Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry
Abstract
:1. Introduction
2. Biological Roles of IBPs
2.1. Antifreeze Protection
2.2. Other Roles
3. Structural Diversity, Characteristics, and Classification of IBPs
3.1. AFPs from Fish
3.2. AFPs of Non-Fish Origin
3.2.1. AFPs from Plants
3.2.2. AFPs from Insects
3.2.3. AFPs of Microbial Origin
AFPs from Fungi
AFPs from Bacteria
3.3. INPs
4. Mechanisms of Action of IBPs
4.1. Antifreeze Activities of AFPs
4.2. Ice Nucleation Activity of INPs
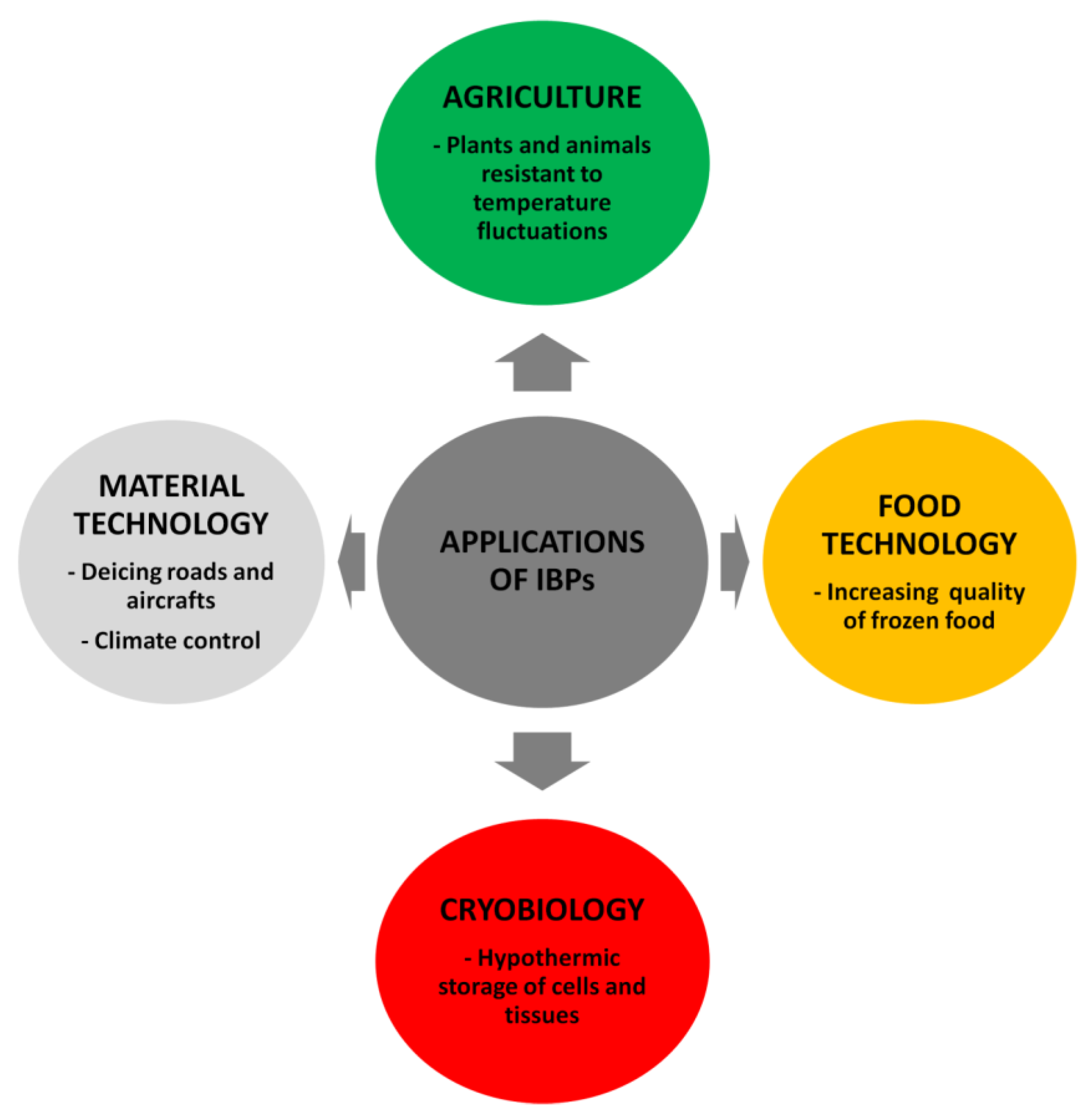
5. Potential Applications of IBPs
5.1. AFPs
5.1.1. Food Processing
5.1.2. Cryopreservation
5.1.3. Agriculture
5.1.4. Other Applications
5.2. INPs
6. Future Outlook
Funding
Conflicts of Interest
References
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef]
- Mason, O.U.; Nakagawa, T.; Rosner, M.; Van Nostrand, J.D.; Zhou, J.; Maruyama, A.; Fisk, M.R.; Giovanonni, S.J. First Investigation of the Microbiology of the Deepest Layer of Ocean Crust. PLoS ONE 2010, 5, e15399. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, T.; Kunze, G. Yeast Biotechnology: Diversity and Applications; Springer: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeast from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef]
- Bakermans, C. Limits for microbial life at subzero temperatures. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Xu, H.; Griffith, M.; Patten, C.L.; Galick, B.R. Isolation and characterization of an antifreeze protein with ice nucleation activity from the plant growth promoting rhizobacterium Pseudomonas putida GR12–2. Can. J. Microbiol. 1998, 44, 64–73. [Google Scholar] [CrossRef]
- Ustun, N.S.; Turhan, S. Antifreeze proteins: Characteristic, function, mechanism of action, sources and application to foods. J. Food Process. Preserv. 2015, 39, 3189–3197. [Google Scholar] [CrossRef]
- Bredow, M.; Walker, V.K. Ice–Binding Proteins in Plants. Front. Plant Sci. 2017, 8, 2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.G.; Koh, H.Y.; Lee, J.H.; Kang, S.H.; Kim, H.J. Cryopreservative Effects of the Recombinant Ice–Binding Protein from the Arctic Yeast Leucosporidium sp. on Red Blood Cells. Appl. Biochem. Biotechnol. 2012, 167, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Youm, H.W.; Lee, H.J.; Jee, B.C.; Suh, C.S.; Kim, S.H. Effect of antifreeze protein on mouse ovarian tissue cryopreservation and transplantation. Yonsei Med. J. 2015, 56, 778–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhach, J. The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues. Organogenesis 2009, 5, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, J.P.; Lee, R.E. Avoidance and tolerance of freezing in ectothermic vertebrates. J. Exp. Biol. 2013, 216, 1961–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antartic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, K.E.; Li, N.G.; Laugsand, A.E.; Kristiansen, E.; Pedersen, S.A. Is the strategy for cold hardiness in insects determined by their water balance? A study on two closely related families of beetles: Cerambycidae and Chrysomelidae. J. Comp. Physiol. B 2008, 178, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Davies, P.; Laybourn–Parry, J. A hyperactive, Ca2+–dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol. Lett. 2005, 145, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnham, C.P.; Campbell, R.L.; Davies, P.L. Anchored clathrate waters bind antifreeze proteins to ice. Proc. Natl. Acad. Sci. USA 2011, 108, 7363–7367. [Google Scholar] [CrossRef] [Green Version]
- Vance, T.D.R.; Olijve, L.L.C.; Campbell, R.L.; Voets, I.K.; Davies, P.L.; Guo, S. Ca2+-stabilized adhesin helps an Antarctic bacterium reach out and bind ice. Biosci. Rep. 2014, 34, 357–368. [Google Scholar] [CrossRef]
- Raymond, J.A.; Christner, B.C.; Schuster, S.C. A bacterial ice–binding protein from Vostok ice core. Extremophiles 2008, 12, 713–717. [Google Scholar] [CrossRef]
- Raymond, J.A.; Fritsen, C.; Shen, K. An ice–binding protein from an Antarctic sea ice bacterium. FEMS Microbiol. Ecol. 2007, 61, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Kondo, H.; Hanada, Y.; Sugimoto, H.; Hoshino, T.; Garnham, C.P.; Davies, P.L.; Tsuda, S. Ice–binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc. Natl. Acad. Sci. USA 2012, 109, 9360–9365. [Google Scholar] [CrossRef] [Green Version]
- Xiao, N.; Suzuki, N.; Nishimiya, Y.; Kondo, H.; Miura, A.; Tsuda, D.; Hoshino, T. Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces psychrotrophicus and Typhula ishikariensis. FEBS J. 2010, 277, 394–403. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, K.S.; Park, S.; Park, H.; Song, Y.H.; Kang, S.H.; Kim, H.J. An extracellular ice–binding glycoprotein from Arctic psychrophilic yeast. Cryobiology 2010, 60, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Do, H.; Lee, J.H.; Park, S.I.; Kim, E.J.; Kim, S.J.; Kang, S.H.; Kim, H.J. Characterization of the ice–binding protein from Arctic yeast Leucosproidium sp. AY30. Cryobiology 2012, 64, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Bayer–Giraldi, M.; Weikusat, I.; Besir, H.; Dieckman, G. Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryiobiology 2011, 63, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Hon, W.C.; Griffith, M.; Chong, P.; Yang, D. Extraction and Isolation of Antifreeze Proteins from Winter Rye (Secale cereale L.) Leaves. Plant Physiol. Mar. 1994, 104, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, M.; Yaish, M.W. Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef]
- Hakim, A.; Nguyen, J.B.; Basu, K.; Zhu, D.F.; Thakral, D.; Davies, P.L.; Isaacs, F.J.; Modis, Y.; Meng, W. Crystal Structure of an Insect Antifreeze Protein and its Implications for Ice Binding. J. Biol. Chem. 2013, 288, 12295–12304. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.L. Ice–binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef]
- Deville, S.; Viazzi, C.; Leloup, J.; Lasalle, A.; Guizard, C.; Maire, E.; Adrien, J.; Gremillard, L. Ice Shaping Properties, Similar to That of Antifreeze Proteins, of a Zirconium Acetate Complex. PLoS ONE 2011, 6, e26474. [Google Scholar] [CrossRef] [Green Version]
- Middleton, A.J.; Marshall, C.B.; Faucher, F.; Bar–Dolev, M.; Braslavsky, I.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Antifreeze protein from freeze–tolerant grass has a beta–roll fold with an irregularly structured ice–binding site. J. Mol. Biol. 2012, 416, 713–724. [Google Scholar] [CrossRef]
- Sicheri, F.; Yang, D.S. Ice–binding structure and mechanism of an antifreeze protein from winter flounder. Nature 1995, 375, 427–431. [Google Scholar] [CrossRef]
- Chen, L.; DeVries, A.; Cheng, C.-H.C. Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod. Proc. Natl. Acad.Sci. USA 1997, 94, 3817–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice–Binding Proteins and Their Function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef]
- Pentelute, B.L.; Gates, Z.P.; Tereshko, V.; Dashnau, J.L.; Vanderkooi, J.M.; Kossiakoff, A.A.; Kent, S.B.H. X–ray Structure of Snow Flea Antifreeze Protein Determined by Racemic Crystallization of Synthetic Protein Enantiomers. J. Am. Chem. Soc. 2008, 130, 9695–9701. [Google Scholar] [CrossRef] [Green Version]
- Hanada, Y.; Nishimiya, Y.; Miura, A.; Tsuda, S.; Kondo, H. Hyperactive antifreeze protein from an Antarctic sea ice bacterium Colwellia sp. has a compound ice–binding site without repetitive sequences. FEBS J. 2014, 281, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L.; Komatsu, S.K.; Feeney, R.E. Chemical and physical properties of freezing point–depressing glycoproteins from Antarctic fishes. J. Biol. Chem. 1970, 245, 2901–2908. [Google Scholar] [PubMed]
- Logsdon, J.M.; Doolittle, W.F. Origin of antifreeze protein genes: A cool tale in molecular evolution. Proc.Natl. Acad. Sci. USA 1997, 94, 3485–3487. [Google Scholar] [CrossRef] [Green Version]
- Harding, M.M.; Anderberg, P.I.; Haymet, A.D.J. ‘Antifreeze’ glycoproteins from polar fish. Eur. J. Biochem. 2003, 270, 1381–1392. [Google Scholar] [CrossRef]
- Sun, T.; Lin, F.-H.; Campbell, R.L.; Allingham, J.S.; Davies, P.L. An Antifreeze Protein Folds with an Interior Network of More Than 400 Semi–Clathrate Waters. Science 2014, 343, 795–798. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Lin, Q.; Kosinski, J.; Seethataman, J.; Bujnicki, J.M.; Sivaraman, J.; Hew, C.-L. Structure and Evolutionary Origin of Ca2+–Dependent Herring Type II Antifreeze Protein. PLoS ONE 2007, 2, e548. [Google Scholar] [CrossRef]
- Hew, C.L.; Wang, N.C.; Joshi, S.; Fletcher, G.L.; Scott, G.K.; Hayes, P.H.; Buettner, B.; Davies, P.L. Multiple genes provide the basis for antifreeze protein diversity and dosage in the ocean pout, Macrozoarces americanus. J. Biol. Chem. 1988, 263, 12049–12055. [Google Scholar]
- Levitt, J. Responses of Plants to Environmental Stresses: Chilling, Freezing, and High Temperature Stresses, 2nd ed.; Academic Press: New York, NY, USA; London, UK, 1980. [Google Scholar]
- Griffith, M.; Ala, P.; Yang, D.S.C.; Hon, W.C.; Moffat, B.A. Antifreeze Protein Produced Endogenously in Winter Rye Leaves. Plant Physiol. 1992, 100, 593–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarzabek, M.; Pukacki, P.M.; Nuc, K. Cold–regulated proteins with potent antifreeze and cryoprotective activities in spruces (Picea spp.). Crybiology 2009, 58, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J.; Brown, A.; Middleton, A.; Davies, P.L.; Walker, V.K. Expression and characterization of an antifreeze protein from the perennial rye grass, Lolium perenne. Cryobiology 2011, 62, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.R., Jr.; Serianni, A.S.; Voituron, Y.; Sformo, T.; Barnes, B.M.; Duman, J.G. A thermal hysteresis–producing xylomannan glycolipid antifreeze associated with cold tolerance is found in diverse taxa. J. Comp. Physiol. B 2011, 181, 631–640. [Google Scholar] [CrossRef]
- Yeh, S.; Moffatt, B.A.; Griffith, M.; Xiong, F.; Yang, D.S.C.; Wiseman, S.B.; Sarhan, F.; Danyluk, J.; Xue, Y.Q.; Hew, C.L.; et al. Chitinase Genes Responsive to Cold Encode Antifreeze Proteins in Winter Cereals. Plant Physiol. 2000, 124, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Lindow, S.E.; Arny, D.C.; Upper, C.D. Bacterial Ice Nucleation: A Factor in Frost Injury to Plants. Plant Physiol. 1982, 70, 1084–1089. [Google Scholar] [CrossRef]
- Tursman, D.; Duman, J.G.; Knight, C.A. Freeze tolerance adaptations in the centipede Lithobius forficatus. J. Exp. Zool 1994, 268, 347–353. [Google Scholar] [CrossRef]
- Duman, J.G. Subzero temperature tolerance in spiders: the role of thermal hysteresis factors. J. Comp. Physiol. 1979, 131, 347–352. [Google Scholar] [CrossRef]
- Neelakanta, G.; Sultana, H.; Fish, D.; Anderson, J.F.; Fikring, E. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Invest. 2010, 120, 3179–3190. [Google Scholar] [CrossRef] [Green Version]
- Block, W.; Duman, J.G. Presence of thermal hysteresis producing antifreeze proteins in the antarctic mite, Alaskozetes antarcticus. J. Exp. Zool 1989, 250, 229–231. [Google Scholar] [CrossRef]
- Zettel, J. The significance of temperature and barometric pressure changes for the snow surface activity of Isotomahiemalis (Collembola). Experientia 1984, 40, 1369–1372. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Terblanche, J.S.; Scott, M.B.; Blatch, G.L.; Klok, C.J.; Chown, S.L. Environmental physiology of three species of Collembola at Cape Hallet, North Victoria Land, Antarctica. J. Insect Physiol. 2006, 52, 29–50. [Google Scholar] [CrossRef]
- Graham, L.; Liou, Y.; Walker, V.; Davies, P.L. Hyperactive antifreeze protein from beetles. Nature 1997, 388, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.A. Insect rectum. Phil. Trans. Roy Soc. Lond B 1971, 262, 251–260. [Google Scholar] [CrossRef]
- Duman, J.G.; Bennett, V.; Sformo, T.; Hochstrasser, R.; Barnes, B.M. Antifreeze proteins in Alaskan insects and spiders. J. Insect Physiol. 2004, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tyshenko, M.G.; d’Anjou, M.; Davies, P.L.; Daugulis, A.J.; Walker, V.K. Challenges in the expression of disulfide bonded, threonine–rich antifreeze proteins in bacteria and yeast. Protein Expr. Purif. 2006, 47, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.Y.; Hyuck Lee, J.; Han, S.J.; Park, H.; Lee, S.G. Effect of the Antifreeze Protein from the Arctic Yeast Leucosporidium sp. AY30 on Cryopreservation of the Marine Diatom Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2015, 175, 677–686. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.G.; Do, H.; Park, J.-C.; Kim, E.; Choe, Y.H.; Han, S.J.; Kim, H.J. Optimization of the pilot–scale production of an ice–binding protein by fed–batch culture of Pichia pastoris. Appl. Microbiol. Biotechnol. 2013, 97, 3383. [Google Scholar] [CrossRef]
- Hashim, N.H.; Bharudin, I.; Nguong, D.L.; Higa, S.; Bakar, F.D.; Nathan, S.; Rabu, A.; Kawahara, H.; Illias, R.M.; Najimudin, N.; et al. Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles 2013, 17, 63–73. [Google Scholar] [CrossRef]
- Hashim, N.H.F.; Sulaiman, S.; Abu Bakar, F.D.; Illias, R.M.; Kawahara, H.; Najimudin, N.; Mahadi, N.M.; Murad, A.M.A. Molecular cloning, expression and characterisation of Afp4, an antifreeze protein from Glaciozymaantarctica. Polar Biol. 2014, 37, 1495–1505. [Google Scholar] [CrossRef]
- Villarreal, P.; Carrasco, M.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Antarctic yeasts: Analysis of their freeze–thaw tolerance and production of antifreeze proteins, fatty acids and ergosterol. BMC Microbiol. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hanada, Y.; Miura, A.; Tsuda, S.; Kondo, H. Hydrophobic ice–binding sites confer hyperactivity of an antifreeze protein from a snow mold fungus. Biochem. J. 2016, 473, 4011–4026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshino, T.; Kiriaki, M.; Ohgiya, S.; Fujiwara, M.; Kondo, H.; Nishimiya, Y.; Yumoto, I.; Tsuda, S. Antifreeze proteins from snow mold fungi. Can. J. Bot. 2003, 81, 1175–1181. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Hill, P.J.; Dodd, C.E.R.; Laybourn-Parry, J. Demonstration of Antifreeze protein activity in Anctarctic lake bacteria. Microbiology 2004, 150, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Garnham, C.P.; Gilbert, J.A.; Hartman, C.P.; Campbell, R.L.; Laybourn-Parry, J.; Davies, P.L. A Ca2+–dependent bacterial antifreeze protein domain has a novel β–helical ice–binding fold. Biochem. J. 2008, 411, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Do, H.; Lee, J.H.; Lee, S.G.; Kim, H.J. Crystallization and preliminary X–ray crystallographic analysis of an ice–binding protein (FfIBP) from Flavobacterium frigoris PS1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Do, H.; Kim, S.J.; Kim, H.J.; Lee, J.H. Structure–based characterization and antifreeze properties of a hyperactive ice–binding protein from the Antarctic bacterium Flavobacterium frigoris PS1. Acta Cryst. sect. D 2014, D70, 1061–1073. [Google Scholar] [CrossRef]
- Xiao, N.; Inaba, S.; Tojo, M.; Degawa, Y.; Fujiu, S.; Kudoh, S.; Hoshino, T. Antifreeze activities of various fungi and Stramenopila isolated from Antarctica. N. Am. Fungi 2010, 5, 215–220. [Google Scholar] [CrossRef]
- Smallwood, M.; Worrall, D.; Byass, L.; Elias, L.; Ashford, D.; Doucet, C.J.; Holt, C.; Telford, J.; Lillford, P.; Bowles, D.J. Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota). Biochem. J. 1999, 340, 385–391. [Google Scholar] [CrossRef]
- Liou, Y.C.; Tocilj, A.; Davies, P.L.; Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a beta–helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [CrossRef]
- Nishimiya, Y.; Kondo, H.; Takamichi, M.; Sugimoto, H.; Suzuki, M.; Miura, A.; Tsuda, S. Crystal structure and mutational analysis of Ca2+–independent type II antifreeze protein from longsnout poacher, Brachyopsis rostratus. J. Mol. Biol. 2008, 382, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, C.; Poulsen, J.C.; Ramløv, H.; Lo Leggio, L. Purification, Crystal Structure Determination and Functional Characterization of Type III Antifreeze Proteins from the European Eelpout Zoarces Viviparus. Cryobiology 2014, 69, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, H. Cryoprotectants and ice–binding proteins. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer: Berlin, Germany, 2008; pp. 229–246. [Google Scholar]
- Garnham, C.P.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Novel dimeric β–helical model of an ice nucleation protein with bridged active sites. BMC Struct. Biol. 2011, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yan, Q.; Chen, J.; He, Y.; Wang, J.; Zhang, H.; Yu, Z.; Li, L. Molecular characterization of an ice nucleation protein variant (InaQ) from Pseudomonas syringae and the analysis of its transmembrane transport activity in Escherichia coli. Int. J. Biol. Sci. 2012, 8, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.A. Structural biology. Adding to the antifreeze agenda. Nature 2000, 406, 249–251. [Google Scholar] [CrossRef]
- Celik, Y.; Graham, L.A.; Mok, Y.F.; Bar, M.; Davies, P.L.; Braslavsky, I. Superheating of ice crystals in antifreeze protein solutions. Proc. Natl. Acad. Sci. USA 2010, 107, 5423–5428. [Google Scholar] [CrossRef] [Green Version]
- Doucet, D.; Tyshenko, M.G.; Kuiper, M.J.; Graether, S.P.; Sykes, B.D.; Daugulis, A.J.; Davies, P.L.; Walker, V.K. Structure–function relationship in spruce budworm antifreeze protein revealed by isoform diversity. Eur. J. Biochem. 2000, 267, 6082–6088. [Google Scholar] [CrossRef] [Green Version]
- Duman, J.G. Animal ice–binding (antifreeze) proteins and glycolipids: an overview with emphasis on physiological function. J. Exp. Biol. 2015, 218, 1846–1855. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Park, A.K.; Do, H.; Park, K.S.; Moh, S.H.; Chi, Y.M.; Kim, H.J. Structural Basis for Antifreeze Activity of Ice–binding Protein from Arctic Yeast. J. Biol. Chem. 2012, 287, 11460–11468. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, P.; Fondi, M.; Tutino, M.L.; Lo Giudice, A.; de Pascale, D.; Fani, R. The art of adapting to extreme environments: The model system Pseudoalteromonas. Phys. Life Rev. 2019, in press. [Google Scholar] [CrossRef]
- Knight, C.A.; DeVries, A.L.; Oolman, L.D. Fish antifreeze protein and the freezing and recrystallization of ice. Nature 1984, 308, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Inglis, S.R.; Turner, J.J.; Harding, M.M. Applications of Type I Antifreeze Proteins: Studies with Model Membranes & Cryoprotectant Properties. Curr. Protein Pept. Sci. 2006, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Deswal, R. Antifreeze Proteins in Plants: An overview with an insight into the detection techniques including nanobiotechnology. J. Proteins Proteom. 2014, 5, 89–107. [Google Scholar]
- Ramløv, H.; Johnsen, J.L. Controlling the Freezing Process with Antifreeze Proteins. In Emerging Technologies for Food Processing, 2nd ed.; Sun, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 539–562. [Google Scholar] [CrossRef]
- Olijve, L.L.C.; Meister, K.; DeVries, A.L.; Duman, J.G.; Guo, S.; Bakker, H.J.; Voets, I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef] [Green Version]
- Haji-Akbari, A. Rating antifreeze proteins: Not a breeze. Proc. Natl. Acad. Sci. USA 2016, 113, 3714–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotter, A.J.; Marshall, C.B.; Graham, L.A.; Gilbert, J.A.; Granham, C.P.; Davies, P.L. The basis of hyperactivity of antifreeze proteins. Cryobiology 2006, 53, 229–239. [Google Scholar] [CrossRef]
- Smolin, N.; Daggett, V. Formation of ice–like water structure on the surface of an antifreeze protein. J. Phys. Chem. B 2008, 112, 6193–6202. [Google Scholar] [CrossRef]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [Green Version]
- Vrielink, O.A.S.; Aloi, A.; Olijve, L.L.C.; Voets, I.K. Interaction of ice binding proteins with ice, water and ions. Biointerphases 2016, 11, 018906. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.N.; Graether, S.P. Increased flexibility decreases antifreeze protein activity. Protein Sci. 2010, 19, 2356–2365. [Google Scholar] [CrossRef] [Green Version]
- Meister, K.; Strazdaite, S.; DeVries, A.L.; Lotze, S.; Olijve, L.L.C.; Voets, I.K.; Bakker, H.J. Observation of ice–like water layers at an aqueous protein surface. Proc. Natl. Acad. Sci. USA 2014, 111, 17732–17736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertaya, N.; Marshall, C.B.; DiPrinzio, C.L.; Wilen, L.; Thomson, E.S.; Wettlaufer, J.S.; Davies, P.L.; Braslavsky, I. Fluorescence Microscopy Evidence for Quasi–Permanent Attachment of Antifreeze Proteins to Ice Surfaces. Biophys. J. 2007, 92, 3663–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristiansen, E.; Zachariassen, K.E. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Crybiology 2005, 51, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, C.; Ma, J.; Shi, G.; Yao, X.; Fang, H.; Song, Y.; Wang, J. Janus effect of antifreeze proteins on ice nucleation. Proc. Natl. Acad. Sci. USA 2016, 113, 14739–14744. [Google Scholar] [CrossRef] [Green Version]
- Meister, K.; DeVries, A.; Bakker, H.J.; Drori, R. Antifreeze Glycoproteins Bind Irreversibly to Ice. J. Am. Chem. Soc. 2018, 140, 9365–9368. [Google Scholar] [CrossRef]
- Lorv, J.S.H.; Rose, D.R.; Glick, B.R. Bacterial Ice Crystal Controlling Proteins. Scientifica 2014, 20, 976895. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Safety of Ice Structuring Protein (ISP)—Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies and of the Panel on Genetically Modified Organisms. EFSA J. 2008, 768, 1–18. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Agency Response Letter GRAS Notice No. GRN 000117. 2003. Available online: https://wayback.archive-it.org/7993/20171031032532/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm153931.htm (accessed on 17 April 2003).
- Regand, A.; Goff, H.D. Ice Recrystallization Inhibition in Ice Cream as Affected by Ice Structuring Proteins from Winter Wheat Grass. J. Dairy Sci. 2006, 89, 49–57. [Google Scholar] [CrossRef]
- Muse, M.R.; Hartel, R.W. Ice cream structural elements that affect melting rate and hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef]
- Cao, H.; Zhao, Y.; Zhu, Y.B.; Xu, F.; Yu, J.S.; Yuan, M. Antifreeze and cryoprotective activities of ice–binding collagen peptides from pig skin. Food Chem. 2016, 194, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Ding, X.; Cheng, L.; Wang, L.; Qian, H.; Qi, H.; Song, C. Purification and Identification of Antifreeze Protein From Cold–Acclimated Oat (Avena sativa L.) and the Cryoprotective Activities in Ice Cream. Food Bioprocess. Technol. 2016, 9, 1746–1755. [Google Scholar] [CrossRef]
- Valera, P.; Pintor, A.; Fiszman, S. How hydrocolloids affect the temporal oral perception of ice cream. Food Hydrocoll. 2014, 36, 220–228. [Google Scholar] [CrossRef]
- Kaleda, A.; Tsanev, R.; Klesment, T.; Vilu, R.; Laos, K. Ice cream structure modification by ice–binding proteins. Food Chem. 2017, 246, 164–171. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, J.H.; Kang, S.H.; Kim, H.J. Marine Antifreeze Proteins: Types, Functions and Applications. In Marine Proteins and Peptides: Biological Activities and Applications; Kim, S.K., Ed.; John Wiley & Sons, Incorporated: Chichester, UK, 2013; pp. 667–694. [Google Scholar]
- Fletcher, G.L.; Hew, C.L.; Joshi, S.B.; Wu, Y. Antifreeze Polypeptide-Expressing Microorganisms Useful in Fermentation and Frozen Storage of Foods. U.S. Patent 6174550, 16 January 2001. [Google Scholar]
- Omedi, J.O.; Huang, W.; Zhang, B.; Li, Z.; Zheng, J. Advances in present-day frozen dough technology and its improver and novel biotech ingredients development trends—A review. Cereal Chem. 2019, 96, 34–56. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Yu, H.; Lee, T.C. A novel approach for improving yeast viability and baking quality of frozen dough by adding biogenic ice nucleators from Erwinia herbicola. J. Cereal Sci. 2012, 57, 237–243. [Google Scholar] [CrossRef]
- Xu, H.N.; Huang, W.; Jia, C.; Kim, Y.; Liu, H. Evaluation of water holding capacity and breadmaking properties for frozen dough containing ice structuring proteins from winter wheat. J. Cereal Sci. 2009, 49, 250–253. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Wang, L. Effect of carrot (Daucus carota) antifreeze proteins on the fermentation capacity of frozen dough. Food Res. Int. 2007, 40, 763–769. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Chen, H.; Wang, L.; Qian, H.; Qi, X. Extraction, purification and identification of antifreeze proteins from cold acclimated malting barley (Hordeum vulgare L.). Food Chem. 2015, 175, 74–81. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Liu, W.; Wang, L.; Qian, H.; Qi, X. Extraction of carrot (Daucus carota) antifreeze proteins and evaluation of their effects on frozen white salted noodles. Food Bioprocess. Tech. 2014, 7, 842–852. [Google Scholar] [CrossRef]
- Feeney, R.E.; Yeh, Y. Antifreeze proteins: current status and possible food uses. Trends Food Sci. Tech. 1998, 9, 102–106. [Google Scholar] [CrossRef]
- Payne, S.R.; Young, O.A. Effects of pre–slaughter administration of antifreeze proteins on frozen meat quality. Meat Sci. 1993, 41, 147–155. [Google Scholar] [CrossRef]
- MacDonald, G.A.; Lanier, T.C. Actomyosin stabilization to freeze–thaw and heat denaturation by lactate salts. J. Food Sci. 1994, 59, 101–105. [Google Scholar] [CrossRef]
- Kong, C.H.Z.; Hamid, N.; Liu, T.; Sarojini, V. Effect of Antifreeze Peptide Pretreatment on Ice Crystal Size, Drip Loss, Texture, and Volatile Compounds of Frozen Carrots. J. Agric. Food Chem. 2016, 64, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Vidanarachchi, J.K.; Ranadheera, C.S.; Wijerathne, T.D.; Udayangani, R.M.C.; Himali, S.M.C.; Pickova, J. Applications of Seafood By–products in the Food Industry and Human Nutrition. In Seafood Processing By–Products; Kim, S.K., Ed.; Springer: New York, NY, USA, 2014; pp. 463–528. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Fletcher, G.L. Hypothermic protection — A fundamental property of “Antifreeze” proteins. Biochem. Biophys. Res. Commun. 1991, 180, 566–571. [Google Scholar] [CrossRef]
- Brockbank, K.G.M.; Campbell, L.H.; Greene, E.D.; Brockbank, M.C.G.; Duman, J.G. Lessons from nature for preservation of mammalian cells, tissues, and organs. In Vitro Cell Dev. Biol. Anim. 2011, 47, 210–217. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, J.H.; Park, G.H.; Bae, S.H.; Kim, H.J.; Kim, M.S.; Hwang, Y.J.; Kim, D.Y. Influence of antifreeze proteins on boar sperm DNA damaging during cryopreservation. Dev. Biol. 2011, 356, 195. [Google Scholar] [CrossRef] [Green Version]
- Hightower, R.; Baden, C.; Lund, P.; Dunsmuir, P. Expression of antifreeze proteins in transgenic plants. Plant Mol. Biol. 1991, 17, 1013–1021. [Google Scholar] [CrossRef]
- Kenward, K.D.; Altschuler, M.; Hildebrand, D.; Davies, P.L. Accumulation of type I fish antifreeze protein in transgenic tobacco is cold–specific. Plant Mol. Biol. 1993, 23, 377–385. [Google Scholar] [CrossRef]
- Wallis, J.G.; Wang, H.; Guerra, D.J. Expression of a synthetic antifreeze protein in potato reduces electrolyte release at freezing temperatures. Plant Mol. Biol. 1997, 35, 323–330. [Google Scholar] [CrossRef]
- Holmberg, N.; Farrés, J.; Bailey, J.E.; Kallio, P.T. Targeted expression of a synthetic codon optimized gene, encoding the spruce budworm antifreeze protein, leads to accumulation of antifreeze activity in the apoplasts of transgenic tobacco. Gene Sep. 2001. [Google Scholar] [CrossRef]
- Zhang, C.; Fei, S.Z.; Arora, R.; Hannapel, D.J. Ice recrystallization inhibition proteins of perennial ryegrass enhance freezing tolerance. Planta 2010, 232, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bredow, M.; Vanderbeld, B.; Walker, V.K. Ice-binding proteins confer freezing tolerance in transgenic Arabidopsis thaliana. Plant Biotechnol. J. 2017, 15, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Strong, C.; McCabe, G.J. Observed variations in U.S. frost timing linked to atmospheric circulation patterns. Nat. Commun. 2017, 23, 15307. [Google Scholar] [CrossRef] [Green Version]
- Gwak, Y.; Park, J.I.; Kim, M.; Kim, H.S.; Kwon, M.J.; Oh, S.J.; Kim, Y.P.; Jin, E. Creating Anti–icing Surfaces via the Direct Immobilization of Antifreeze Proteins on Aluminum. Sci. Rep. 2015, 8, 12019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, L.E.; Hasan, M.; Fayter, A.E.R.; Biggs, C.; Walker, M.; Gibson, M.I. Site-specific conjugation of antifreeze proteins onto polymer-stabilized nanoparticles. Polym. Chem. 2019, 10, 2986–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voets, I.K. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter 2017, 13, 4808–4823. [Google Scholar] [CrossRef] [Green Version]
- Cochet, N.; Widehem, P. Ice crystallization by Pseudomonas syringae. Appl. Microbiol. Biotechnol. 2000, 54, 153–161. [Google Scholar] [CrossRef]
- Available online: http://www.snomax.com/product/snomax.html (accessed on 30 January 2020).
- Bae, W.; Mulchandani, A.; Chen, W. Cell surface display of synthetic phytochelatins using ice nucleation protein for enhanced heavy metal bioaccumulation. J. Inorg. Biochem. 2002, 88, 223–227. [Google Scholar] [CrossRef]
- Lindgren, P.B.; Frederic, R.; Govindarajan, A.G.; Panopoulos, N.J.; Staskawicz, B.J.; Lindow, S.E. An ice nucleation reporter gene system: Identification of inducible pathogenicity genes in Pseudomonas syringae pv. phaseolicola. EMBO J. 1989, 8, 1291–1301. [Google Scholar] [CrossRef]
- Arvanitis, N.; Vargas, C.; Tegos, G.; Perysinakis, A.; Nieto, J.J.; Ventosa, A.; Drainas, C. Development of a gene reporter system in moderately halophilic bacteria by employing the ice nucleation gene of Pseudomonas syringae. Appl. Environ. Microbiol. 1995, 61, 3821–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drainas, C.; Vartholomatos, G.; Panopoulos, N.J. The Ice Nucleation Gene from Pseudomonas syringae as a Sensitive Gene Reporter for Promoter Analysis in Zymomonas mobilis. Appl. Environ. Microbiol. 1995, 61, 273–277. [Google Scholar] [CrossRef] [Green Version]


| Organism (Protein) | Molecular Mass (kDa) | TH/IRI Activity | Gene/Protein Accession Nr | Place of Isolation | Ref. |
|---|---|---|---|---|---|
| YEAST | |||||
| Glaciozyma sp. AY30 (LeIBP) | 26.80 | +/+ | ACU30807.1/C7F6X3 | a freshwater pond, Ny–Ålesund, Svalbard archipelago, Norway | [23] |
| Glaciozyma antarctica PI12 (Afp1) | 18.24 | +/+ | ACX31168.1/D0EKL2 | sea ice, Casey Research Station, Antarctica | [62] |
| Rhodotorula glacialis | NA * | +/+ | NA/NA | soils, mosses and algal mats, Great Wall Station, King George Island, South Shetland Islands; Zhongshan Station, Larsemann Hills, Prydz Bay and Soya coast, East Antarctica | [71] |
| FUNGI | |||||
| Typhula ishikariensis (TisAFP) | 24.09 | +/+ | BAD02893.1/Q76CE6 | Finnmark (northern Norway), Svalbard, Iceland, western Greenland, Siberia | [66] |
| Antarctomyces psychrotrophicus | 28.00 | +/+ | NA/NA | mosses, soils and algal mats, Great Wall station, King George Island, South Shetland Islands; Zhongshan Station, Larsemann Hills, Prydz Bay, East Antarctica | [22] |
| Coprinus psychromorbidus | 23.00 | +/+ | NA/NA | Finnmark (northern Norway), Svalbard, Iceland, western Greenland, and Siberia | [66] |
| BACTERIA | |||||
| Collwellia SLW05 (ColAFP) | 26.35 | +/+ | ABH08428.1/A5XB26 | winter sea ice, the west side of the Antarctic Peninsula | [20] |
| Marinomonas primoryensis (MpAFP) | 1500 | +/+ | ABL74378.1/A1YIY3 | Antarctic Lakes, Vestfold Hills, East Antarctica | [16] |
| Flavobacterium frigoris PS1 (FfIBP) | 25.46 | +/+ | AFK13196.1/H7FWB6 | sea ice, shore of McMurdo Sound, Antarctica | [69] |
| PLANTS | |||||
| ryegrass Lolium perenne | 13.30 | +/+ | ACG63783.1/B5T007 | native to Europe, temperate Asia, and North Africa; widely distributed throughout the world, including North and South America, Europe, New Zealand, and Australia. | [31] |
| wild carrot (Daucus carota) | 36.80 | +/+ | AAV66074.1/Q5RLY3 | Europe, southwest Asia, North America and Australia | [72] |
| INSECTS | |||||
| beetle (mealworm) Tenebrio molitor | 8.40 | +/+ | AF160494.1/O16119 | areas associated with human activity | [73] |
| longhorn beetle Rhagium inquisitor | 12.54 | +/+ | HQ540314.1/E5LR38 | Holarctic | [28] |
| snow flea Hypogastrura harveyi | 6.50 | +/+ | ABB03725.1/Q38PT6 | North America | [35] |
| FISH | |||||
| winter flounder Pseudopleuronectes americanus | 19.34 | +/+ | ABX38716.1/B1P0S1 | waters of the western north Atlantic coast, from Labrador, Canada to Georgia, United States of America | [40] |
| longsnout poacher Brachyopsis segaliensis | 18.02 | +/+ | BAF37106.1/A0ZT93 | Northwest Pacific Ocean | [74] |
| European eelpout Zoarces viviparus | 6.90 | +/+ | ABN42205.1/A3EYI7 | Northeast Atlantic; Baltic, Barents, Irish, North, and White Seas | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białkowska, A.; Majewska, E.; Olczak, A.; Twarda-Clapa, A. Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry. Biomolecules 2020, 10, 274. https://doi.org/10.3390/biom10020274
Białkowska A, Majewska E, Olczak A, Twarda-Clapa A. Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry. Biomolecules. 2020; 10(2):274. https://doi.org/10.3390/biom10020274
Chicago/Turabian StyleBiałkowska, Aneta, Edyta Majewska, Aleksandra Olczak, and Aleksandra Twarda-Clapa. 2020. "Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry" Biomolecules 10, no. 2: 274. https://doi.org/10.3390/biom10020274
APA StyleBiałkowska, A., Majewska, E., Olczak, A., & Twarda-Clapa, A. (2020). Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry. Biomolecules, 10(2), 274. https://doi.org/10.3390/biom10020274






