Brewer’s Spent Grains—Valuable Beer Industry By-Product
Abstract
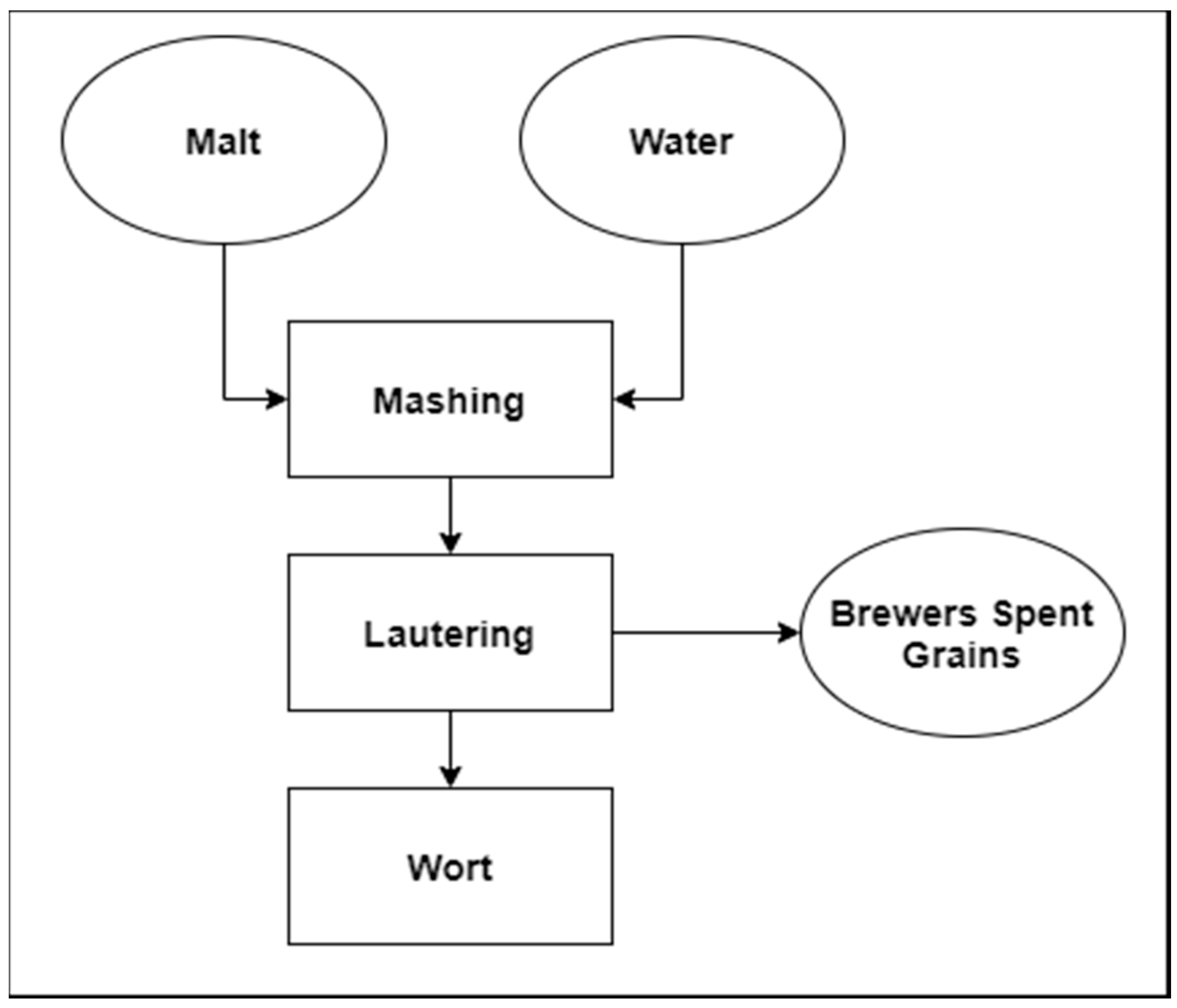
:1. Introduction
2. Activated Carbon Production and Sorption Properties
3. Biomethane Production
4. Thermal Valorization of BSG
4.1. BSG as a Solid Fuel
4.2. Hydrothermal Carbonization as a Thermal Valorization Method for Wet Types of Biomass
4.3. The Effect of Hydrothermal Carbonization of BSG
5. Extraction of High-Value Compounds from BSG
5.1. Arabinoxylans, Polyphenol, Antioxidants and Glucose
5.2. Proteins
6. Sustainable Materials
7. Use of BSG in Agriculture
7.1. Animal Nutrition
7.2. BSG as a Sustainable Fertilizer and Soil Amendment
8. Human Nutrition
8.1. Beer Production
8.2. Flour, Pasta and Bread Production
8.3. Cookies
8.4. Snacks
8.5. Frankfurters
8.6. High Fibre Products and High Protein Products
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haas, M.; Schreiber, M.; Mascher, M. Domestication and crop evolution of wheat and barley: Genes, genomics, and future directions. J. Integr. Plant Biol. 2019, 61, 204–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.L.; Ye, H.; Liu, L.; Wu, J.H.; Ru, W.M.; Sun, G.L. Molecular Insights on the Domestication of Barley (Hordeum vulgare L.). CRC. Crit. Rev. Plant Sci. 2019, 38, 280–294. [Google Scholar] [CrossRef]
- Badr, A.; Müller, K.; Schäfer-Pregl, R.; El Rabey, H.; Effgen, S.; Ibrahim, H.H.; Pozzi, C.; Rohde, W.; Salamini, F. On the origin and domestication history of barley (Hordeum vulgare). Mol. Biol. Evol. 2000, 17, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiśniewski, P. Piwa Historie Niezwykłe; Print Shops PREGO—Polska: Warszawa, Poland, 1993; ISBN 83-85830-00-6. [Google Scholar]
- Valamoti, S.M. Brewing beer in wine country? First archaeobotanical indications for beer making in Early and Middle Bronze Age Greece. Veg. Hist. Archaeobot. 2018, 27, 611–625. [Google Scholar] [CrossRef]
- Perruchini, E.; Glatz, C.; Hald, M.M.; Casana, J.; Toney, J.L. Revealing invisible brews: A new approach to the chemical identification of ancient beer. J. Archaeol. Sci. 2018, 100, 176–190. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J.; Kaminski, C. Water a Comprehensive Guide for Brewerse; Brewers Publications: Boulder, CO, USA, 2013; ISBN 978-0-937381-99-1. [Google Scholar]
- Mallet, J. Malt A Practical Guide from Field to Brewhouse; Brewers Publications: Boulder, CO, USA, 2014; ISBN 978-1-938469-12-1. [Google Scholar]
- Esslinger, H.M. Handbook of Brewing; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; Volume 1, ISBN 9788578110796. [Google Scholar]
- Calado, L.S.; Lacerda, A.L.F.; Fiaux, S.B.; Sphaier, L.A.; Silva, V.N.H.; Peixoto, F.C. Low-cost fluorescence-based method for beer bitterness measurement. J. Food Eng. 2019, 262, 9–12. [Google Scholar] [CrossRef]
- Lewis, M.; Young, T. Piwowarstwo; Mostowik, K., Ed.; Wydawnictwo Naukowe PWN S.A.: Warszawa, Poland, 2001; ISBN 83-01-13472-0. [Google Scholar]
- Happy International Beer Day! Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/EDN-20200807-1 (accessed on 23 October 2020).
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a functional germinated barley foodstuff from brewer’s spent grain for the treatment of ulcerative colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Esteves, M.P.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Silva, J.P.; Sousa, S.; Rodrigues, J.; Antunes, H.; Porter, J.J.; Gonçalves, I.; Ferreira-Dias, S. Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. Sep. Purif. Technol. 2004, 40, 309–315. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical characterization and liberation of pentose sugars from brewer’s spent grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. The effects of malting and mashing on barley protein extractability. J. Cereal Sci. 2006, 44, 203–211. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Jay, A.J.; Parker, M.L.; Faulks, R.; Husband, F.; Wilde, P.; Smith, A.C.; Faulds, C.B.; Waldron, K.W. A systematic micro-dissection of brewers’ spent grain. J. Cereal Sci. 2008, 47, 357–364. [Google Scholar] [CrossRef]
- Treimo, J.; Westereng, B.; Horn, S.J.; Forssell, P.; Robertson, J.A.; Faulds, C.B.; Waldron, K.W.; Buchert, J.; Eijsink, V.G.H. Enzymatic solubilization of brewers’ spent grain by combined action of carbohydrases and peptidases. J. Agric. Food Chem. 2009, 57, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Khidzir, K.M.; Noorlidah, A.; Agamuthu, P. Brewery Spent Grain: Chemical Characteristics and utilization as an Enzyme Substrate. Malays. J. Sci. 2019, 29, 41–51. [Google Scholar]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Sobukola, O.P.; Babajide, J.M.; Ogunsade, O. Effect of brewers spent grain addition and extrusion parameters on some properties of extruded yam starch-based pasta. J. Food Process. Preserv. 2013, 37, 734–743. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam explosion of Brewer’s spent grain improves enzymatic digestibility of carbohydrates and affects solubility and stability of proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of protein hydrolysates from brewer’s spent grain using enzyme and ultrasonication. Int. J. Food Sci. Technol. 2020, 55, 357–368. [Google Scholar] [CrossRef]
- Allen, S.J.; Whitten, L.; Mckay, G. The Production and Characterisation of Activated Carbons: A Review. Dev. Chem. Eng. Miner. Process. 2008, 6, 231–261. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2018, 35, 777–815. [Google Scholar] [CrossRef]
- Keirsse, H.; Hartoyo, W.; Buekens, A.; Schoeters, J.; Janssens, J. Preparation of Activated Carbon by the Partial Gasification of Charcoal. In Research in Thermochemical Biomass Conversion; Springer: Dordrecht, The Netherlands, 1988; pp. 531–541. [Google Scholar]
- Dai, X.; Antal, M.J. Synthesis of a high-yield activated carbon by air gasification of macadamia nut shell charcoal. Ind. Eng. Chem. Res. 1999, 38, 3386–3395. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Gasification Char as a Potential Substitute of Activated Carbon in Adsorption Applications. Energy Procedia 2017, 105, 712–717. [Google Scholar] [CrossRef]
- Anderson, N.; Jones, J.G.; Page-Dumroese, D.; McCollum, D.; Baker, S.; Loeffler, D.; Chung, W. A comparison of producer gas, biochar, and activated carbon from two distributed scale thermochemical conversion systems used to process forest biomass. Energies 2013, 6, 164–183. [Google Scholar] [CrossRef]
- Ng, C.; Marshall, W.E.; Rao, R.M.; Bansode, R.R.; Losso, J.N. Activated carbon from pecan shell: Process description and economic analysis. Ind. Crops Prod. 2003, 17, 209–217. [Google Scholar] [CrossRef]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (furfural) adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef] [Green Version]
- Shamsuddin, M.S.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and Characterization of Activated Carbon Produced from Kenaf Core Fiber Using H3PO4 Activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef] [Green Version]
- Balogun, A.O.; Sotoudehniakarani, F.; McDonald, A.G. Thermo-kinetic, spectroscopic study of brewer’s spent grains and characterisation of their pyrolysis products. J. Anal. Appl. Pyrolysis 2017, 127, 8–16. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Rocha, G.J.M.; Órfão, J.J.M.; Teixeira, J.A.; Roberto, I.C. Production, characterization and application of activated carbon from Brewer’s spent grain lignin. Bioresour. Technol. 2010, 101, 2450–2457. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.I.; O’Connor, E.; McSpadden, G.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Upcycling brewer’s spent grain waste into activated carbon and carbon nanotubes for energy and other applications via two-stage activation. J. Chem. Technol. Biotechnol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A.S. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Vanderheyden, S.R.H.; Vanreppelen, K.; Yperman, J.; Carleer, R.; Schreurs, S. Chromium(VI) removal using in-situ nitrogenized activated carbon prepared from Brewers’ spent grain. Adsorption 2018, 24, 147–156. [Google Scholar] [CrossRef]
- Wierzba, S.; Rajfur, M.; Nabrdalik, M.; Kłos, A. Assessment of the influence of counter ions on biosorption of copper cations in brewer’s spent grain—Waste product generated during beer brewing process. Microchem. J. 2019, 145, 196–203. [Google Scholar] [CrossRef]
- Wierzba, S.; Kłos, A. Heavy metal sorption in biosorbents—Using spent grain from the brewing industry. J. Clean. Prod. 2019, 225, 112–120. [Google Scholar] [CrossRef]
- De Araújo, T.P.; de Oliveira Tavares, F.; Vareschini, D.T.; Barros, M.A.S.D. Biosorption mechanisms of cationic and anionic dyes in a low-cost residue from brewer’s spent grain. Environ. Technol. 2020. [Google Scholar] [CrossRef]
- Safarik, I.; Horska, K.; Safarikova, M. Magnetically modified spent grain for dye removal. J. Cereal Sci. 2011, 53, 78–80. [Google Scholar] [CrossRef]
- Slavík, J.; Rybová, K.; Dolejš, M. Biowaste Separation at Source and Its Limitations Based on Spatial Conditions. Detritus 2019, 5, 1. [Google Scholar] [CrossRef]
- Moretti, P.; Morais de Araujo, J.; Borges de Castilhos, A.; Buffière, P.; Gourdon, R.; Bayard, R. Characterization of municipal biowaste categories for their capacity to be converted into a feedstock aqueous slurry to produce methane by anaerobic digestion. Sci. Total Environ. 2020, 716, 137084. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tao, Y.; Temudo, M.; Schooneveld, M.; Bijl, H.; Ren, N.; Wolf, M.; Heine, C.; Foerster, A.; Pelenc, V.; et al. An integrated approach for efficient biomethane production from solid bio-wastes in a compact system. Biotechnol. Biofuels 2015, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitanza, R.; Cortesi, A.; Gallo, V.; Colussi, I.; De Arana-Sarabia, M.E. Biovalorization of brewery waste by applying anaerobic digestion. Chem. Biochem. Eng. Q. 2016, 30, 351–357. [Google Scholar] [CrossRef]
- Kan, X.; Zhang, J.; Wah, Y.; Wang, C. Overall evaluation of microwave-assisted alkali pretreatment for enhancement of biomethane production from brewers’ spent grain. Energy Convers. Manag. 2018, 158, 315–326. [Google Scholar] [CrossRef]
- Dudek, M.; Świechowski, K.; Manczarski, P.; Koziel, J.A.; Białowiec, A. The effect of biochar addition on the biogas production kinetics from the anaerobic digestion of brewers’ spent grain. Energies 2019, 12, 1518. [Google Scholar] [CrossRef] [Green Version]
- Bougrier, C.; Dognin, D.; Laroche, C.; Gonzalez, V.; Benali-Raclot, D.; Cacho Rivero, J.A. Anaerobic digestion of Brewery Spent Grains: Trace elements addition requirement. Bioresour. Technol. 2018, 247, 1193–1196. [Google Scholar] [CrossRef]
- Sperandio, G.; Amoriello, T.; Carbone, K.; Fedrizzi, M.; Monteleone, A.; Tarangioli, S.; Pagano, M. Increasing the value of spent grain from craft microbreweries for energy purposes. Chem. Eng. Trans. 2017, 58, 487–492. [Google Scholar]
- Enweremadu, C.; Waheed, M.A.; Adekunle, A.A.; Adeala, A. The Energy Potential of Brewer’s Spent Grain for Breweries in Nigeria. Eng. Appl. Sci. 2008, 3, 175–177. [Google Scholar]
- Chetrariu, A.; Dabija, A. Brewer’s spent grains: Possibilities of valorization, a review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolopoulos, N.; Agraniotis, M.; Violidakis, I.; Karampinis, E.; Nikolopoulos, A.; Grammelis, P.; Papapavlou, C.; Tzivenis, S.; Kakaras, E. Parametric investigation of a renewable alternative for utilities adopting the co-firing lignite/biomass concept. Fuel 2013, 113, 873–897. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Piotrowski, K.; Wolska, B.; Debowski, M.; Zielinski, M.; Dziugan, P.; Szufa, S. Stimulating Effect of Ash from Sorghum on the Growth of Lemnaceae—A New Source of Energy Biomass; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 341–349. ISBN 9783030138882. [Google Scholar]
- Bala-Litwiniak, A.; Zajemska, M. Computational and experimental study of pine and sunflower husk pellet combustion and co-combustion with oats in domestic boiler. Renew. Energy 2020, 162, 151–159. [Google Scholar] [CrossRef]
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Grzesik, M.; Cebula, A.; Kowalczyk, S. Experimental Studies on Energy Crops Torrefaction Process Using Batch Reactor to Estimate Torrefaction Temperature and Residence Time. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 365–373. ISBN 978-3-319-72370-9. [Google Scholar]
- Szufa, S.; Wielgosiński, G.; Piersa, P.; Czerwińska, J.; Dzikuć, M.; Adrian, Ł.; Lewandowska, W.; Marczak, M. Torrefaction of straw from oats and maize for use as a fuel and additive to organic fertilizers-TGA analysis, kinetics as products for agricultural purposes. Energies 2020, 13, 2064. [Google Scholar] [CrossRef] [Green Version]
- Jewiarz, M.; Wróbel, M.; Mudryk, K.; Szufa, S. Impact of the drying temperature and grinding technique on biomass grindability. Energies 2020, 13, 3392. [Google Scholar] [CrossRef]
- Trinh, T.T.; Werle, S.; Tran, K.Q.; Magdziarz, A.; Sobek, S.; Pogrzeba, M. Energy crops for sustainable phytoremediation – Thermal decomposition kinetics. Energy Procedia 2019, 158, 873–878. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Sepúlveda, F.J.; Montero, I.; Rojas, C.V. Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467. [Google Scholar] [CrossRef] [Green Version]
- Stroem, L.K.; Desai, D.K.; Hoadley, A.F.A. Superheated steam drying of Brewer’s spent grain in a rotary drum. Adv. Powder Technol. 2009, 20, 240–244. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonisation of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of wet and high ash biomass: Wet torrefaction—A review. J. Power Technol. 2017, 97, 354–369. [Google Scholar]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Jackowski, M.; Semba, D.; Trusek, A.; Wnukowski, M.; Niedzwiecki, L.; Baranowski, M.; Krochmalny, K.; Pawlak-Kruczek, H. Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Persson, H.; Yang, W.; Jönsson, P.G. Pyrolysis study of hydrothermal carbonization-treated digested sewage sludge using a Py-GC/MS and a bench-scale pyrolyzer. Fuel 2019. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M.; Wądrzyk, M. Pyrolysis of hydrochar derived from biomass—Experimental investigation. Fuel 2020, 267, 117246. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Mihajlović, M.; Petrović, J.; Maletić, S.; Isakovski, M.K.; Stojanović, M.; Lopičić, Z.; Trifunović, S. Hydrothermal carbonization of Miscanthus × giganteus: Structural and fuel properties of hydrochars and organic profile with the ecotoxicological assessment of the liquid phase. Energy Convers. Manag. 2018, 159, 254–263. [Google Scholar] [CrossRef]
- Shafie, S.A.; Al-attab, K.A.; Zainal, Z.A. Effect of hydrothermal and vapothermal carbonization of wet biomass waste on bound moisture removal and combustion characteristics. Appl. Therm. Eng. 2018, 139, 187–195. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Louwes, A.C.; Halfwerk, R.B.; Bramer, E.A.; Brem, G. Experimental Study on Fast Pyrolysis of Raw and Torrefied Woody Biomass. Energy Technol. 2020, 8, 1900799. [Google Scholar] [CrossRef] [Green Version]
- Louwes, A.C.; Basile, L.; Yukananto, R.; Bhagwandas, J.C.; Bramer, E.A.; Brem, G. Torrefied biomass as feed for fast pyrolysis: An experimental study and chain analysis. Biomass Bioenergy 2017, 105, 116–126. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus – Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Wedwitschka, H.; Baskyr, I.; Koehler, R.; Kopinke, F.D. Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresour. Technol. 2014, 164, 162–169. [Google Scholar] [CrossRef]
- Arauzo, P.; Olszewski, M.; Kruse, A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef] [Green Version]
- Jackowski, M.; Niedzwiecki, L.; Lech, M.; Wnukowski, M.; Arora, A.; Tkaczuk-Serafin, M.; Baranowski, M.; Krochmalny, K.; Veetil, V.K.; Seruga, P.; et al. HTC of Wet Residues of the Brewing Process: Comprehensive Characterization of Produced Beer, Spent Grain and Valorized Residues. Energies 2020, 13, 2058. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, M.P.; Arauzo, P.J.; Wądrzyk, M.; Kruse, A. Py-GC-MS of hydrochars produced from brewer’s spent grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.M.; Kruse, A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 260, 121101. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer’s spent grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzyme Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Lecce, L.; Padalino, L.; Del Nobile, M.A. Supercritical carbon dioxide extraction of brewer’s spent grain. J. Supercrit. Fluids 2016, 107, 69–74. [Google Scholar] [CrossRef]
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrason. Sonochem. 2015, 24, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Łukasiak, J.; Olsen, K.; Georgiou, C.A.; Georgakopoulos, D.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Eur. Food Res. Technol. 2013, 237, 33–48. [Google Scholar]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.P.L.V.O.; Coimbra, M.A. Valuation of brewer’s spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Ind. Crops Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Kitryte, V.; Šaduikis, A.; Venskutonis, P.R. Assessment of antioxidant capacity of brewer’s spent grain and its supercritical carbon dioxide extract as sources of valuable dietary ingredients. J. Food Eng. 2015, 167, 18–24. [Google Scholar] [CrossRef]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioprocess Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Riaño, P.; Sanz Diez, M.T.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of polyphenols from brewer’s spent grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid state fermentation of Brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17. Fermentation 2019, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water-organic solvent extraction of phenolic antioxidants from brewers’ spent grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Arauzo, P.J.; Meza Zavala, M.F.; Cao, Z.; Olszewski, M.P.; Kruse, A. Towards the properties of different biomass-derived proteins via various extraction methods. Molecules 2020, 25, 488. [Google Scholar] [CrossRef] [Green Version]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of different pretreatment strategies for protein extraction from brewer’s spent grains. Ind. Crops Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High Yield Protein Extraction from Brewer’s Spent Grain with Novel Carboxylate Salt—Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Hamada, H.M.; Jokhio, G.A.; Al-Attar, A.A.; Yahaya, F.M.; Muthusamy, K.; Humada, A.M.; Gul, Y. The use of palm oil clinker as a sustainable construction material: A review. Cem. Concr. Compos. 2020, 106, 103447. [Google Scholar] [CrossRef]
- Olacia, E.; Pisello, A.L.; Chiodo, V.; Maisano, S.; Frazzica, A.; Cabeza, L.F. Sustainable adobe bricks with seagrass fibres. Mechanical and thermal properties characterization. Constr. Build. Mater. 2020, 239, 117669. [Google Scholar] [CrossRef]
- Zedler, L.; Colom, X.; Cañavate, J.; Saeb, M.R.; Haponiuk, J.T.; Formela, K. Investigating the impact of curing system on structure-property relationship of natural rubber modified with brewery by-product and ground tire rubber. Polymers 2020, 12, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, thermal and physico-mechanical properties of polyurethane/brewers’ spent grain composite foams modified with ground tire rubber. Ind. Crops Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Ferraz, E.; Coroado, J.; Gamelas, J.; Silva, J.; Rocha, F.; Velosa, A. Spent brewery grains for improvement of thermal insulation of ceramic bricks. J. Mater. Civ. Eng. 2013, 25, 1638–1646. [Google Scholar] [CrossRef]
- Russ, W.; Mörtel, H.; Meyer-Pittroff, R. Application of spent grains to increase porosity in bricks. Constr. Build. Mater. 2005, 19, 117–126. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Biosourced disposable trays made of brewer’s spent grain and potato starch. Polymers 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from brewers’ spent grain: A green and low-cost smart material to modify screen-printed electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef]
- Jamroz, D. Żywienie Zwierząt I Paszoznawstwo, Tom 3; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2013; ISBN 978-83-01-18227-4. [Google Scholar]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain; Bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Ben-Hamed, U.; Seddighi, H.; Thomas, K. Economic returns of using Brewery’s spent grain in animal feed. World Acad. Sci. Eng. Technol. 2011, 50, 695–698. [Google Scholar]
- Czekała, W.; Pawlisiak, A. Produkcja i Wykorzystanie Wysłodzin Browarnianych. Technika Rolnicza Ogrodnicza Leśna 2017, 5, 23–25. [Google Scholar]
- Dulcet, E. Metody i techniki zakiszania młóta browarnianego w belach cylindrycznych. J. Res. Appl. Agric. Eng. 2008, 53, 59–62. [Google Scholar]
- Faccenda, A.; Zambom, M.A.; Castagnara, D.D.; de Avila, A.S.; Fernandes, T.; Eckstein, E.I.; Anschau, F.A.; Schneider, C.R. Use of dried brewers’ grains instead of soybean meal to feed lactating cows. Rev. Bras. Zootec. 2017, 46, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, J.; Martin, D.S.; Perez-Vendrell, A.M.; Padrell, L.; Iñarra, B.; Orive, M.; Estévez, A. Apparent digestibility coefficients of brewer’s by-products used in feeds for rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata). Aquaculture 2021, 530, 735796. [Google Scholar] [CrossRef]
- San Martin, D.; Orive, M.; Iñarra, B.; Castelo, J.; Estévez, A.; Nazzaro, J.; Iloro, I.; Elortza, F.; Zufía, J. Brewers’ Spent Yeast and Grain Protein Hydrolysates as Second-Generation Feedstuff for Aquaculture Feed. Waste Biomass Valorization 2020, 11, 5307–5320. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessalew, G.; Beyene, A.; Nebiyu, A.; Ruelle, M.L. Use of industrial diatomite wastes from beer production to improve soil fertility and cereal yields. J. Clean. Prod. 2017, 157, 22–29. [Google Scholar] [CrossRef]
- Alayu, E.; Leta, S. Brewery sludge quality, agronomic importance and its short-term residual effect on soil properties. Int. J. Environ. Sci. Technol. 2020, 17, 2337–2348. [Google Scholar] [CrossRef] [Green Version]
- Ojeniyi, S.O.; Awodun, M.A.; Odedina, S.A. Effect of Animal Manure Ammended Spent Grain and Cocoa Husk on Nutrient Status Growth and Yield of Tomato. Int. J. Agric. Res. 2007, 2, 406–410. [Google Scholar]
- Nsoanya, L.N. Effect of integrated use of spent grain and NPK (20:10:10) fertilizer on soil chemical properties and maize (Zea Mays L) growth. Int. J. Res. Agric. For. 2015, 2, 14–19. [Google Scholar]
- Qiu, L.; Li, J.J.; Li, Z.; Wang, J.J. Production and characterization of biocontrol fertilizer from brewer’s spent grain via solid-state fermentation. Sci. Rep. 2019, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Mbagwu, J.S.C.; Ekwealor, G.C. Agronomic potential of brewers’ spent grains. Biol. Wastes 1990, 34, 335–347. [Google Scholar] [CrossRef]
- Lal, R. Sequestering carbon and increasing productivity by conservation agriculture. J. Soil Water Conserv. 2015, 70, 55A–62A. [Google Scholar] [CrossRef] [Green Version]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; Nair, P.K.R.; Dari, B.; Freitas, A.M.; Chatterjee, N.; Pinheiro, F.M. Biochar in the agroecosystem-climate-change-sustainability Nexus. Front. Plant Sci. 2017, 8, 1051. [Google Scholar] [CrossRef]
- Baltrėnaitė, E.; Baltrėnas, P.; Bhatnagar, A.; Vilppo, T.; Selenius, M.; Koistinen, A.; Dahl, M.; Penttinen, O.P. A multicomponent approach to using waste-derived biochar in biofiltration: A case study based on dissimilar types of waste. Int. Biodeterior. Biodegrad. 2017, 119, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Abd-Allah, E.F.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7, 209. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, J.R.; Viger, M.; Arnold, E.C.; Harris, Z.M.; Ventura, M.; Miglietta, F.; Girardin, C.; Edwards, R.J.; Rumpel, C.; Fornasier, F.; et al. Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. GCB Bioenergy 2017, 9, 591–612. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.; Liang, C.; Luo, Y.; Xu, Q.; Han, C.; Zhao, Q.; Sun, B. Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 2019, 7, 77. [Google Scholar] [CrossRef]
- Obia, A.; Cornelissen, G.; Martinsen, V.; Smebye, A.B.; Mulder, J. Conservation tillage and biochar improve soil water content and moderate soil temperature in a tropical Acrisol. Soil Tillage Res. 2020, 197, 104521. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, G.; Jubaedah; Nurida, N.L.; Hale, S.E.; Martinsen, V.; Silvani, L.; Mulder, J. Fading positive effect of biochar on crop yield and soil acidity during five growth seasons in an Indonesian Ultisol. Sci. Total Environ. 2018, 634, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Scholes, R.; Brainich, A. IPBES (2018): The IPBES Assessment Report on Land Degradation and Restoration; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018. [Google Scholar]
- George, C.; Wagner, M.; Kücke, M.; Rillig, M.C. Divergent consequences of hydrochar in the plant–soil system: Arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl. Soil Ecol. 2012, 59, 68–72. [Google Scholar] [CrossRef]
- Amoriello, T.; Fiorentino, S.; Vecchiarelli, V.; Pagano, M. Evaluation of spent grain biochar impact on hop (Humulus lupulus L.) growth by multivariate image analysis. Appl. Sci. 2020, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Fărcaş, A.; Tofană, M.; Socaci, S.; Mudura, E.; Scrob, S.; Salanţă, L.; Mureşan, V. Brewers’ spent grain—A new potential ingredient for functional foods. Hop Med. Plants 2014, 22, 44–50. [Google Scholar]
- Muller, R. The Effects of Mashing Temperature and Mash Thickness on Wort Carbohydrate Composition. J. Inst. Brew. 1991, 97, 85–92. [Google Scholar] [CrossRef]
- Zürcher, C.; Gruss, R. Method of Making Alcohol-Free or Nearly Alcohol-Free Beer. United. States Patent 5077061, 21 December 1990. [Google Scholar]
- Petitot, M.; Boyer, L.; Minier, C.; Micard, V. Fortification of pasta with split pea and faba bean flours: Pasta processing and quality evaluation. Food Res. Int. 2010, 43, 634–641. [Google Scholar] [CrossRef]
- Nocente, F.; Taddei, F.; Galassi, E.; Gazza, L. Upcycling of brewers ’ spent grain by production of dry pasta with higher nutritional potential. LWT Food Sci. Technol. 2019, 114, 108421. [Google Scholar] [CrossRef]
- Steinmacher, N.C.; Honna, F.A.; Gasparetto, A.V.; Anibal, D.; Grossmann, M.V.E. Bioconversion of brewer’s spent grains by reactive extrusion and their application in bread-making. LWT Food Sci. Technol. 2012, 46, 542–547. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P. The effect of different enzymes on the quality of high-fibre enriched brewer’s spent grain breads. Food Chem. 2008, 110, 865–872. [Google Scholar] [CrossRef]
- Butt, M.S.; Tahir-Nadeem, M.; Ahmad, Z.; Sultan, M.T. Xylanases and their applications in baking industry. Food Technol. Biotechnol. 2008, 46, 22–31. [Google Scholar]
- Wang, X.; Pei, D.; Teng, Y.; Liang, J. Effects of enzymes to improve sensory quality of frozen dough bread and analysis on its mechanism. J. Food Sci. Technol. 2018, 55, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, J.; Pajin, B.; Tanackov-Kocic, S.; Pejin, J.; Fistes, A.; Bojanic, N.; Loncarevic, I. Quality properties of cookies supplemented with fresh brewer’s spent grain. Food Feed Res. 2017, 44, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Kirjoranta, S.; Tenkanen, M.; Jouppila, K. Effects of process parameters on the properties of barley containing snacks enriched with brewer’s spent grain. J. Food Sci. Technol. 2016, 53, 775–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieroń, R. Pieczemy Ciastka Wysłodkowe. Available online: https://www.sodr.pl/swietokrzyski-portal-rolny/aktualnosci/Pieczemy-ciastka-wyslodkowe/idn:960 (accessed on 3 November 2020).
- Spent Grain Cookies. Available online: https://www.pageandplate.com/spent-grain-cookies/ (accessed on 4 November 2020).
- 9 Spent Grain Cookie Recipes. Available online: https://brooklynbrewshop.com/blogs/themash/9-spent-grain-cookie-recipes (accessed on 3 November 2020).
- Ktenioudaki, A.; Crofton, E.; Scannell, A.G.M.; Hannon, J.A.; Kilcawley, K.N.; Gallagher, E. Sensory properties and aromatic composition of baked snacks containing brewer’s spent grain. J. Cereal Sci. 2013, 57, 384–390. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanoǧlu, S. The recycling of brewer’s processing by-product into ready-to-eat snacks using extrusion technology. J. Cereal Sci. 2008, 47, 469–479. [Google Scholar] [CrossRef]
- Özvural, E.B.; Vural, H.; Gökbulut, I.; Özboy-Özbaş, Ö. Utilization of brewer’s spent grain in the production of Frankfurters. Int. J. Food Sci. Technol. 2009, 44, 1093–1099. [Google Scholar] [CrossRef]


| Lignin | Cellulose | Hemicellulose | Ash | Protein | Lipids | Phenolics | Starch | |
|---|---|---|---|---|---|---|---|---|
| Kanauchi et al., (2001) [13] | 11.9 | 25.4 | 21.8 | 2.4 | 24.0 | 10.6 | N.D. | N.D. |
| Carvalheiro et al., (2004) [14] | 21.7 | 21.9 | 29.6 | 1.2 | 24.6 | N.D. | N.D. | N.D. |
| Silva et al., (2004) [15] | 16.9 | 25.3 | 41.9 | 4.6 | N.D. | N.D. | N.D. | N.D. |
| Mussatto and Roberto, (2006) [16] | 27.8 | 16.8 | 28.4 | 4.6 | 15.2 | N.D. | N.D. | N.D. |
| Celus et al., (2006) [17] | N.D. | 0.3 | 22.5 | 3.3 | 26.7 | N.D. | N.D. | 1 |
| Xiros et al., (2008) [18] | 11.5 | 12 | 40 | 3.3 | 14.2 | 13 | 2.0 | 2.7 |
| Jay et al., (2008) [19] | 20–22 | 31–33 | N.D. | N.D. | 15–17 | 6–8 | 1.0–1.5 | 10–12 |
| Treimo et al., (2009) [20] | 12.6 ± 0.1 | 45.9 * | 23.4 ± 1.4 | N.D. | N.D. | 7.8 ± 0.2 | ||
| Robertson et al., (2010) [21] | 13–17 | N.D. | 22–29 | N.D. | 20–24 | N.D. | N.D. | 2–8 |
| Khidzir et al., (2010) [22] | 56.74 ± 9.38 | 40.20 ± 17.71 | N.D. | 2.27 ± 0.76 | 6.41 ± 0.31 | 2.50 ± 0.11 | N.D. | 0.28 ± 0.06 |
| Waters et al., (2012) [23] | N.D. | 26.0 | 22.2 | 1.1 | 22.1 | N.D. | N.D. | N.D. |
| Nuno et al., (2013) [24] | 19.40 ± 0.34 | 21.73 ± 1.36 | 19.27 ± 1.18 | 4.18 ± 0.03 | 24.69 ± 1.04 | N.D. | N.D. | N.D. |
| Sobukola et al., (2012) [25] | 9.19 ± 0.011 | 60.64 ± 0.26 * | 2.48 ± 0.02 | 24.39 ± 0.46 | 6.18 ± 0.13 | N.D. | N.D. | |
| Kemppai-nen et al., (2016) [26] | 19.6 | 45 * | 4.1 | 20.3 | N.D. | N.D. | N.D. | |
| Yu et al., (2020) [27] | N.D. | 51.0 ± 0.7 * | 4.1 ± 0.1 | 23.4 ± 0.2 | 9.4 ± 0.1 | N.D. | N.D. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. https://doi.org/10.3390/biom10121669
Jackowski M, Niedźwiecki Ł, Jagiełło K, Uchańska O, Trusek A. Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules. 2020; 10(12):1669. https://doi.org/10.3390/biom10121669
Chicago/Turabian StyleJackowski, Mateusz, Łukasz Niedźwiecki, Kacper Jagiełło, Oliwia Uchańska, and Anna Trusek. 2020. "Brewer’s Spent Grains—Valuable Beer Industry By-Product" Biomolecules 10, no. 12: 1669. https://doi.org/10.3390/biom10121669
APA StyleJackowski, M., Niedźwiecki, Ł., Jagiełło, K., Uchańska, O., & Trusek, A. (2020). Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules, 10(12), 1669. https://doi.org/10.3390/biom10121669






