The Role of Retinoic Acid in Establishing the Early Limb Bud
Abstract
:1. Introduction
2. Limb Bud Induction, Initiation, and Outgrowth
3. Tbx5 and Tbx4 Are Not Sufficient for Limb Bud Induction
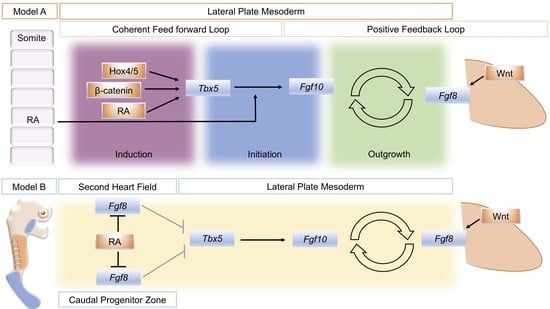
4. Retinoic Acid is Required for Limb Induction and Initiation
5. An Alternative Model for RA Action in Forelimb Initiation
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Maden, M. Vitamin A and pattern formation in the regenerating limb. Nature 1982, 295, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tickle, C.; Alberts, B.; Wolpert, L.; Lee, J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature 1982, 296, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Tickle, C. The early history of the polarizing region: from classical embryology to molecular biology. Int. J. Dev. Boil. 2002, 46, 847–852. [Google Scholar]
- Tickle, C. How the embryo makes a limb: determination, polarity and identity. J. Anat. 2015, 227, 418–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercader, N.; Leonardo, E.; E Piedra, M.; Martínez-A, C.; A Ros, M.; Torres, M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 2000, 127, 3961–3970. [Google Scholar]
- Rosello-Diez, A.; Arques, C.G.; Delgado, I.; Giovinazzo, G.; Torres, M. Diffusible signals and epigenetic timing cooperate in late proximo-distal limb patterning. Development 2014, 141, 1534–1543. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, T.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Boil. 2015, 16, 110–123. [Google Scholar] [CrossRef] [Green Version]
- Stratford, T.; Horton, C.; Maden, M. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr. Boil. 1996, 6, 1124–1133. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.; Yakushiji-Kaminatsui, N.; Atsuta, Y.; Andrey, G.; Schorderet, P.; Duboule, D.; Tabin, C.J. Integration of Shh and Fgf signaling in controlling Hox gene expression in cultured limb cells. Proc. Natl. Acad. Sci. 2017, 114, 3139–3144. [Google Scholar] [CrossRef] [Green Version]
- Tickle, C.; Towers, M. Sonic Hedgehog Signaling in Limb Development. Front. Cell Dev. Boil. 2017, 5, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Weinstein, M.; Li, C.; Naski, M.; I Cohen, R.; Ornitz, D.M.; Leder, P.; Deng, C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 1998, 125, 753–765. [Google Scholar]
- Boulet, A.M.; Moon, A.M.; Arenkiel, B.; Capecchi, M.R. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev. Boil. 2004, 273, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohuchi, H.; Nakagawa, T.; Yamamoto, A.; Araga, A.; Ohata, T.; Ishimaru, Y.; Yoshioka, H.; Kuwana, T.; Nohno, T.; Yamasaki, M.; et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development 1997, 124, 2235–2244. [Google Scholar]
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141. [Google Scholar] [CrossRef]
- Duboc, V.; Logan, M. Regulation of limb bud initiation and limb-type morphology. Dev. Dyn. 2011, 240, 1017–1027. [Google Scholar] [CrossRef]
- Fischer, S.; Draper, B.W.; Neumann, C.J. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development 2003, 130, 3515–3524. [Google Scholar] [CrossRef] [Green Version]
- Min, H.; Danilenko, D.; Scully, S.A.; Bolon, B.; Ring, B.D.; Tarpley, J.E.; De Rose, M.; Simonet, W.S. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genome Res. 1998, 12, 3156–3161. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.K.; Kawakami, Y.; Büscher, D.; Raya, A.; Itoh, T.; Koth, C.M.; Esteban, C.R.; Rodríguez-León, J.; Garrity, D.M.; Fishman, M.C.; et al. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development 2002, 129, 5161–5170. [Google Scholar]
- Agarwal, P.; Wylie, J.N.; Galceran, J.; Arkhitko, O.; Li, C.; Deng, C.; Grosschedl, R.; Bruneau, B.G. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development 2003, 130, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Naiche, L.A.; Papaioannou, V. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 2003, 130, 2681–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rallis, C. Tbx5 is required for forelimb bud formation and continued outgrowth. Development 2003, 130, 2741–2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, J.K.; Koshiba-Takeuchi, K.; Suzuki, T.; Kamimura, M.; Ogura, K. Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade. Development 2003, 130, 2729–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimoto, S.; Wilde, S.M.; Wood, S.; Logan, M. RA Acts in a Coherent Feed-Forward Mechanism with Tbx5 to Control Limb Bud Induction and Initiation. Cell Rep. 2015, 12, 879–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Sirbu, I.O.; Mic, F.A.; Molotkova, N.; Molotkov, A.; Kumar, S.; Duester, G. Retinoic Acid Promotes Limb Induction through Effects on Body Axis Extension but Is Unnecessary for Limb Patterning. Curr. Boil. 2009, 19, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minguillon, C.; Del Buono, J.; Logan, M. Tbx5 and Tbx4 Are Not Sufficient to Determine Limb-Specific Morphologies but Have Common Roles in Initiating Limb Outgrowth. Dev. Cell 2005, 8, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohn, M.J.; Izpisua-Belmonte, J.C.; Abud, H.E.; Heath, J.K.; Tickle, C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 1995, 80, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Murillo-Ferrol, N.L. [Causal study of the earliest differentiation of the morphological rudiments of the extremities. Experimental analysis on bird embryos]. Acta Anatom 1965, 62, 80–103. [Google Scholar] [CrossRef]
- Stephens, T.D.; Beier, R.L.; Bringhurst, D.C.; Hiatt, S.R.; Prestridge, M.; Pugmire, D.E.; Willis, H.J. Limbness in the early chick embryo lateral plate. Dev. Boil. 1989, 133, 1–7. [Google Scholar] [CrossRef]
- Kieny, M. [On the relations between somatic and somatopleural mesoderm before and during primary induction of chick embryo limbs]. Comptes rendus Hebd. des seances de l’Academie des Sci. Ser. D: Sci. Nat. 1969, 268, 3183–3186. [Google Scholar]
- Pinot, M. [The role of the somitic mesoderm in the early morphogenesis of the limbs in the fowl embryo]. J. Embryol. Exp. Morphol. 1970, 23, 109–151. [Google Scholar] [PubMed]
- Begemann, G.; Schilling, T.F.; Rauch, G.J.; Geisler, R.; Ingham, P.W. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 2001, 128, 3081–3094. [Google Scholar]
- Grandel, H.; Lun, K.; Rauch, G.-J.; Rhinn, M.; Piotrowski, T.; Houart, C.; Sordino, P.; Küchler, A.M.; Schulte-Merker, S.; Geisler, R.; et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 2002, 129, 2851–2865. [Google Scholar]
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Gibert, Y. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development 2006, 133, 2649–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minguillon, C.; Nishimoto, S.; Wood, S.; Vendrell, E.; Gibson-Brown, J.J.; Logan, M. Hox genes regulate the onset of Tbx5 expression in the forelimb. Development 2012, 139, 3180–3188. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, S.; Minguillon, C.; Wood, S.; Logan, M. A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of Tbx5 and Reveals Hox Protein Specificity. PLoS Genet. 2014, 10, e1004245. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, Y.; Capdevila, J.; Büscher, D.; Itoh, T.; Esteban, C.R.; Belmonte, J.C.I. WNT Signals Control FGF-Dependent Limb Initiation and AER Induction in the Chick Embryo. Cell 2001, 104, 891–900. [Google Scholar] [CrossRef]
- Cunningham, T.; Lancman, J.J.; Berenguer, M.; Dong, P.D.S.; Duester, G. Genomic Knockout of Two Presumed Forelimb Tbx5 Enhancers Reveals They Are Nonessential for Limb Development. Cell Rep. 2018, 23, 3146–3151. [Google Scholar] [CrossRef]
- Adachi, N.; Robinson, M.; Goolsbee, A.; Shubin, N.H. Regulatory evolution of Tbx5 and the origin of paired appendages. Proc. Natl. Acad. Sci. 2016, 113, 10115–10120. [Google Scholar] [CrossRef] [Green Version]
- Alon, U. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 2007, 8, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Mic, F.A.; Haselbeck, R.J.; Cuenca, A.E.; Duester, G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 2002, 129, 2271–2282. [Google Scholar]
- Cunningham, T.; Zhao, X.; Duester, G. Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension. Genesis 2011, 49, 776–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandell, L.; Sanderson, B.W.; Moiseyev, G.; Johnson, T.; Mushegian, A.R.; Young, K.; Rey, J.-P.; Ma, J.-X.; Staehling-Hampton, K.; A Trainor, P. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007, 21, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, T.; Zhao, X.; Sandell, L.; Evans, S.M.; A Trainor, P.; Duester, G. Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Rep. 2013, 3, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Rhinn, M.; Schuhbaur, B.; Niederreither, K.; Dollé, P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl. Acad. Sci. 2011, 108, 16687–16692. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feneck, E.; Logan, M. The Role of Retinoic Acid in Establishing the Early Limb Bud. Biomolecules 2020, 10, 312. https://doi.org/10.3390/biom10020312
Feneck E, Logan M. The Role of Retinoic Acid in Establishing the Early Limb Bud. Biomolecules. 2020; 10(2):312. https://doi.org/10.3390/biom10020312
Chicago/Turabian StyleFeneck, Eleanor, and Malcolm Logan. 2020. "The Role of Retinoic Acid in Establishing the Early Limb Bud" Biomolecules 10, no. 2: 312. https://doi.org/10.3390/biom10020312
APA StyleFeneck, E., & Logan, M. (2020). The Role of Retinoic Acid in Establishing the Early Limb Bud. Biomolecules, 10(2), 312. https://doi.org/10.3390/biom10020312