Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Actinomycetes
2.2. Morphological and Biochemical Characterization of the Isolated Actinomycetes
2.3. Molecular Identification of the Biologically Active Actinomycete Strains
2.4. Experimental Setup, Plant Materials and Growth Conditions
2.5. Soil Analysis
2.6. Microbial Counts
2.7. Photosynthesis Rate and Pigment Analysis
2.8. Metabolite Profiling
2.8.1. Metabolite Profiling of Isolated Strains
2.8.2. Metabolite Profiling of Isolated Treated Legumes
2.9. Minerals Content Determination
2.10. Enzyme Activity Determination
2.11. Assessment of Biological Activities
2.11.1. In Vitro Antioxidant Activity
2.11.2. Antimicrobial Assay
2.11.3. Antiprotozoal Activity
2.12. Statistical Analyses
3. Results
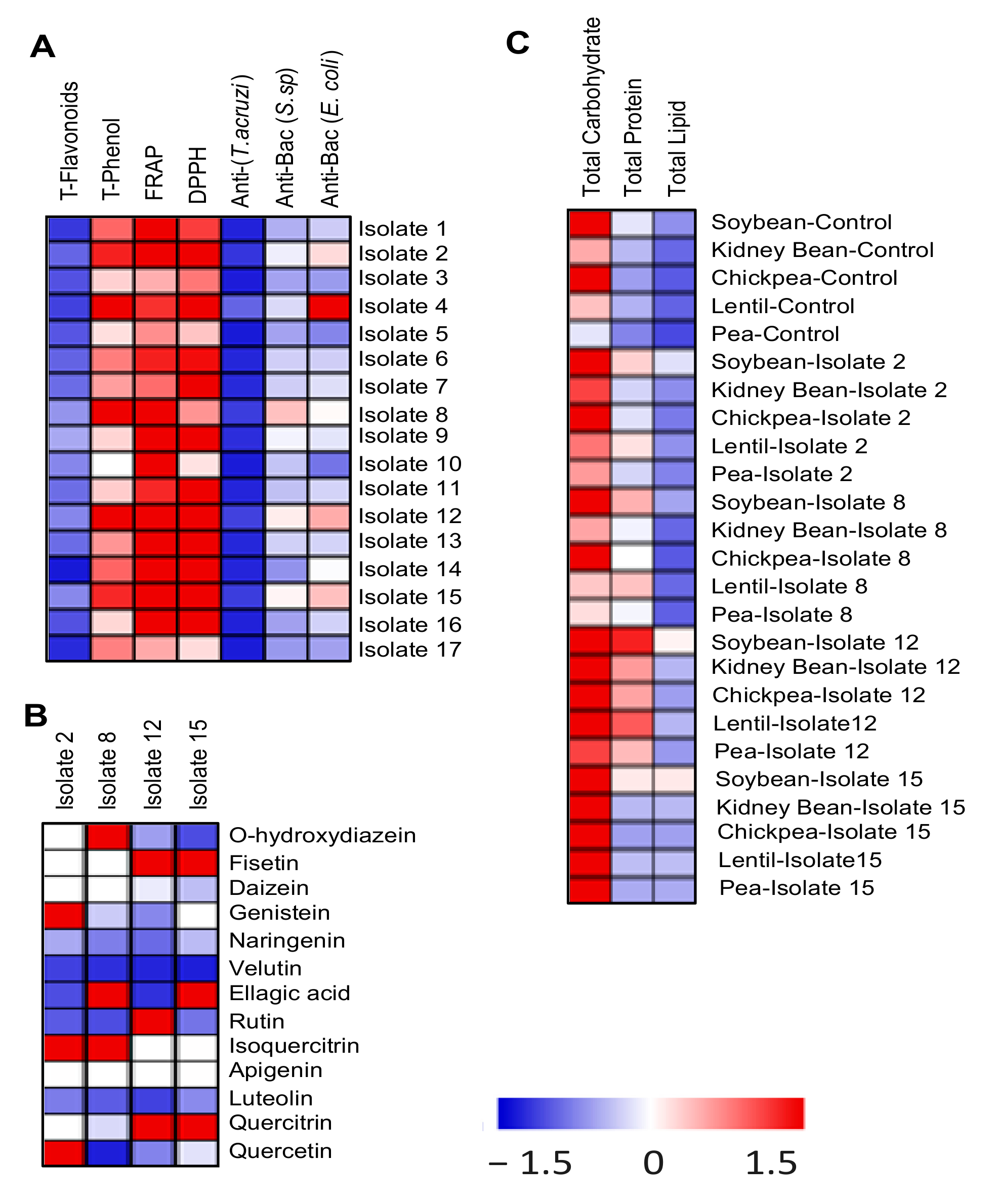
3.1. Characterization of the Isolated Actinomycetes
3.2. Selection of the Potent Biologically Active Actinomycete Isolates
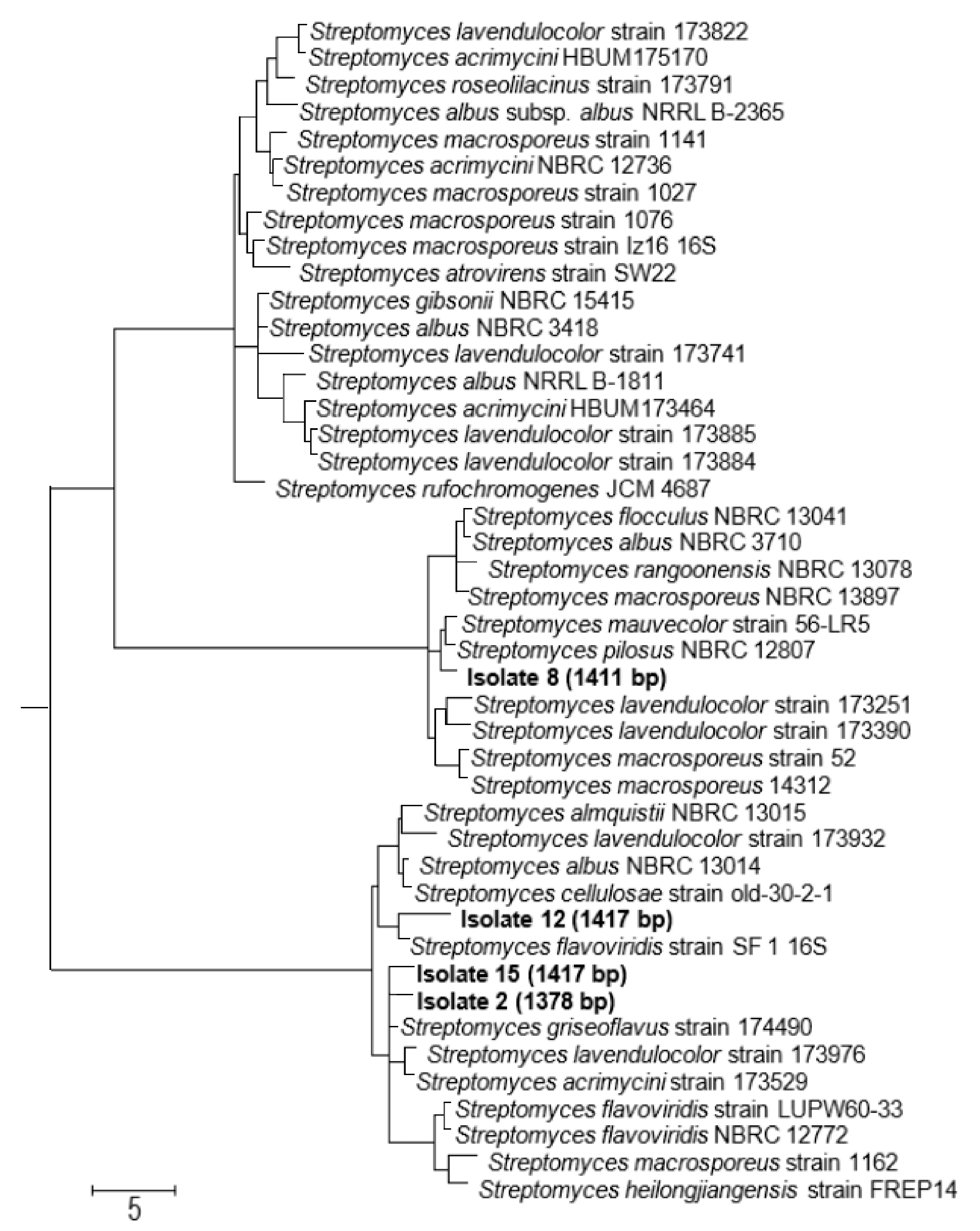
3.3. The Four Selected Biologically Active Isolates belong to the Genus Streptomyces
3.4. Actinomycetes Improved Soil Fertility
3.5. Nitrogen Availability and Metabolism
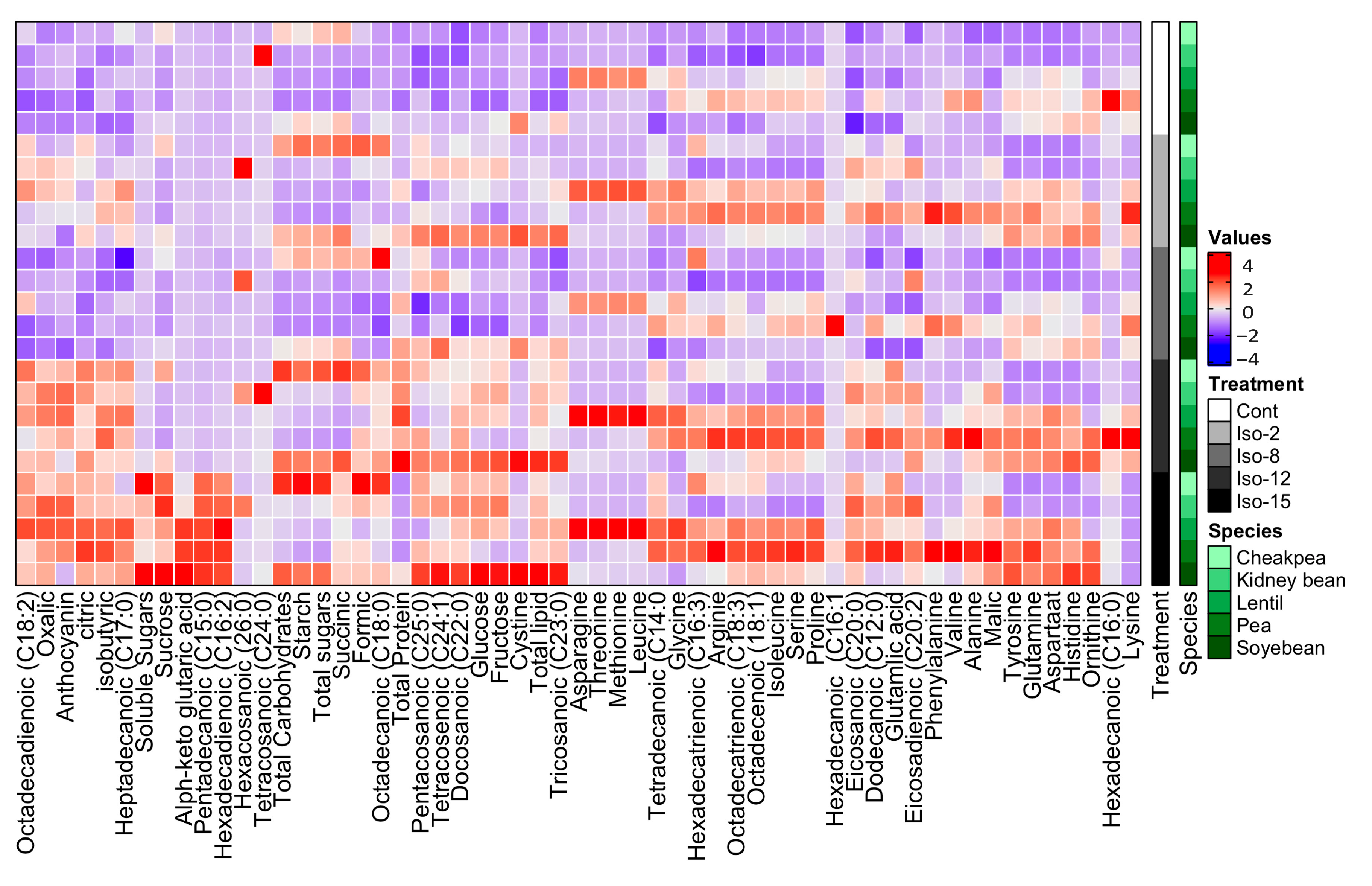
3.7. Amino Acid Composition
3.8. Organic Acid Composition
3.9. Fatty Acid Composition
3.10. Actinomycetes Enhanced the Nutritional Value of the Treated Legumes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, T.; Acosta-Martinez, V.; Senwo, Z.; Dowd, S.E. Soil Rhizosphere Microbial Communities and Enzyme Activities under Organic Farming in Alabama. Diversity 2011, 3, 308. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, M.E.; Haroun, S.A.; Gomah, A.A.; El-Naggar, N.E.; Badr, H.H. Application of actinomycetes as biocontrol agents in the management of onion bacterial rot diseases. Arch. Phytopathol. Plant Prot. 2013, 46, 1797–1808. [Google Scholar] [CrossRef]
- Behie, S.W.; Bonet, B.; Zacharia, V.M.; McClung, D.J.; Traxler, M.F. Molecules to Ecosystems: Actinomycete Natural Products In situ. Front. Microbiol. 2017, 7, 2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, Z.; Mohamed, M.; Namoury, N. Biosynthesis, characterization, and antimicrobial activity of silver nanoparticles from actinomycetes. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 119–127. [Google Scholar]
- Das, S.; Lyla, P.S.; Ajmal Khan, S. Distribution and generic composition of culturable marine actinomycetes from the sediments of Indian continental slope of Bay of Bengal. Chin. J. Oceanol. Limnol. 2008, 26, 166–177. [Google Scholar] [CrossRef]
- Sousa, C.d.S.; Soares, A.C.F.; Garrido, M.d.S. Characterization of streptomycetes with potential to promote plant growth and biocontrol. Sci. Agric. 2008, 65, 50–55. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Sayed, E.-S.A.; Rasmey, A.-H.M. Indole-3-acetic acid (IAA) production by Streptomyces atrovirens isolated from rhizospheric soil in Egypt. J. Biol. Earth Sci. 2013, 3, 12. [Google Scholar]
- Arasu, M.V.; Esmail, G.A.; Al-Dhabi, N.A.; Ponmurugan, K. Managing Pests and Diseases of Grain Legumes with Secondary Metabolites from Actinomycetes. In Plant Growth Promoting Actinobacteria: A New Avenue for Enhancing the Productivity and Soil Fertility of Grain Legumes; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer: Singapore, 2016; pp. 83–98. [Google Scholar] [CrossRef]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl. Soil Ecol. 2008, 40, 510–517. [Google Scholar] [CrossRef]
- Hong, T.-Y.; Cheng, C.-W.; Huang, J.-W.; Meng, M. Isolation and biochemical characterization of an endo-1,3-β-glucanase from Streptomyces sioyaensis containing a C-terminal family 6 carbohydrate-binding module that binds to 1,3-β-glucan. Microbiology 2002, 148, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreevidya, M.; Gopalakrishnan, S.; Kudapa, H.; Varshney, R.K. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz. J. Microbiol. 2016, 47, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poovarasan, S.; Mohandas, S.; Paneerselvam, P.; Saritha, B.; Ajay, K.M. Mycorrhizae colonizing actinomycetes promote plant growth and control bacterial blight disease of pomegranate (Punica granatum L. cv Bhagwa). Crop Prot. 2013, 53, 175–181. [Google Scholar] [CrossRef]
- Warrad, M.; Hassan, Y.M.; Mohamed, M.S.M.; Hagagy, N.; Al-Maghrabi, O.A.; Selim, S.; Saleh, A.M.; AbdElgawad, H. A Bioactive Fraction from Streptomyces sp. Enhances Maize Tolerance against Drought Stress. J. Microbiol. Biotechnol. 2020, 30, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; Nassar, A.H.; Sivasithamparam, K. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl. Soil Ecol. 2008, 39, 161–171. [Google Scholar] [CrossRef]
- Boonkerd, N. Symbiotic association between Frankia and actinorhizal plants. In Nitrogen Fixation with Non-Legumes, Proceedings of the 7th International Symposium on Nitrogen Fixation with Non-Legumes, Faisalabad, Pakistan, 16–21 October 1996; Malik, K.A., Mirza, M.S., Ladha, J.K., Eds.; Malik, K.A., Mirza, M.S., Ladha, J.K., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 327–331. [Google Scholar] [CrossRef]
- Weston, D.J.; Pelletier, D.A.; Morrell-Falvey, J.L.; Tschaplinski, T.J.; Jawdy, S.S.; Lu, T.-Y.; Allen, S.M.; Melton, S.J.; Martin, M.Z.; Schadt, C.W.; et al. Pseudomonas fluorescens Induces Strain-Dependent and Strain-Independent Host Plant Responses in Defense Networks, Primary Metabolism, Photosynthesis, and Fitness. Mol. Plant-Microbe Interact. 2012, 25, 765–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, A.M.; Murphy, T.M.; Okubara, P.A.; Jacobsen, K.R.; Swensen, S.M.; Pawlowski, K. Novel Expression Pattern of Cytosolic Gln Synthetase in Nitrogen-Fixing Root Nodules of the Actinorhizal Host, Datisca glomerata. Plant Physiol. 2004, 135, 1849–1862. [Google Scholar] [CrossRef] [Green Version]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Große, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-Induced Resistance Against Oak Powdery Mildew Involves Host Plant Responses in Defense, Photosynthesis, and Secondary Metabolism Pathways. Mol. Plant-Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Waksman, S.A. The Actinomycetes. Vol. II. Classification, Identification and Descriptions of Genera and Species; Baillière, Tindall & Cox, Ltd.: London, UK, 1961. [Google Scholar]
- Hozzein, W.N.; Abuelsoud, W.; Wadaan, M.A.M.; Shuikan, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. Exploring the potential of actinomycetes in improving soil fertility and grain quality of economically important cereals. Sci. Total Environ. 2019, 651, 2787–2798. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species1. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar]
- Kawato, M.; Shinobu, R. On Streptomyces herbaricolor nov. sp.: Supplement A simple technique for the microscopical observation. Suppl. Simple Tech. Microsc. Obs. Mem. Osaka Univ. Lib. Arts Educ. B 1959, 8, 114–119. [Google Scholar]
- Oskay, M.; Tamer, A.; Azeri, C. Antibacterial activity of some actinomycetes isolated from farming soils of Turkey. Afr. J. Biotech. 2004, 3, 441–446. [Google Scholar]
- Hozzein, W.N.; Goodfellow, M. Streptomyces synnematoformans sp. nov., a novel actinomycete isolated from a sand dune soil in Egypt. Int. J. Syst. Evol. Microbiol. 2007, 57, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.; Barrow, C. A Simple 96-Well Microplate Method for Estimation of Total Polyphenol Content in Seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Agusa, T.; Kunito, T.; Yasunaga, G.; Iwata, H.; Subramanian, A.; Ismail, A.; Tanabe, S. Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar. Pollut. Bull. 2005, 51, 896–911. [Google Scholar] [CrossRef]
- Brown, M.E.; Burlingham, S.K.; Jackson, R.M. Studies on Azotobacter species in soil. Plant Soil 1962, 17, 309–319. [Google Scholar] [CrossRef]
- AbdElgawad, H.; De Vos, D.; Zinta, G.; Domagalska, M.A.; Beemster, G.T.; Asard, H. Grassland species differentially regulate proline concentrations under future climate conditions: An integrated biochemical and modelling approach. New Phytol. 2015, 208, 354–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620. [Google Scholar] [CrossRef] [PubMed]
- Madany, M.M.Y.; Obaid, W.A.; Hozien, W.; AbdElgawad, H.; Hamed, B.A.; Saleh, A.M. Salicylic acid confers resistance against broomrape in tomato through modulation of C and N metabolism. Plant Physiol. Biochem. 2020, 147, 322–335. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Van den Ende, W.; Janssens, I.A.; Asard, H. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: A comparison of fructan and non-fructan accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef] [PubMed]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO(2) induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224, 121–131. [Google Scholar] [CrossRef]
- Robinson, S.A.; Slade, A.P.; Fox, G.G.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. The role of glutamate dehydrogenase in plant nitrogen metabolism. Plant Physiol. 1991, 95, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, W.M.; Brendle-Behnisch, E. Rapid modulation of spinach leaf nitrate reductase activity by photosynthesis: I. Modulation in vivo by CO2 availability. Plant Physiol. 1991, 96, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Williams, S.T.; Goodfellow, M.; Alderson, G.; Wellington, E.M.H.; Sneath, P.H.A.; Sackin, M.J. Numerical Classification of Streptomyces and Related Genera. Microbiology 1983, 129, 1743–1813. [Google Scholar] [CrossRef] [Green Version]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of secondary metabolism in actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Janardhan, A.; Kumar, A.P.; Viswanath, B.; Saigopal, D.V.R.; Narasimha, G. Production of Bioactive Compounds by Actinomycetes and Their Antioxidant Properties. Biotechnol. Res. Int. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrey, S.D.; Erkenbrack, E.; Früh, E.; Fengler, S.; Hommel, K.; Horlacher, N.; Schulz, D.; Ecke, M.; Kulik, A.; Fiedler, H.-P.; et al. Production of fungal and bacterial growth modulating secondary metabolites is widespread among mycorrhiza-associated streptomycetes. BMC Microbiol. 2012, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jog, R.; Nareshkumar, G.; Rajkumar, S. Enhancing Soil Health and Plant Growth Promotion by Actinomycetes. In Plant Growth Promoting Actinobacteria: A New Avenue for Enhancing the Productivity and Soil Fertility of Grain Legumes; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer: Singapore, 2016; pp. 33–45. [Google Scholar] [CrossRef]
- Korkina, L.G.; Afanas’Ev, I.B. Antioxidant and Chelating Properties of Flavonoids. In Advances in Pharmacology; Sies, H., Ed.; Academic Press: New York, NY, USA, 1996; Volume 38, pp. 151–163. [Google Scholar]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L. Effects of a Microbial Inoculant and Organic Fertilizers on the Growth, Photosynthesis and Yield of Sweet Corn. J. Crop Prod. 2001, 3, 183–214. [Google Scholar] [CrossRef]
- Leaungvutiviroj, C.; Piriyaprin, S.; Limtong, P.; Sasaki, K. Relationships between soil microorganisms and nutrient contents of Vetiveria zizanioides (L.) Nash and Vetiveria nemoralis (A.) Camus in some problem soils from Thailand. Appl. Soil Ecol. 2010, 46, 95–102. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Pande, S.; Sharma, M.; Humayun, P.; Kiran, B.K.; Sandeep, D.; Vidya, M.S.; Deepthi, K.; Rupela, O. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011, 30, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Nimnoi, P.; Pongsilp, N.; Lumyong, S. Co-Inoculation of Soybean (Glycine max) with Actinomycetes and Bradyrhizobium Japonicum Enhances Plant Growth, Nitrogenase Activity and Plant Nutrition. J. Plant Nutr. 2014, 37, 432–446. [Google Scholar] [CrossRef]
- Soe, K.M.; Bhromsiri, A.; Karladee, D. Effects of selected endophytic actinomycetes (Streptomyces sp.) and Bradyrhizobia from Myanmar on growth, nodulation, nitrogen fixation and yield of different soybean varieties. Chiang Mai Univ. J. Nat. Sci. 2010, 9, 95–110. [Google Scholar]
- Madhaiyan, M.; Peng, N.; Te, N.S.; Hsin, I.C.; Lin, C.; Lin, F.; Reddy, C.; Yan, H.; Ji, L. Improvement of plant growth and seed yield in Jatropha curcas by a novel nitrogen-fixing root associated Enterobacter species. Biotechnol. Biofuels 2013, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Gupta, A.K.; Gaur, V.S.; Kumar, A. Relationship of Nitrogen Use Efficiency with the Activities of Enzymes Involved in Nitrogen Uptake and Assimilation of Finger Millet Genotypes Grown under Different Nitrogen Inputs. Sci. World J. 2012, 2012, 625731. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.-J.; Miki, T. Incorporating the soil environment and microbial community into plant competition theory. Front. Microbiol. 2015, 6, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Różycki, H.; Strzelczyk, E. Free amino acids production by actinomycetes, isolated from soil, rhizosphere, and mycorrhizosphere of pine (Pinns sylvestris L.). Zent. Mikrobiol. 1986, 141, 423–429. [Google Scholar] [CrossRef]
- Vinall, K.; Schmidt, S.; Brackin, R.; Lakshmanan, P.; Robinson, N. Amino acids are a nitrogen source for sugarcane. Funct. Plant Biol. 2012, 39, 503–511. [Google Scholar] [CrossRef]
- Rodrı́guez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. South Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Jog, R.; Nareshkumar, G.; Rajkumar, S. Plant growth promoting potential and soil enzyme production of the most abundant Streptomyces spp. from wheat rhizosphere. J. Appl. Microbiol. 2012, 113, 1154–1164. [Google Scholar] [CrossRef]
- Sakuradani, E.; Kobayashi, M.; Shimizu, S. Δ6-Fatty acid desaturase from an arachidonic acid-producing Mortierella fungus: Gene cloning and its heterologous expression in a fungus, Aspergillus. Gene 1999, 238, 445–453. [Google Scholar] [CrossRef]
- Çakir, Ö.; Uçarli, C.; Tarhan, Ç.a.; Pekmez, M.; Turgut-Kara, N. Nutritional and health benefits of legumes and their distinctive genomic properties J. Food Sci. Technol. 2019, 39, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Strzelczyk, E.; Leniarska, U. Production of B-group vitamins by mycorrhizal fungi and actinomycetes isolated from the root zone of pine (Pinus sylvestris L.). Plant Soil 1985, 86, 387–394. [Google Scholar] [CrossRef]
- Mozafar, A.; Oertli, J.J. Uptake of a microbially-produced vitamin (B12) by soybean roots. Plant Soil 1992, 139, 23–30. [Google Scholar] [CrossRef]

| Nitrogen | Carbon | ||||||
|---|---|---|---|---|---|---|---|
| Plant-Isolate | Root | Shoot | Seed | Soil | Root | Shoot | Seed |
| Soybean-Cont | 34.8 ± 0.8 a | 51.5 ± 1.1 a | 29.1± 0.8 a | 0.4 ± 0.01 a | 239.5 ± 9.60 a | 557.0 ± 90.5 a | 104.8 ± 0.40 a |
| Soybean-I2 | 51.1 ± 3.5 a | 65.8 ± 1.5 b | 41.2 ± 2.1 b | 0.6 ± 0.01 b | 329.6 ± 13.0 b | 374.7 ± 0.00 a | 131.2 ± 5.40 b |
| Soybean-I8 | 52.4 ± 4.0 a | 48.1 ± 1.1 a | 38.3 ± 2.1 a | 0.4 ± 0.20 a | 281.0 ± 11.3 a | 390.9 ± 0.00 a | 136.2 ± 1.70 b |
| Soybean-I12 | 55.7 ± 1.6 b | 71.4 ± 1.6 b | 50.9 ± 0.5 b | 1.0 ± 0.20 a | 404.3 ± 16.0 b | 426.1 ± 0.00 a | 159.5 ± 5.20 b |
| Soybean-I15 | 66.8 ± 3.7 b | 86.7 ± 1.9 b | 55.8 ± 0.5 b | 1.0 ± 0.10 a | 434.3 ± 17.0 b | 425.1 ± 0.00 a | 112.0 ± 20.7 a |
| Kidney Bean-Cont | 48.3 ± 3.9 a | 53.5 ± 2.9 a | 37.4 ± 1.5 a | 0.4 ± 0.03 a | 292.2 ± 1.10 a | 349.8 ± 8.10 a | 158.7 ± 1.40 a |
| Kidney Bean-I2 | 58.9 ± 7.3 a | 55.8 ± 3.0 a | 38.8 ± 0.2 a | 0.5 ± 0.00 a | 331.0 ± 1.30 b | 421.4 ± 0.00 a | 161.3 ± 2.90 a |
| Kidney Bean-I8 | 51.6 ± 7.3 a | 40.8 ± 2.2 a | 37.9 ± 2.6 a | 0.5 ± 0.00 a | 290.9 ± 1.00 a | 392.3 ± 0.00 a | 219.1 ± 1.30 a |
| Kidney Bean-I12 | 62.1 ± 9.2 a | 57.9 ± 3.2 a | 38.5 ± 0.2 a | 0.6 ± 0.00 b | 327.5 ± 1.00 b | 433.9 ± 0.00 a | 160.8 ± 3.60 a |
| Kidney Bean-I15 | 61.3 ± 6.4 a | 73.5 ± 4.0 a | 33.6 ± 0.3 a | 0.7 ± 0.00 b | 262.3 ± 1.00 b | 404.3 ± 0.00 a | 235.5 ± 6.50 a |
| Chickpea-Cont | 44.4 ± 1.4 a | 54.0 ± 2.9 a | 36.2 ± 0.6 a | 0.3 ± 0.10 a | 295.0 ± 1.20 a | 236.9 ± 38.0 a | 170.8 ± 2.80 a |
| Chickpea-I2 | 56.2 ± 5.1 a | 83.0 ± 4.5 b | 37.5 ± 0.3 a | 0.7 ± 0.01 b | 292.0 ± 1.10 a | 92.8 ± 0.00 a | 219.0 ± 2.60 a |
| Chickpea-I8 | 48.8 ± 3.8 a | 60.7 ± 3.3 a | 37.4 ± 1.4 a | 0.6 ± 0.01 a | 285.0 ± 1.10 b | 496.9 ± 0.00 a | 232.1 ± 1.50 a |
| Chickpea-I12 | 41.7 ± 0.5 a | 61.9 ± 3.4 a | 34.8 ± 0.2 a | 0.6 ± 0.00 b | 285.8 ± 1.00 b | 158.9 ± 0.00 a | 237.6 ± 3.50 a |
| Chickpea-I15 | 51.9 ± 1.0 a | 109.4 ± 6.0 b | 40.9 ± 0.5 b | 0.9 ± 0.10 b | 299.6 ± 1.00 a | 105.3 ± 0.00 a | 197.6 ± 1.70 b |
| Lentil-Cont | 37.0 ± 0.9 a | 54.5 ± 1.2 a | 30.9 ± 0.9 a | 0.3 ± 0.10 a | 254.7 ± 10.0 a | 304.7 ± 34.0 a | 357.7 ± 2.10 a |
| Lentil-I2 | 41.3 ± 2.2 a | 57.8 ± 1.3 a | 41.9 ± 1.3 b | 0.6 ± 0.01 a | 361.2 ± 14.0 b | 2172.8 ± 0.00 b | 458.0 ± 16.8 b |
| Lentil-I8 | 35.0 ± 0.6 a | 53.1 ± 1.2 a | 31.7 ± 0.9 a | 0.5 ± 0.00 a | 264.0 ± 10.6 a | 2267.2 ± 0.00 b | 480.2 ± 9.60 b |
| Lentil-I12 | 57.7 ± 1.6 b | 65.6 ± 1.5 b | 55.6 ± 1.8 b | 1.1 ± 0.20 a | 435.3 ± 18.0 b | 2497.7 ± 0.00 b | 533.0 ± 19.0 b |
| Lentil-I15 | 63.1 ± 1.8 b | 71.2 ± 1.3 b | 60.8 ± 2.0 b | 0.7 ± 0.00 b | 476.0 ± 19.1 b | 2465.2 ± 0.00 b | 392.0 ± 60.5 a |
| Pea-Cont | 32.0 ± 0.7 a | 52.8 ± 1.2 a | 26.3 ± 0.7 a | 0.3 ± 0.10 a | 210.5 ± 8.00 a | 435.3 ± 50.0 a | 158.7 ± 21.0 a |
| Pea-I2 | 43.5 ± 3.0 a | 63.1 ± 1.4 b | 42.6 ± 1.3 b | 0.7 ± 0.00 b | 363.2 ± 15.0 b | 1497.3 ± 0.00 b | 337.2 ± 13.0 b |
| Pea-I8 | 35.9 ± 0.7 a | 57.6 ± 1.3 a | 32.3 ± 0.9 b | 0.5 ± 0.10 a | 265.4 ± 10.0 a | 1562.3 ± 0.00 b | 293.2 ± 23.0 a |
| Pea-I12 | 53.9 ± 1.4 b | 69.9 ± 1.6 b | 51.3 ± 1.6 b | 0.8 ± 0.10 b | 392.1 ± 16.0 b | 728.1 ± 0.00 a | 268.3 ± 9.60 a |
| Pea-I15 | 75.9 ± 1.8 b | 72.7 ± 1.6 b | 57.4 ± 3.1 b | 1.1 ± 0.20 a | 478.6 ± 19.0 b | 1698.9 ± 0.00 b | 305.0 ± 41.3 a |
| GDH | GS | NR | GOGAT | |||||
|---|---|---|---|---|---|---|---|---|
| Plant-Isolate | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot |
| Soybean-Cont | 0.97 ± 0.09 a | 0.47 ± 0.03 a | 1.99 ± 0.49 a | 0.95 ± 0.21 a | 0.84 ± 0.01 a | 0.22 ± 0.01 a | 1.82 ± 0.06 a | 0.59 ± 0.05 a |
| Soybean-I2 | 0.91 ± 0.08 a | 0.49 ± 0.08 a | 6.56 ± 0.39 b | 1.45 ± 0.04 a | 1.53 ± 0.15 a | 0.20 ± 0.02 a | 2.39 ± 0.04 b | 0.63 ± 0.05 a |
| Soybean-I8 | 0.93 ± 0.09 a | 0.80 ± 0.11 a | 6.70 ± 0.39 b | 2.32 ± 0.08 b | 1.09 ± 0.18 a | 0.27 ± 0.01 a | 1.53 ± 0.02 b | 0.64 ± 0.05 a |
| Soybean-I12 | 1.20 ± 0.11 a | 0.69 ± 0.08 a | 8.31 ± 0.49 b | 1.63 ± 0.09 a | 1.56 ± 0.02 b | 0.32 ± 0.02 b | 3.22 ± 0.10 b | 0.75 ± 0.06 a |
| Soybean-I15 | 0.97 ± 0.09 a | 0.97 ± 0.18 a | 6.99 ± 0.41 b | 1.17 ± 0.11 a | 1.87 ± 0.08 b | 0.28 ± 0.01 a | 3.80 ± 0.25 b | 0.67 ± 0.05 a |
| Kidney Bean-Cont | 0.92 ± 0.05 a | 0.51 ± 0.00 a | 2.63 ± 0.21 a | 1.67 ± 0.24 a | 0.05 ± 0.01 a | 0.13 ± 0.02 a | 1.59 ± 0.23 a | 6.91 ± 0.65 a |
| Kidney Bean-I2 | 1.07 ± 0.08 a | 0.91 ± 0.13 a | 7.56 ± 0.45 b | 1.69 ± 0.11 a | 0.15 ± 0.00 b | 0.20 ± 0.02 a | 2.54 ± 0.04 a | 9.83 ± 0.76 a |
| Kidney Bean-I8 | 1.10 ± 0.08 a | 1.43 ± 0.23 a | 7.72 ± 0.45 b | 2.81 ± 0.24 a | 0.09 ± 0.01 a | 0.31 ± 0.02 b | 1.62 ± 0.08 a | 10.05 ± 0.78 a |
| Kidney Bean-I12 | 1.36 ± 0.10 a | 1.00 ± 0.12 a | 9.66 ± 0.57 b | 2.50 ± 0.10 a | 0.17 ± 0.00 b | 0.41 ± 0.02 b | 3.38 ± 0.06 b | 10.05 ± 0.78 a |
| Kidney Bean-I15 | 1.14 ± 0.09 a | 0.80 ± 0.02 b | 8.05 ± 0.47 b | 1.73 ± 0.06 a | 0.20 ± 0.00 b | 0.32 ± 0.02 b | 3.38 ± 0.05 b | 10.48 ± 0.81 a |
| Chickpea-Cont | 1.07 ± 0.08 a | 0.49 ± 0.01 a | 2.37 ± 0.49 a | 1.33 ± 0.13 a | 0.15 ± 0.05 a | 0.15 ± 0.01 a | 0.85 ± 0.15 a | 0.76 ± 0.06 a |
| Chickpea-I2 | 0.90 ± 0.07 a | 0.73 ± 0.17 a | 6.03 ± 0.36 b | 1.15 ± 0.04 a | 0.52 ± 0.01 b | 0.13 ± 0.01 a | 1.83 ± 0.03 b | 0.93 ± 0.07 a |
| Chickpea-I8 | 0.92 ± 0.07 a | 1.03 ± 0.12 a | 6.16 ± 0.36 b | 1.67 ± 0.05 a | 0.36 ± 0.02 a | 0.14 ± 0.01 a | 1.27 ± 0.04 a | 0.95 ± 0.07 a |
| Chickpea-I12 | 1.29 ± 0.10 a | 0.80 ± 0.10 a | 7.90 ± 0.46 b | 1.95 ± 0.08 a | 0.49 ± 0.01 b | 0.18 ± 0.01 a | 2.00 ± 0.03 b | 1.00 ± 0.08 a |
| Chickpea-I15 | 0.96 ± 0.07 a | 0.79 ± 0.09 a | 6.42 ± 0.38 b | 0.97 ± 0.03 a | 0.70 ± 0.01 b | 0.14 ± 0.01 a | 2.48 ± 0.04 b | 0.99 ± 0.08 a |
| Lentil-Cont | 0.88 ± 0.05 a | 0.47 ± 0.02 a | 2.07 ± 0.29 a | 1.31 ± 0.07 a | 0.39 ± 0.07 a | 0.16 ± 0.01 a | 0.41 ± 0.01 a | 1.43 ± 0.36 a |
| Lentil-I2 | 1.05 ± 0.09 a | 0.91 ± 0.07 b | 5.08 ± 0.30 b | 1.06 ± 0.08 a | 1.43 ± 0.18 b | 0.19 ± 0.04 a | 0.60 ± 0.03 b | 1.23 ± 0.31 a |
| Lentil-I8 | 1.07 ± 0.09 a | 0.96 ± 0.18 a | 5.19 ± 0.31 b | 1.42 ± 0.15 a | 0.72 ± 0.08 a | 0.22 ± 0.03 a | 0.50 ± 0.04 a | 3.25 ± 0.23 a |
| Lentil-I12 | 1.31 ± 0.11 a | 0.58 ± 0.07 a | 6.28 ± 0.37 b | 1.22 ± 0.07 a | 1.73 ± 0.20 b | 0.28 ± 0.04 a | 0.73 ± 0.02 b | 1.74 ± 0.44 a |
| Lentil-I15 | 1.12 ± 0.09 a | 0.80 ± 0.05 b | 5.41 ± 0.32 b | 0.93 ± 0.04 a | 1.34 ± 0.21 a | 0.23 ± 0.03 a | 0.78 ± 0.01 b | 1.43 ± 0.31 a |
| Pea-Cont | 0.62 ± 0.09 a | 0.55 ± 0.02 a | 2.71 ± 0.44 a | 1.03 ± 0.09 a | 0.30 ± 0.07 a | 0.12 ± 0.01 a | 1.21 ± 0.11a | 3.87 ± 0.39 a |
| Pea-I2 | 1.27 ± 0.08 b | 0.89 ± 0.07 b | 6.37 ± 0.38 b | 1.22 ± 0.09 a | 1.33 ± 0.21 a | 0.20 ± 0.03 a | 4.81 ± 0.22 b | 6.70 ± 0.52 a |
| Pea-I8 | 1.30 ± 0.09 b | 0.72 ± 0.03 a | 6.51 ± 0.38 b | 1.66 ± 0.17 a | 0.98 ± 0.19 a | 0.27 ± 0.01 b | 4.02 ± 0.32 b | 6.84 ± 0.53 a |
| Pea-I12 | 1.47 ± 0.10 b | 0.60 ± 0.07 a | 6.76 ± 0.40 b | 1.25 ± 0.07 a | 1.98 ± 0.01 b | 0.29 ± 0.01 b | 5.70 ± 0.18 b | 9.55 ± 0.74 b |
| Pea-I15 | 1.36 ± 0.09 b | 0.86 ± 0.04 b | 6.79 ± 0.40 b | 1.11 ± 0.04 a | 1.82 ± 0.35 a | 0.28 ± 0.01 b | 5.91 ± 0.10 b | 7.13 ± 0.55 b |
| Plant-Isolate | Photosynthetic Rate | Ch a+b Content |
|---|---|---|
| Soybean-Cont | 15.17 ± 1.20 a | 4.26 ± 0.20 a |
| Soybean-I2 | 21.82 ± 1.20 a | 6.30 ± 0.91 a |
| Soybean-I8 | 18.55 ± 3.30 b | 6.71 ± 0.20 ab |
| Soybean-I12 | 26.68 ± 0.03 b | 8.87 ± 0.50 b |
| Soybean-I15 | 22.30 ± 1.33 b | 4.48 ± 0.70 b |
| Kidney Bean-Cont | 17.93 ± 1.60 a | 4.92 ± 0.31 a |
| Kidney Bean-I2 | 26.23 ± 2.03 a | 5.14 ± 0.42 a |
| Kidney Bean-I8 | 19.01 ± 2.10 a | 5.55 ± 1.00 b |
| Kidney Bean-I12 | 20.03 ± 4.25 a | 5.40 ± 1.10 b |
| Kidney Bean-I15 | 16.00 ± 2.61 a | 5.14 ± 1.50 b |
| Chickpea-Cont | 21.26 ± 1.40 a | 3.87 ± 0.77 a |
| Chickpea-I2 | 23.31 ± 2.43 b | 7.26 ± 0.86 b |
| Chickpea-I8 | 19.29 ± 3.80 a | 4.13 ± 0.20 a |
| Chickpea-I12 | 19.05 ± 1.54 a | 4.70 ± 0.80 ab |
| Chickpea-I15 | 21.29 ± 2.10 a | 5.60 ± 1.70 b |
| Lentil-Cont | 18.11 ± 0.80 a | 4.76 ± 0.51 a |
| Lentil-I2 | 31.27 ± 1.13 b | 7.44 ± 0.97 b |
| Lentil-I8 | 22.11 ± 1.50 b | 6.41 ± 0.70 a |
| Lentil-I12 | 30.28 ± 1.21 b | 8.23 ± 0.09 a |
| Lentil-I15 | 38.97 ± 1.10 b | 9.30 ± 1.14 a |
| Pea-Cont | 16.09 ± 0.60 a | 5.02 ± 0.84 a |
| Pea-I2 | 26.51 ± 1.10 b | 7.12 ± 1.30 b |
| Pea-I8 | 21.54 ± 0.60 a | 6.34 ± 0.30 b |
| Pea-I12 | 23.34 ± 2.21 b | 9.46 ± 0.91 a |
| Pea-I15 | 37.40 ± 2.12 b | 9.10 ± 0.85 b |
| Plant-Isolate | Seed Yield (DW/100 Seed) |
|---|---|
| Soybean-Cont. | 15.77 ± 0.8 a |
| Soybean-I2 | 20.18 ± 0.1 b |
| Soybean-I8 | 27.01 ± 0.2 b |
| Soybean-I12 | 13.68 ± 3.70 a |
| Soybean-I15 | 19.86 ± 0.50 b |
| Kidney Bean-Cont. | 16.91 ± 0.10 a |
| Kidney Bean-I2 | 17.60 ± 0.70 a |
| Kidney Bean-I8 | 15.78 ± 0.90 a |
| Kidney Bean-I12 | 14.14 ± 3.00 a |
| Kidney Bean-I15 | 24.31 ± 17.0 a |
| Chickpea-Cont. | 23.37 ± 0.17 a |
| Chickpea-I2 | 25.20 ± 1.90 a |
| Chickpea-I8 | 32.35 ± 1.10 b |
| Chickpea-I12 | 29.70 ± 0.41 b |
| Chickpea-I15 | 20.03 ± 3.80 a |
| Lentil-Cont. | 2.10 ± 0.10 a |
| Lentil-I2 | 4.28 ± 0.50 b |
| Lentil-I8 | 3.01 ± 0.90 b |
| Lentil-I12 | 5.66 ± 0.81 b |
| Lentil-I15 | 5.90 ± 0.70 b |
| Pea-Cont. | 12.78 ± 1.10 a |
| Pea-I2 | 19.65 ± 0.57 b |
| Pea-I8 | 15.39 ± 2.00 a |
| Pea-I12 | 14.19 ± 2.21 a |
| Pea-I15 | 23.56 ± 2.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.Y.; Selim, S.; Zinta, G.; Mousa, A.S.M.; Hozzein, W.N. Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism. Biomolecules 2020, 10, 1675. https://doi.org/10.3390/biom10121675
AbdElgawad H, Abuelsoud W, Madany MMY, Selim S, Zinta G, Mousa ASM, Hozzein WN. Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism. Biomolecules. 2020; 10(12):1675. https://doi.org/10.3390/biom10121675
Chicago/Turabian StyleAbdElgawad, Hamada, Walid Abuelsoud, Mahmoud M. Y. Madany, Samy Selim, Gaurav Zinta, Ahmed S. M. Mousa, and Wael N. Hozzein. 2020. "Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism" Biomolecules 10, no. 12: 1675. https://doi.org/10.3390/biom10121675
APA StyleAbdElgawad, H., Abuelsoud, W., Madany, M. M. Y., Selim, S., Zinta, G., Mousa, A. S. M., & Hozzein, W. N. (2020). Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism. Biomolecules, 10(12), 1675. https://doi.org/10.3390/biom10121675






