Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus
Abstract
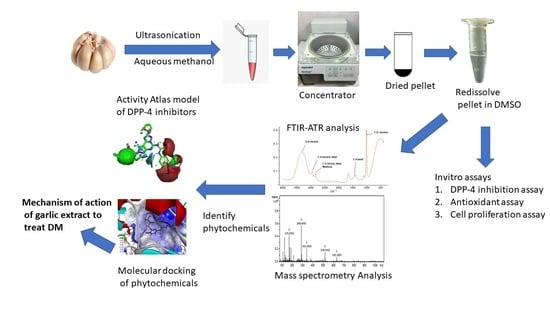
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Preparation of Garlic Extract
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. DPPH Radical Scavenging Activity
2.6. Human Dipeptidyl Peptidase-4 (DPP-4) Inhibition Assay
2.7. Identification of Phytochemicals in Garlic Extract
2.8. Activity Atlas Model and Molecular Docking Simulations
2.9. Cell Culture, Treatment, and Cell Proliferation Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dávila-Cervantes, C.A.; Pardo-Montaño, A.M. Diabetes mellitus: Contribution to changes in the life expectancy in Mexico 1990, 2000, and 2010. Revista de Salud Publica 2014, 16, 910–923. [Google Scholar] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Med. (United Kingdom) 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, S.A.; Ekoé, J.-M. Incretin agents in type 2 diabetes. Can. Fam. Physician Med. Fam. Can. 2010, 56, 639–648. [Google Scholar]
- Matheeussen, V.; Jungraithmayr, W.; De Meester, I. Dipeptidyl peptidase 4 as a therapeutic target in ischemia/reperfusion injury. Pharmacol. Ther. 2012, 136, 267–282. [Google Scholar] [CrossRef]
- Fujiwara, K.; Inoue, T.; Henmi, Y.; Hirata, Y.; Naka, Y.; Hara, A.; Kakimoto, K.; Nouda, S.; Okada, T.; Kawakami, K.; et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, suppresses CXCL5 and sdf-1 and does not accelerate intestinal neoplasia formation in Apc min/+ mice fed a high-fat diet. Oncol. Lett. 2017, 14, 4355–4360. [Google Scholar] [CrossRef]
- Iwata, S.; Yamaguchi, N.; Munakata, Y.; Ikushima, H.; Lee, J.F.; Hosono, O.; Schlossman, S.F.; Morimoto, C. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: possible mechanism for the switch from innate to acquired immune response. Int. Immunol. 1999, 11, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Struyf, S.; Proost, P.; Schols, D.; De Clercq, E.; Opdenakker, G.; Lenaerts, J.P.; Detheux, M.; Parmentier, M.; De Meester, I.; Scharpé, S.; et al. CD26/dipeptidyl-peptidase IV down-regulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor. J. Immunol. 1999, 162, 4903–4909. [Google Scholar]
- Nauck, M.A. Incretin-Based Therapies for Type 2 Diabetes Mellitus: Properties, Functions, and Clinical Implications. Am. J. Med. 2011, 124, S3–S18. [Google Scholar] [CrossRef]
- Al-Sabah, S. Molecular Pharmacology of the Incretin Receptors. Med. Princ. Pract. 2016, 25, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Luque, M.; Gonzalez, N.; Marquez, L.; Acitores, A.; Redondo, A.; Morales, M.; Valverde, I.; Villanueva-Penacarrillo, M. Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human myocytes. J. Endocrinol. 2002, 173, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boschmann, M.; Engeli, S.; Dobberstein, K.; Budziarek, P.; Strauss, A.; Boehnke, J.; Sweep, F.C.G.J.; Luft, F.C.; He, Y.L.; Foley, J.E.; et al. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2009, 94, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Chrysin in Combination with Insulin Promotes Glucose Uptake in Skeletal Muscle Cell: Impact of Combination Therapy in Diabetes Myopathy (P01-031-19). Curr. Dev. Nutr. 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Discovery of Galangin as a Potential DPP-4 Inhibitor That Improves Insulin-Stimulated Skeletal Muscle Glucose Uptake: A Combinational Therapy for Diabetes. Int. J. Mol. Sci. 2019, 20, 1228. [Google Scholar] [CrossRef] [Green Version]
- Finley, J. Functional Foods. In Adequate Food for All; Informa UK Limited: Colchester, UK, 2009; pp. 201–216. [Google Scholar]
- Ekayanti, M.; Sauriasari, R.; Elya, B. Dipeptidyl peptidase IV inhibitory activity of fraction from white tea ethanolic extract (Camellia sinensis (L.) Kuntze) ex vivo. Pharmacogn. J. 2018, 10, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Elya, B.; Handayani, R.; Sauriasari, R.; Hasyyati, U.S.; Permana, I.T.; Permatasar, Y.I. Antidiabetic Activity and Phytochemical Screening of Extracts from Indonesian Plants by Inhibition of Alpha Amylase, Alpha Glucosidase and Dipeptidyl Peptidase IV. Pak. J. Biol. Sci. 2015, 18, 279–284. [Google Scholar] [CrossRef]
- Rachpirom, M.; Ovatlarnporn, C.; Thengyai, S.; Sontimuang, C.; Puttarak, P. Dipeptidyl peptidase-IV (DPP-IV) inhibitory activity, antioxidant property and phytochemical composition studies of herbal constituents of thai folk anti-diabetes remedy. Walailak J. Sci. Technol. 2016, 13, 803–814. [Google Scholar]
- Purnomo, Y.; Soeatmadji, D.W.; Sumitro, S.B.; Widodo, M.A. Anti-diabetic potential of Urena lobata leaf extract through inhibition of dipeptidyl peptidase IV activity. Asian Pac. J. Trop. Biomed. 2015, 5, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.H.; Ullah, M.F.; Abu-Duhier, F.M. Bioactive extract of Artemisia judaica causes in vitro inhibition of dipeptidyl peptidase IV and pancreatic/intestinal enzymes of the carbohydrate absorption cascade: Implication for anti-diabetic new molecular entities (NMEs). Orient. Pharm. Exp. Med. 2019, 19, 71–80. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; Garcia-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Arif, T.; Bhosale, J.; Kumar, N.; Mandal, T.; Bendre, R.; Lavekar, G.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepúlveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Ried, K.; Fakler, P. Potential of garlic (Allium sativum) in lowering high blood pressure: Mechanisms of action and clinical relevance. Integr. Blood Press. Control 2014, 7, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.-Y.; Liu, L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J. Nutr. 2001, 131, 989–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abubakar, E.M.M. Efficacy of crude extracts of garlic (Allium sativum Linn.) against nosocomial Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniea and Pseudomonas aeruginosa. J. Med. Plants Res. 2009, 3, 179–185. [Google Scholar]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivlin, R.S.; Budoff, M.; Amagase, H. Significance of Garlic and Its Constituents in Cancer and Cardiovascular Disease. J. Nutr. 2006, 136. [Google Scholar] [CrossRef] [Green Version]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, C.W.; Oh, S.J.; Yun, J.; Kang, M.R.; Han, S.-B.; Park, H.; Jung, J.C.; Chung, Y.H.; Kang, J.S. Hepatoprotective Effect of Aged Black Garlic Extract in Rodents. Toxicol. Res. 2014, 30, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Worku, M.; Franco, R.; Baldwin, K. Efficacy of garlic as an anthelmintic in adult Boer goats. Arch. Biol. Sci. 2009, 61, 135–140. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; Lopez-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagun, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Rahman, M.M.; Fazlic, V.; Saad, N.W. Antioxidant properties of raw garlic (Allium sativum) extract. Int. Food Res. J. 2012, 19, 589–591. [Google Scholar]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phyther. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]
- Ejaz, S.; Chekarova, I.; Cho, J.W.; Lee, S.Y.; Ashraf, S.; Lim, C.W. Effect of aged garlic extract on wound healing: A new frontier in wound management. Drug Chem. Toxicol. 2009, 32, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Ghosh, S.; Acharya, K. Simplified methods for microtiter based analysis of in vitro antioxidant activity. Asian J. Pharm. 2017, 11, S327–S335. [Google Scholar]
- Kalhotra, P.; Chittepu, V.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Structure–Activity Relationship and Molecular Docking of Natural Product Library Reveal Chrysin as a Novel Dipeptidyl Peptidase-4 (DPP-4) Inhibitor: An Integrated In Silico and In Vitro Study. Molecules 2018, 23, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro, R.E.; Baudry, J.; Chodera, J.; Demir, Ö.; McCammon, J.A.; Miao, Y.; Smith, J.C. Ensemble Docking in Drug Discovery. Biophys. J. 2018, 114, 2271–2278. [Google Scholar] [CrossRef]
- Chittepu, V.C.S.R.; Kalhotra, P.; Gallardo-Velázquez, T.; La Torre, R.R.-D.; Osorio-Revilla, G.; La Torre, R.R.R.-D. Designed Functional Dispersion for Insulin Protection from Pepsin Degradation and Skeletal Muscle Cell Proliferation: In Silico and In Vitro Study. Nanomaterials 2018, 8, 852. [Google Scholar] [CrossRef] [Green Version]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. BIO-PROTOCOL 2016, 6, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, J.R.; Duffy, N.A.; McKillop, A.M.; Ardill, J.; O’Harte, F.P.M.; Flatt, P.R.; Bell, P.M. Inhibition of dipeptidyl peptidase IV activity by oral metformin in Type 2 diabetes. Diabet. Med. 2005, 22, 654–657. [Google Scholar] [CrossRef]
- Deacon, C.F.; Hughes, T.E.; Holst, J.J. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes 1998, 47, 764–769. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Rao, P.S.; Kalva, S.; Yerramilli, A.; Mamidi, S. Free Radicals and Tissue Damage: Role of Antioxidants. Free Radic. Antioxidants 2011, 1, 2–7. [Google Scholar]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes/Metabol. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Zatalia, S.R.; Sanusi, H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones. 2013, 45, 141–147. [Google Scholar]
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Woo, S.; Dong, H.; Lu, F.; Lange, A.J.; Wu, C. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin. B 2012, 2, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Noipha, K.; Ratanachaiyavong, S.; Ninla-aesong, P. Enhancement of glucose transport by selected plant foods in muscle cell line L6. Diabetes Res. Clin. Pract. 2010, 89, e22–e26. [Google Scholar]
- Thomson, M.; Al-Amin, Z.M.; Al-Qattan, K.K.; Shaban, L.H.; Ali, M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int. J. Diabetes Metab. 2007, 15, 108–115. [Google Scholar]
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of Total Phenolic Content and Antioxidant Activity of Garlic (Allium sativum) and Elephant Garlic (Allium ampeloprasum) by Attenuated Total Reflectance–Fourier Transformed Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukić, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Rasul Suleria, H.A.; Sadiq Butt, M.; Muhammad Anjum, F.; Saeed, F.; Batool, R.; Nisar Ahmad, A. Aqueous garlic extract and its phytochemical profile; Special reference to antioxidant status. Int. J. Food Sci. Nutr. 2012, 63, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Kempegowda, P.K.; Zameer, F.; Murari, S.K. Delineating antidiabetic proficiency of catechin from Withania somnifera and its Inhibitory action on dipeptidyl peptidase-4 (DPP-4). Biomed. Res. 2018, 29, 3192–3200. [Google Scholar] [CrossRef] [Green Version]
- Chittepu, V.C.S.R.; Kalhotra, P.; Osorio-Gallardo, T.; Gallardo-Velázquez, T.; Osorio-Revilla, G. Repurposing of FDA-Approved NSAIDs for DPP-4 Inhibition as an Alternative for Diabetes Mellitus Treatment: Computational and in Vitro Study. Pharmaceutics 2019, 11, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead Finder: An Approach To Improve Accuracy of Protein−Ligand Docking, Binding Energy Estimation, and Virtual Screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Karimi, P.; Alihemmati, A.; Reza Alipour, M.; Habibi, P.; Ahmadiasl, N. Therapeutic potential of genistein in ovariectomy-induced pancreatic injury in diabetic rats: The regulation of mapk pathway and apoptosis. Iran. J. Basic Med. Sci. 2017, 20, 1009–1015. [Google Scholar]
- Meshram, G.A.; Khamkar, S.S. Effect Of Oleanolic Acid Isolated From Garlic Leaves On Carbohydrate Metabolizing Enzymes, In Vitro. Int. J. Pharma Sci. Res. 2014, 5, 988–991. [Google Scholar]
- Wang, X.; Li, Y.L.; Wu, H.; Liu, J.Z.; Hu, J.X.; Liao, N.; Peng, J.; Cao, P.P.; Liang, X.; Hai, C.X. Antidiabetic effect of oleanolic acid: A promising use of a traditional pharmacological agent. Phyther. Res. 2011, 25, 1031–1040. [Google Scholar] [CrossRef]












| Phytochemical Name | Similarity Score to Field Template | Novelty Score |
|---|---|---|
| Caffeic acid 3-glucoside | 0.43 | Very high |
| Calenduloside E | 0.4 | Very high |
| Malonylgenistin | 0.41 | Very high |
| Phytochemical Name | Protein–Ligand Binding Free Energy ΔG (kcal/mol) |
|---|---|
| Caffeic acid 3-glucoside | −7.436 |
| Calenduloside E | −10.172 |
| Malonylgenistin | −7.438 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus. Biomolecules 2020, 10, 305. https://doi.org/10.3390/biom10020305
Kalhotra P, Chittepu VCSR, Osorio-Revilla G, Gallardo-Velazquez T. Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus. Biomolecules. 2020; 10(2):305. https://doi.org/10.3390/biom10020305
Chicago/Turabian StyleKalhotra, Poonam, Veera C.S.R. Chittepu, Guillermo Osorio-Revilla, and Tzayhri Gallardo-Velazquez. 2020. "Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus" Biomolecules 10, no. 2: 305. https://doi.org/10.3390/biom10020305
APA StyleKalhotra, P., Chittepu, V. C. S. R., Osorio-Revilla, G., & Gallardo-Velazquez, T. (2020). Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus. Biomolecules, 10(2), 305. https://doi.org/10.3390/biom10020305