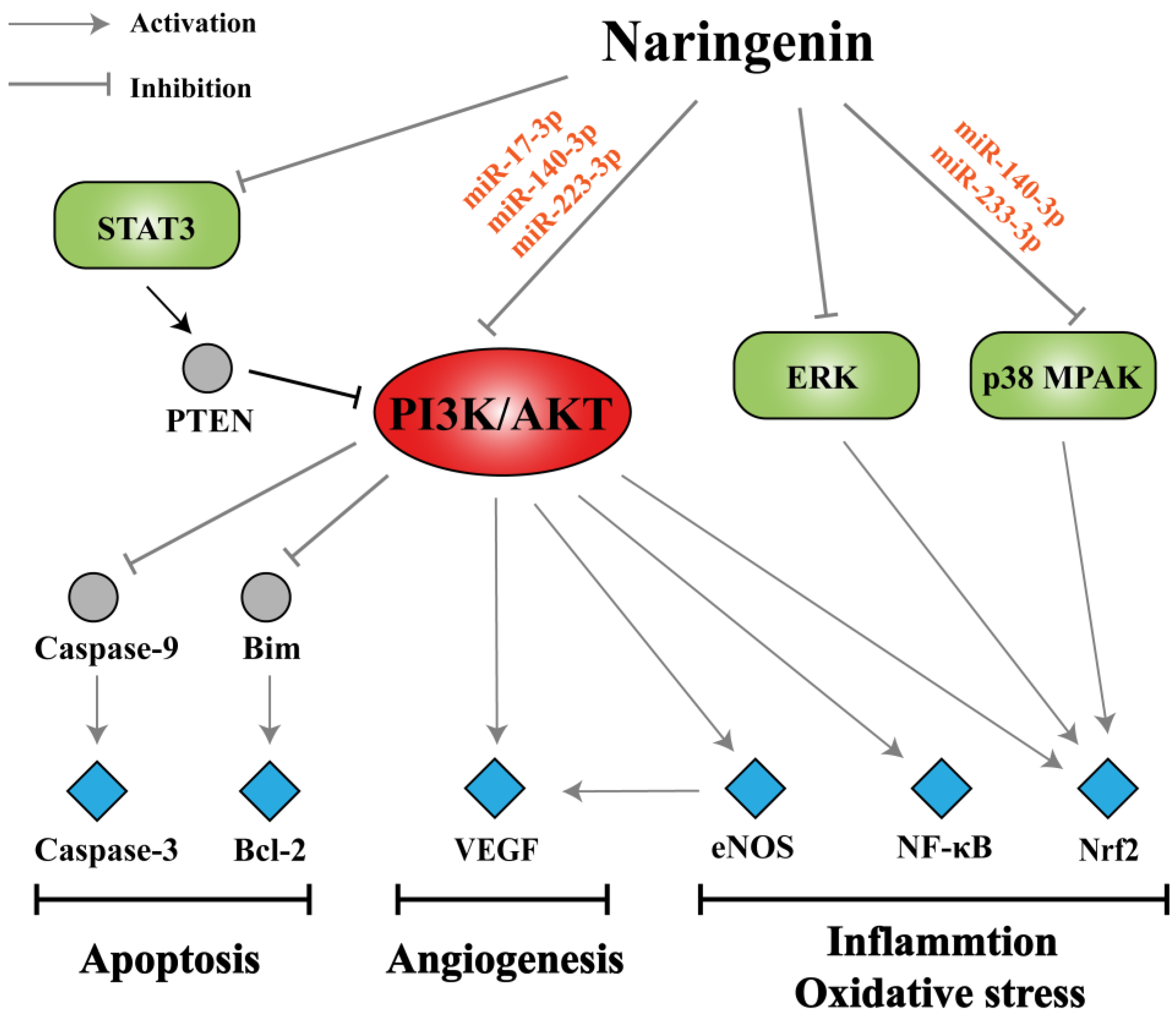
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Anti-inflammation | In vivo | LPS-induced acute lung injury mice | Naringin; 15, 30, and 60 mg/kg, p.o. | Pulmonary neutrophil infiltration and TNF-α, MPO, iNOS, and NF-κB activities ↓ | [23] |
| In vivo | CS-exposed rats | Naringin; 20, 40, and 80 mg/kg, p.o. | Infiltration of neutrophils and MPO, MMP-9, TNF-α, and IL-8 levels ↓; Level of IL-10 ↑ | [24] | |
| In vivo | Staphylococcus aureus-induced pneumonia mice | Naringenin; 100 mg/kg, i.h. | Pulmonary inflammation and inflammatory cells infiltration ↓ | [25] | |
| Both in vitro and in vivo | LPS-induced RAW 264.7 cell line; CLP-induced mice | Naringin; 50, 100, 200 μM (in vitro) 200 mg/kg, i.p. (in vivo) | TNF-α expression and HMGB1 release ↓; HO-1 expression via the AMPK-p38-Nrf2 pathway ↓ (in vitro) Lung injury ↓; TNF-α and HMGB1 expression ↓ (in vivo) | [26] | |
| Both in vitro and in vivo | CS-exposed A549 cell line and mice | Naringenin; 2, 20, 50 mM (in vitro) 20, 40, and 80 mg/kg, p.o. (in vivo) | NF-κB activity ↓; Levels of GR mRNA and protein ↑ (in vitro) Inflammatory cells and the production of IL-8, TNF-α, and MMP-9 ↓ (in vivo) | [33] | |
| In vivo | CS-exposed chronic bronchitis guinea pigs | Naringin; 9.2, 18.4 and 36.8 mg/kg, p.o. | Levels of IL-8 and TNF-α and MPO ↓ | [34] | |
| In vivo | LPS-induced acute lung injury mice | Naringenin; 100 mg/kg, p.o. | Pulmonary edema, neutrophil infiltration and the levels of TNF-α, IL-1β, IL-6, and MIP-2 ↓; The activities of PI3K and AKT ↓ | [36] | |
| In vivo | Radiation-induced lung injury mice | Naringenin; 100 and 200 mg/kg, p.o. | Level of IL-1β ↓ | [37] | |
| In vivo | LPS-induced acute lung injury rats | Naringenin; 50 and 100 mg/kg, p.o. | Levels of IL-6, MPO, TNF-α, and caspase-3 ↓; HSP70 expression ↑ | [12] | |
| In vivo | Carrageenan-induced pleurisy mice | Naringin; 40 and 80 mg/kg, p.o. | Th1 cytokines (TNF-α, IL-2, IL-6, and IL-17) ↓; NF-κB and STAT3 activities↓; Th2 cytokines (IL-4 and IL-10) ↑ | [46] | |
| In vitro | LPS-induced RAW 264.7 cell line | Naringin; 50, 100, and 200 μM | Secretion of IL-8, MCP-1 and MIP-1α ↓; NF-κB and MAPK activities ↓ | [49] | |
| In vivo | Allergen-induced asthma mice | Naringenin; 25, 50, and 100 mg/kg, i.p. | Levels of CCL5 and CCL11 and NF-κB activity ↓ | [50] | |
| In vitro | LPS-induced acute lung injury mice | Naringenin; 100 μM | TSLP production and levels of RIP-2 and caspase-1 ↓ | [53] | |
| In vitro | LPS-induced RAW 264.7 cell line | Naringenin NPs; 25μg/mL | NF-κB and MAPK activities ↓; Levels of TNF-α, IL-6, MCP-1, and IL-1β ↓ | [55] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Antioxidation | In vivo | LPS-induced acute lung injury mice | Naringenin; 100 mg/kg, p.o. | Levels of H2O2 and MDA ↓ | [36] |
| In vivo | Benzo[a]pyrene-induced rats | Naringenin; 100 mg/kg, p.o. | Levels of GSH, GPx, GST, GR, SOD, CAT, and XO ↑; Expression of COX-2 through blockage of NF-κB ↓ | [66] | |
| In vitro | Paraquat-induced BEAS-2B cell line | Naringenin; 100 μM | Generation of ROS ↓; Antioxidant-related genes including GPX2, GPX3, GPX5, and GPX7 and Nrf2 activity ↑ | [67] | |
| In vivo | Wood smoke-exposed rats | Naringin; 80 mg/kg, p.o. | The activities of SOD and CAT ↑; Levels of NO ↓ | [70] | |
| In vivo | Monocrotaline-induced pulmonary hypertension rats | Naringenin; 50 mg/kg, p.o. | GSH content and eNOS protein expression ↑; Expression of iNOS ↓ | [71] | |
| In vitro | LPS-induced RAW 264.7 cell line | Naringenin NPs; 25 μg/mL | Expression of iNOS and COX-2 and the production of NO ↓ | [55] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Anti-Airway Remodeling | In vivo | House dust mite-induced asthma mice | Naringenin; 9 mg/mL, p.o. | Subepithelial fibrosis and smooth muscle hypertrophy ↓ | [76] |
| In vivo | Ovalbumin-induced asthma mice | Naringin; 5 and 10 mg/kg, p.o. | Mean airway resistance and the level of IgE ↓ Percentage of Th1/Th2 cells ↑ | [80] | |
| In vivo | Ovalbumin-induced asthma mice | Naringenin; 50 mg/kg, i.p. | Area of airway fibrosis and the levels of Th2 cytokines ↓ | [81] | |
| In vivo | CS-exposed rats | Naringin; 20, 40, and 80 mg/kg, p.o. | Thickening of the bronchial wall ↓ | [24] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Anti-Pulmonary Fibrosis | In vivo | Paraquat-induced pulmonary fibrosis mice | Naringin; 60 and 120 mg/kg, p.o. | Expression of TNF-α, MMP-9, and TIMP-1 and the pulmonary fibrosis deposition ↓ | [86] |
| In vivo | Bleomycin-induced fibrosis rats | 80 mg/kg, p.o. | Levels of HYP and lung collagen content ↓ | [88] | |
| Both in virto and in vivo | Mycoplasma pneumoniae-induced BEAS-2B cell line and pneumonia mice | Naringenin; 100 μM (in vitro) 100 mg/kg, p.o. (in vivo) | Fibrosis-related proteins (TGF-β, α-SMA, collagen I and collagen III) expression and autophagy ↓ (in vitro) Level of TGF-β and autophagy relative protein LC3 and Beclin-1 expression ↓ (in vivo) | [90] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Expectorant | In vivo | Several animal models | Naringenin; 30–67 mg/kg, p.o. | Volume of airway secretions ↑ (mice); Mucociliary clearability and tracheal mucociliary velocity ↑ (pigeons); Mucin secretion ↓ (rats) | [95] |
| In vitro | EGF-induced A549 cell line | Naringenin; 30 and 100 μM | Expression of MUC5AC and phosphorylation of EGF receptor, MAPK, ERK1/2, JNK, NF-κB p65, and AP1 ↓ | [98] | |
| In vitro | Human neutrophil elastase induced-human airway epithelial cell line | Naringenin; 100 μM | MUC5AC expression, production of ROS and NF-κB activity ↓ | [99] | |
| In vivo | LPS-induced mice and beagle dogs | Naringin; 15 and 60 mg/kg, p.o. (mice); 12.4 mg/kg, p.o. (beagle dogs) | Expression of MUC5AC and goblet cell hyperplasia ↓ (mice); Sputum volume ↓ and elasticity and viscosity of sputum ↑ (beagle dogs) | [102] | |
| In vitro | LPS-induced airway epithelial cell and Calu-3 cell line | Naringenin; 100 μM | CFTR expression ↑ by Na+-K+-2Cl− co-transporters and K+ channels and regulated by intracellular cAMP | [103] | |
| Both in vitro and in vivo | DPM-induced Calu-3 cell line and mice | Naringenin; 25, 50, 100 μM (in vitro); Naringin; 30, 60, and 120 mg/kg, p.o. (in vivo) | Liquid viscosity, MUC5AC and total protein secretion ↓; CFTR, AQP1, and AQP5 expression and intracellular cAMP ↑ | [104] |
| Pharmacological Activity | Type of Study | Study Subject | Pharmacological Aspects | Findings | Ref. |
|---|---|---|---|---|---|
| Antitussive | In vivo | CS-exposed guinea pigs | Naringin; 18.4 mg/kg, p.o. | Airway hyperresponsiveness, chronic cough and expression of SP content, NK-1 receptor and NEP activity ↓ | [107] |
| In vivo | Different cough guinea pig models | Naringin; 15, 30, and 60 mg/kg, i.v. 0.5, 1.0, and 2.0 µM, i.c.v. | Exerted peripheral antitussive effects | [108] | |
| In vivo | Capsaicin-induced cough-variant asthma guinea pigs | Naringin; 18.4 mg/kg, p.o. | Airway hyperresponsiveness and cough ↓ | [111] |
| NO | Gene Name | Protein Name | Degree | NO | Gene Name | Protein Name | Degree |
|---|---|---|---|---|---|---|---|
| 1 | AKT1 | RAC-alpha serine/threonine-protein kinase | 42 | 29 | MMP3 | Stromelysin-1 | 14 |
| 2 | VEGFA | Vascular endothelial growth factor A | 41 | 30 | NOX4 | NADPH oxidase 4 | 14 |
| 3 | MAPK3 | Mitogen-activated protein kinase 3 | 37 | 31 | PPARA | Peroxisome proliferator-activated receptor alpha | 13 |
| 4 | PTGS2 | Prostaglandin G/H synthase 2 | 34 | 32 | HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | 13 |
| 5 | ESR1 | Estrogen receptor | 33 | 33 | INSR | Insulin receptor | 12 |
| 6 | MAPK1 | Mitogen-activated protein kinase 1 | 33 | 34 | MMP13 | Collagenase 3 | 12 |
| 7 | CASP3 | Caspase-3 | 33 | 35 | GSTP1 | Glutathione S transferase P | 12 |
| 8 | SRC | Proto-oncogene tyrosine-protein kinase Src | 30 | 36 | LDLR | Low-density lipoprotein receptor | 11 |
| 9 | MMP2 | 72 kDa type IV collagenase | 28 | 37 | KIT | Mast/stem cell growth factor receptor Kit | 11 |
| 10 | CAT | Catalase | 24 | 38 | CYP1B1 | Cytochrome P450 1B1 | 11 |
| 11 | SERPINE1 | Plasminogen activator inhibitor 1 | 24 | 39 | PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | 10 |
| 12 | APP | Amyloid-beta precursor protein | 24 | 40 | CYP2C9 | Cytochrome P450 2C9 | 10 |
| 13 | KDR | Vascular endothelial growth factor receptor 2 | 22 | 41 | F3 | Tissue factor | 10 |
| 14 | ADIPOQ | Adiponectin | 22 | 42 | GSR | Glutathione reductase, mitochondrial | 9 |
| 15 | PPARG | Peroxisome proliferator-activated receptor gamma | 22 | 43 | FGFR1 | Fibroblast growth factor receptor 1 | 9 |
| 16 | PIK3CA | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | 21 | 44 | SHBG | Sex hormone-binding globulin | 9 |
| 17 | BCL2L1 | Bcl-2-like protein 1 | 21 | 45 | BCL2 | Apoptosis regulator Bcl-2 | 8 |
| 18 | IGF1R | Insulin-like growth factor 1 receptor | 20 | 46 | UGT1A1 | UDP-glucuronosyltransferase 1A1 | 8 |
| 19 | SOD1 | Superoxide dismutase | 18 | 47 | EDNRA | Endothelin-1 receptor | 7 |
| 20 | APOB | Apolipoprotein B-100 | 18 | 48 | PLA2G2A | Phospholipase A2 | 7 |
| 21 | CYP3A4 | Cytochrome P450 3A4 | 18 | 49 | CTSL | Procathepsin L | 7 |
| 22 | IGFBP3 | Insulin-like growth factor-binding protein 3 | 18 | 50 | SYK | Tyrosine-protein kinase SYK | 6 |
| 23 | CYP19A1 | Aromatase | 18 | 51 | VCP | Transitional endoplasmic reticulum ATPase | 6 |
| 24 | ABCG2 | Broad substrate specificity ATP-binding cassette transporter ABCG2 | 17 | 52 | PTGS1 | Prostaglandin G/H synthase 1 | 6 |
| 25 | RELA | Transcription factor p65 | 16 | 53 | ADORA1 | Adenosine receptor A1 | 5 |
| 26 | HNF4A | Hepatocyte nuclear factor 4-alpha | 16 | 54 | BCHE | Cholinesterase | 5 |
| 27 | MET | Hepatocyte growth factor receptor | 15 | 55 | MMP12 | Macrophage metalloelastase | 2 |
| 28 | ESR2 | Estrogen receptor beta | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Chen, P.; Wu, H.; Shi, R.; Su, W.; Wang, Y.; Li, P. Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules 2020, 10, 1644. https://doi.org/10.3390/biom10121644
Chen Z, Chen P, Wu H, Shi R, Su W, Wang Y, Li P. Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules. 2020; 10(12):1644. https://doi.org/10.3390/biom10121644
Chicago/Turabian StyleChen, Zhen, Pan Chen, Hao Wu, Rui Shi, Weiwei Su, Yonggang Wang, and Peibo Li. 2020. "Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology" Biomolecules 10, no. 12: 1644. https://doi.org/10.3390/biom10121644
APA StyleChen, Z., Chen, P., Wu, H., Shi, R., Su, W., Wang, Y., & Li, P. (2020). Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules, 10(12), 1644. https://doi.org/10.3390/biom10121644





