Structural Characteristics and Hypolipidemic Activity of Theabrownins from Dark Tea Fermented by Single Species Eurotium cristatum PW-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Theabrownins
2.3. Chemical Composition Analysis of Theabrownins
2.4. Preparation of Theabrownins with Different Molecular Weights
2.5. UV-Vis Absorption and FT-IR Spectroscopic Analysis of Fractionated Theabrownins
2.6. Hypolipidemic Activity of Fractionated Theabrownins in Zebrafish Model
2.7. Structural Characterization of TBs-10-30k
2.7.1. Thermal Properties
2.7.2. X-Ray Diffraction
2.7.3. Morphological Characterization
2.7.4. Pyrolysis-Gas Chromatography-Mass Spectrometry Analysis
2.8. Statistical Analysis
3. Results
3.1. Compositions and Molecular Weight Distributions of Theabrownins
3.2. Spectroscopic Characteristics of Theabrownins
3.3. Hypolipidemic Activity of Fractionated Theabrownins
3.4. Structure Characteristics of TBs-10-30k
3.4.1. Thermal Properties
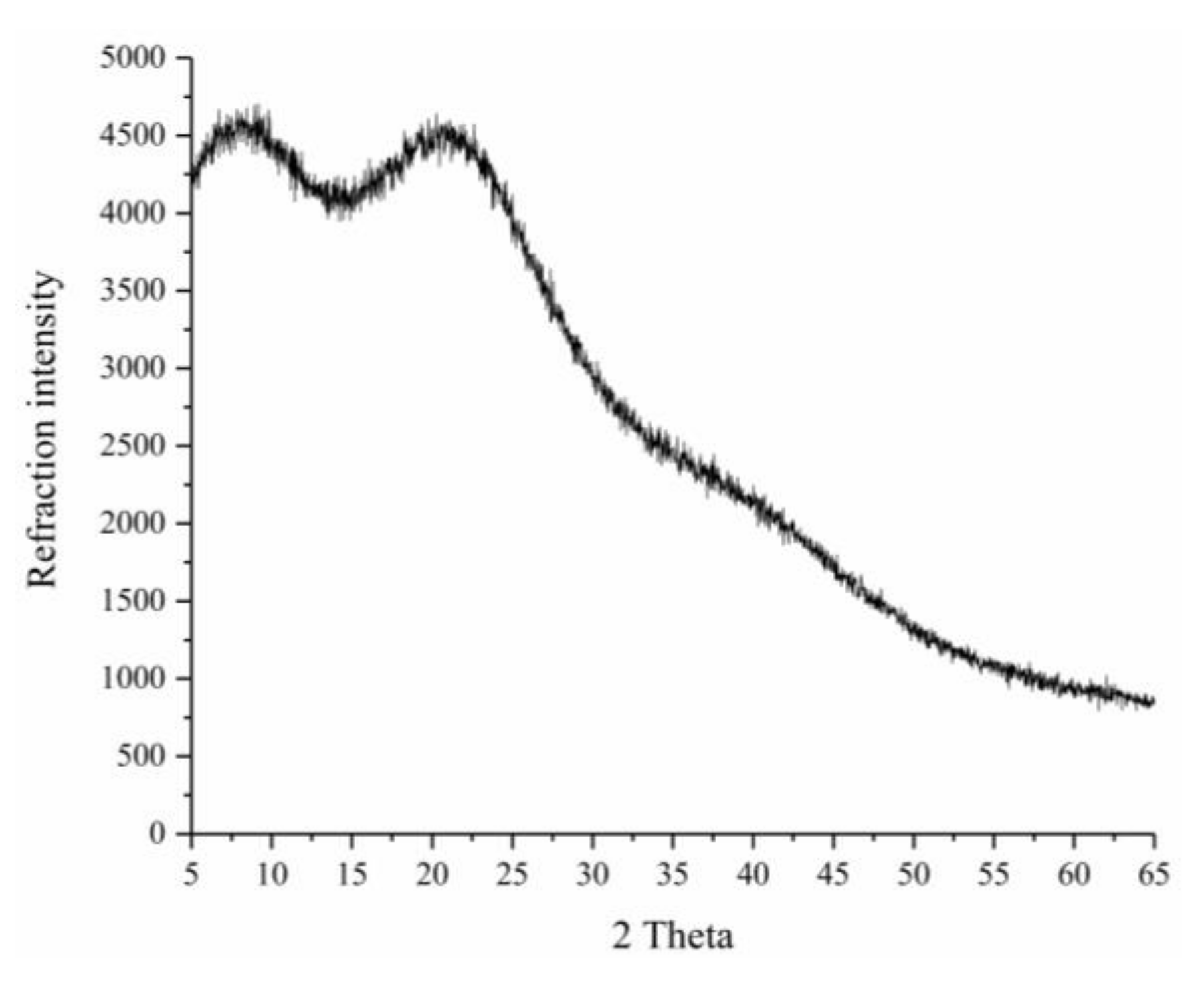
3.4.2. X-Ray Diffraction
3.4.3. Morphological Characteristics
3.4.4. Pyrolysis-Gas Chromatography-Mass Spectrometry Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hasumura, T.; Shimada, Y.; Kuroyanagi, J.; Nishimura, Y.; Meguro, S.; Takema, Y.; Tanaka, T. Green tea extract suppresses adiposity and affects the expression of lipid metabolism genes in diet-induced obese zebrafish. Nutr. Metab. 2012, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Lin, J.K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. 2012, 3, 170–177. [Google Scholar] [CrossRef]
- Ikeda, I.; Yamahira, T.; Kato, M.; Ishikawa, A. Black-Tea Polyphenols Decrease Micellar Solubility of Cholesterol in Vitro and Intestinal Absorption of Cholesterol in Rats. J. Agric. Food Chem. 2010, 58, 8591–8595. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Xu, H.; Zhu, Q.; Lu, H.; Zhang, M.; Hao, S.; Fang, C.; Zhang, D.; Wu, X.; et al. Oxidized tea polyphenols prevent lipid accumulation in liver and visceral white adipose tissue in rats. Eur. J. Nutr. 2017, 56, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N. Unraveling the structure of the black tea thearubigins. Arch. Biochem. Biophys. 2010, 501, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Belščak-Cvitanović, A.; Durgo, K.; Chisti, Y.; Gong, J.; Sirisansaneeyakul, S.; Komes, D. Physicochemical properties and biological activities of a high-theabrownins instant Pu-erh tea produced using Aspergillus tubingensis. LWT Food Sci. Technol. 2018, 90, 598–605. [Google Scholar] [CrossRef]
- Chen, D.; Sun, J.; Dong, W.; Shen, Y.; Xu, Z. Effects of polysaccharides and polyphenolics fractions of Zijuan tea (Camellia sinensis var. kitamura) on α-glucosidase activity and blood glucose level and glucose tolerance of hyperglycaemic mice. Int. J. Food Sci. Technol. 2018, 53, 2335–2341. [Google Scholar] [CrossRef]
- Liu, T.; Xiang, Z.; Chen, F.; Yin, D.; Huang, Y.; Xu, J.; Hu, L.; Xu, H.; Wang, X.; Sheng, J. Theabrownin suppresses in vitro osteoclastogenesis and prevents bone loss in ovariectomized rats. Biomed. Pharmacother. 2018, 106, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhou, L.; Yan, B.; Yan, L.; Liu, F.; Tong, P.; Yu, W.; Dong, X.; Xie, L.; Zhang, J.; et al. Theabrownin triggers DNA damage to suppress human osteosarcoma U2OS cells by activating p53 signalling pathway. J. Cell. Mol. Med. 2018, 22, 4423–4436. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wu, F.; Jin, W.; Yan, B.; Chen, X.; He, Y.; Yang, W.; Du, W.; Zhang, Q.; Guo, Y.; et al. Theabrownin Inhibits Cell Cycle Progression and Tumor Growth of Lung Carcinoma through c-myc-Related Mechanism. Front. Pharmacol. 2017, 8, 75. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.; Jin, W.; Yang, W.; Wang, Y.; Yan, B.; Du, W.; Zhang, Q.; Zhang, L.; Guo, Y.; et al. Anti-Proliferative and Apoptosis-Inducing Effect of Theabrownin against Non-small Cell Lung Adenocarcinoma A549 Cells. Front. Pharmacol. 2016, 7, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Peng, C.-X.; Gao, B.; Gong, J.-S. Serum metabolomics analysis of rat after intragastric infusion of Pu-erh theabrownin. J. Sci. Food Agric. 2016, 96, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Peng, C.; Chen, T.; Gao, B.; Zhou, H. Effects of Theabrownin from Pu-erh Tea on the Metabolism of Serum Lipids in Rats: Mechanism of Action. J. Food Sci. 2010, 75, H182–H189. [Google Scholar] [CrossRef]
- Peng, C.-X.; Wang, Q.-P.; Liu, H.-R.; Gao, B.; Sheng, J.; Gong, J. Effects of Zijuan pu-erh tea theabrownin on metabolites in hyperlipidemic rat feces by Py-GC/MS. J. Anal. Appl. Pyrolysis 2013, 104, 226–233. [Google Scholar] [CrossRef]
- Wang, Q.-P.; Peng, C.-X.; Gao, B.; Gong, J.-S. Influence of large molecular polymeric pigments isolated from fermented Zijuan tea on the activity of key enzymes involved in lipid metabolism in rat. Exp. Gerontol. 2012, 47, 672–679. [Google Scholar] [CrossRef]
- Peng, C.-X.; Liu, J.; Liu, H.-R.; Zhou, H.-J.; Gong, J.-S. Influence of different fermentation raw materials on pyrolyzates of Pu-erh tea theabrownin by Curie-point pyrolysis-gas chromatography–mass spectroscopy. Int. J. Biol. Macromol. 2013, 54, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, Q.; Peng, C.; Fan, J.; Dong, W. Curie-point pyrolysis–gas chromatography–mass spectroscopic analysis of theabrownins from fermented Zijuan tea. J. Anal. Appl. Pyrolysis 2012, 97, 171–180. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.-R.; Wu, Y.-P.; Zhang, J.-Q.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. LWT Food Sci. Technol. 2020, 117, 108629. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Chen, K.; Wang, C.Q.; Fan, Y.Q.; Xie, Y.S.; Yin, Z.F.; Xu, Z.J.; Zhang, H.L.; Cao, J.T.; Han, Z.H.; Wang, Y.; et al. Optimizing methods for the study of intravascular lipid metabolism in zebrafish. Mol. Med. Rep. 2015, 11, 1871–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.Z.; Meng, M.; Duan, S.Q.; Feng, C.C.; Wang, C.L. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int. J. Biol. Macromol. 2019, 126, 796–804. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z.J. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Trigui, I.; Yaich, H.; Sila, A.; Cheikh-Rouhou, S.; Bougatef, A.; Blecker, C.; Attia, H.; Ayadi, M.A. Physicochemical properties of water-soluble polysaccharides from black cumin seeds. Int. J. Biol. Macromol. 2018, 117, 937–946. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Ji, X.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv. Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Huang, J.; Luo, G.; Liang, Q.; Wang, D.; Ye, X.; Wu, C.; Wang, L.; Hu, J. Anti-obesity and hypolipidemic effects of Fuzhuan brick tea water extract in high-fat diet-induced obese rats. J. Sci. Food Agric. 2013, 93, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gong, J.; Chisti, Y.; Sirisansaneeyakul, S. Production of theabrownins using a crude fungal enzyme concentrate. J. Biotechnol. 2016, 231, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gong, J.; Chisti, Y.; Sirisansaneeyakul, S. Fungal Isolates from a Pu-Erh Type Tea Fermentation and Their Ability to Convert Tea Polyphenols to Theabrownins. J. Food Sci. 2015, 80, M809–M817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Zhang, Z.; Lu, H.; Gao, X.; Yue, P. High-theabrownins instant dark tea product by Aspergillus niger via submerged fermentation: α-glucosidase and pancreatic lipase inhibition and antioxidant activity. J. Sci. Food Agric. 2017, 97, 5100–5106. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Menet, M.-C.; Sang, S.; Yang, C.S.; Ho, C.-T.; Rosen, R.T. Analysis of Theaflavins and Thearubigins from Black Tea Extract by MALDI-TOF Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 2455–2461. [Google Scholar] [CrossRef]




| No. | Rt (min) 1 | Compound | CAS | Content (%) 2 | SI 3 |
|---|---|---|---|---|---|
| 1 | 7.67 | Phenol | 108-95-2 | 14.90 | 25 |
| 2 | 8.47 | 4-Amino-6-hydroxypyrimidine | 1193-22-2 | 0.91 | 60 |
| 3 | 9.14 | 2-Acetyl pyrrole | 1072-83-9 | 0.38 | 58 |
| 4 | 9.54 | 3-Methylhex-3-en-2-one | 1187-80-0 | 0.36 | 38 |
| 5 | 9.94 | Nonanal | 124-19-6 | 2.22 | 91 |
| 6 | 10.15 | 3,4-Dimethyl-3-pyrrolin-2-one | 4030-22-2 | 0.59 | 64 |
| 7 | 11.17 | 2-Pyranone-6-carboxylic acid | 672-67-3 | 1.21 | 58 |
| 8 | 11.60 | 9-[1-Hydroxy-2-dibutylaminoethyl]-10-chloro phenanthrene | 52979-76-7 | 0.72 | 78 |
| 9 | 11.83 | Decanal | 112-31-2 | 1.31 | 91 |
| 10 | 12.05 | 2,3-Dihydrobenzofuran | 496-16-2 | 0.24 | 76 |
| 11 | 12.86 | Nonanoic acid | 112-05-0 | 0.42 | 53 |
| 12 | 13.47 | Indole | 120-72-9 | 0.22 | 46 |
| 13 | 14.42 | 1,3-Diisocyanato-2-methylbenzene | 91-08-7 | 1.82 | 98 |
| 14 | 15.20 | 7-Amino-1,3-dihydro-indol-2-one | 1000303-02-6 | 0.61 | 64 |
| 15 | 15.30 | 1,3-Dihydro-5-methyl-2H-benzimidazol-2-one | 5400-75-9 | 0.67 | 91 |
| 16 | 16.27 | 1-Chlorododecane | 112-52-7 | 1.76 | 91 |
| 17 | 16.50 | Dihydrocoumarin, 4,4,5,7,8-pentamethyl | 39170-97-3 | 0.53 | 50 |
| 18 | 16.93 | 2,4-Bis(1,1-dimethylethyl)-phenol | 96-76-4 | 1.16 | 96 |
| 19 | 17.58 | Dodecanoic acid | 143-07-7 | 0.35 | 64 |
| 20 | 18.24 | Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester | 1000140-77-5 | 0.97 | 91 |
| 21 | 19.30 | Oxalic acid, cyclobutyl octadecyl ester | 1000309-70-8 | 0.51 | 38 |
| 22 | 19.90 | l-Gala-l-ido-octose | 1000130-12-1 | 0.56 | 32 |
| 23 | 20.40 | Tetradecanoic acid | 544-63-8 | 1.73 | 99 |
| 24 | 20.87 | N-Methylmaleimide | 930-88-1 | 0.44 | 38 |
| 25 | 20.94 | Octadecane | 593-45-3 | 6.04 | 98 |
| 26 | 21.13 | 1-Hexadecanol, 2-methyl- | 2490-48-4 | 0.27 | 18 |
| 27 | 21.34 | Melibiose | 585-99-9 | 0.31 | 32 |
| 28 | 21.75 | Pentadecanoic acid | 1002-84-2 | 1.19 | 98 |
| 29 | 22.00 | Phthalic acid, butyl 2-methylpent-3-yl ester | 1000356-90-8 | 0.40 | 98 |
| 30 | 22.45 | Quinoline-5,8-dione-6-ol, 7-[[(4-cyclohexylbutyl) amino] methyl]- | 1000252-66-9 | 0.30 | 37 |
| 31 | 23.05 | Palmitoleic acid | 373-49-9 | 0.74 | 43 |
| 32 | 23.48 | Hexadecanoic acid | 57-10-3 | 33.72 | 99 |
| 33 | 25.97 | Butyl 9-tetradecenoate | 1000336-51-4 | 0.41 | 53 |
| 34 | 26.27 | Octadecanoic acid | 57-11-4 | 3.52 | 99 |
| 35 | 27.82 | Tetracosane | 646-31-1 | 3.72 | 98 |
| 36 | 29.73 | Eicosane | 112-95-8 | 12.95 | 97 |
| 37 | 30.21 | Diisooctyl phthalate | 131-20-4 | 1.12 | 76 |
| 38 | 32.27 | 2-Ethylacridine | 55751-83-2 | 0.34 | 30 |
| 39 | 34.52 | Benzo[h]quinoline, 2,4-dimethyl- | 605-67-4 | 0.35 | 46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Li, M.; Wu, Y.; Zhong, K.; Gao, H. Structural Characteristics and Hypolipidemic Activity of Theabrownins from Dark Tea Fermented by Single Species Eurotium cristatum PW-1. Biomolecules 2020, 10, 204. https://doi.org/10.3390/biom10020204
Xiao Y, Li M, Wu Y, Zhong K, Gao H. Structural Characteristics and Hypolipidemic Activity of Theabrownins from Dark Tea Fermented by Single Species Eurotium cristatum PW-1. Biomolecules. 2020; 10(2):204. https://doi.org/10.3390/biom10020204
Chicago/Turabian StyleXiao, Yue, Maoyun Li, Yanping Wu, Kai Zhong, and Hong Gao. 2020. "Structural Characteristics and Hypolipidemic Activity of Theabrownins from Dark Tea Fermented by Single Species Eurotium cristatum PW-1" Biomolecules 10, no. 2: 204. https://doi.org/10.3390/biom10020204
APA StyleXiao, Y., Li, M., Wu, Y., Zhong, K., & Gao, H. (2020). Structural Characteristics and Hypolipidemic Activity of Theabrownins from Dark Tea Fermented by Single Species Eurotium cristatum PW-1. Biomolecules, 10(2), 204. https://doi.org/10.3390/biom10020204







