Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Animal Experiments
2.3. Biochemical Analysis
2.4. Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.5. Western Blot Analysis
2.6. Small Interfering RNA (siRNA) Transfection
2.7. Statistical Analysis
3. Results
3.1. Downregulation of Adipsin is Associated with Increased ER Stress in the Adipose Tissues of Obese Mice
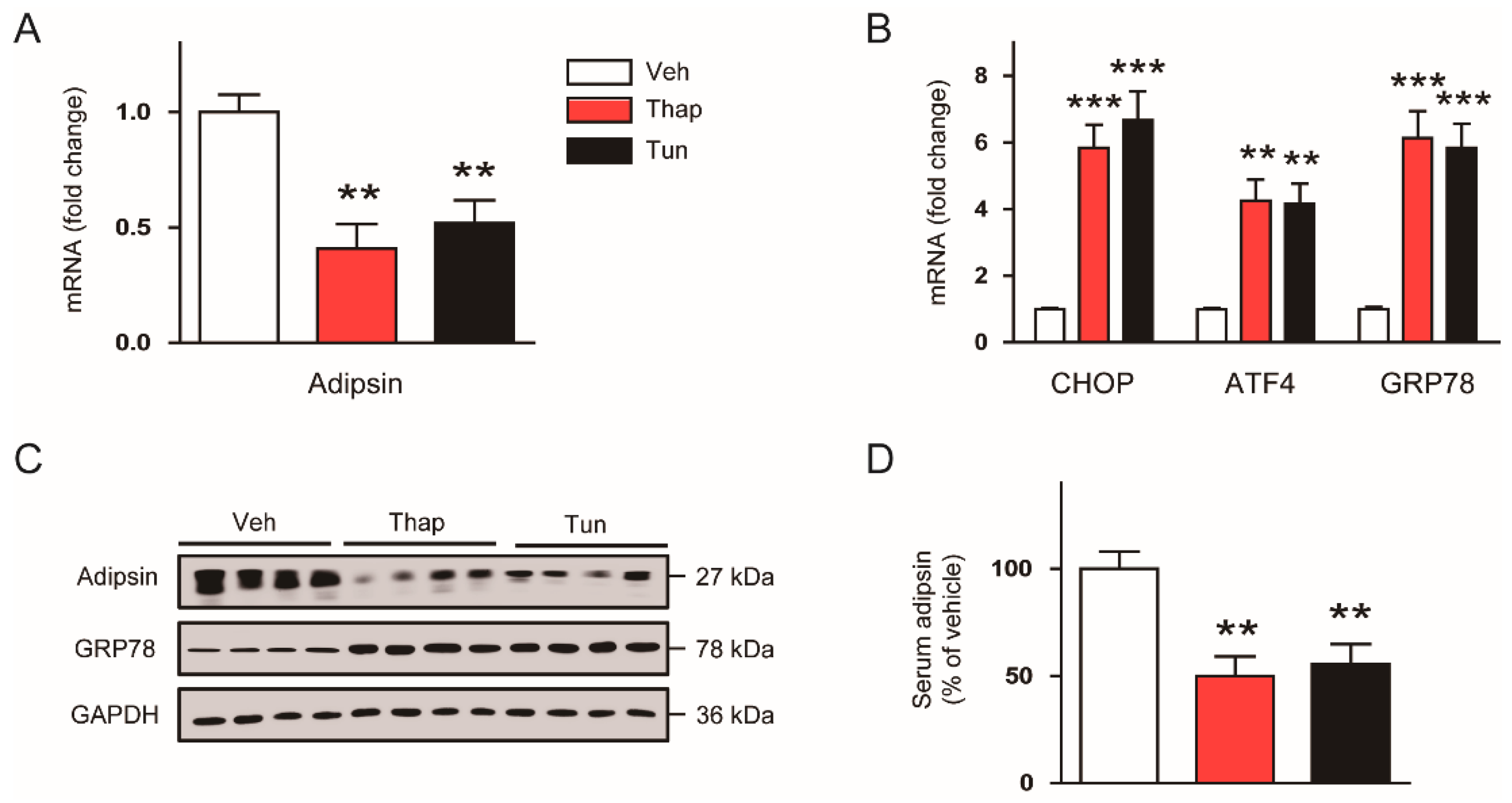
3.2. ER Stress Suppresses Adipsin Expression in Adipose Tissues
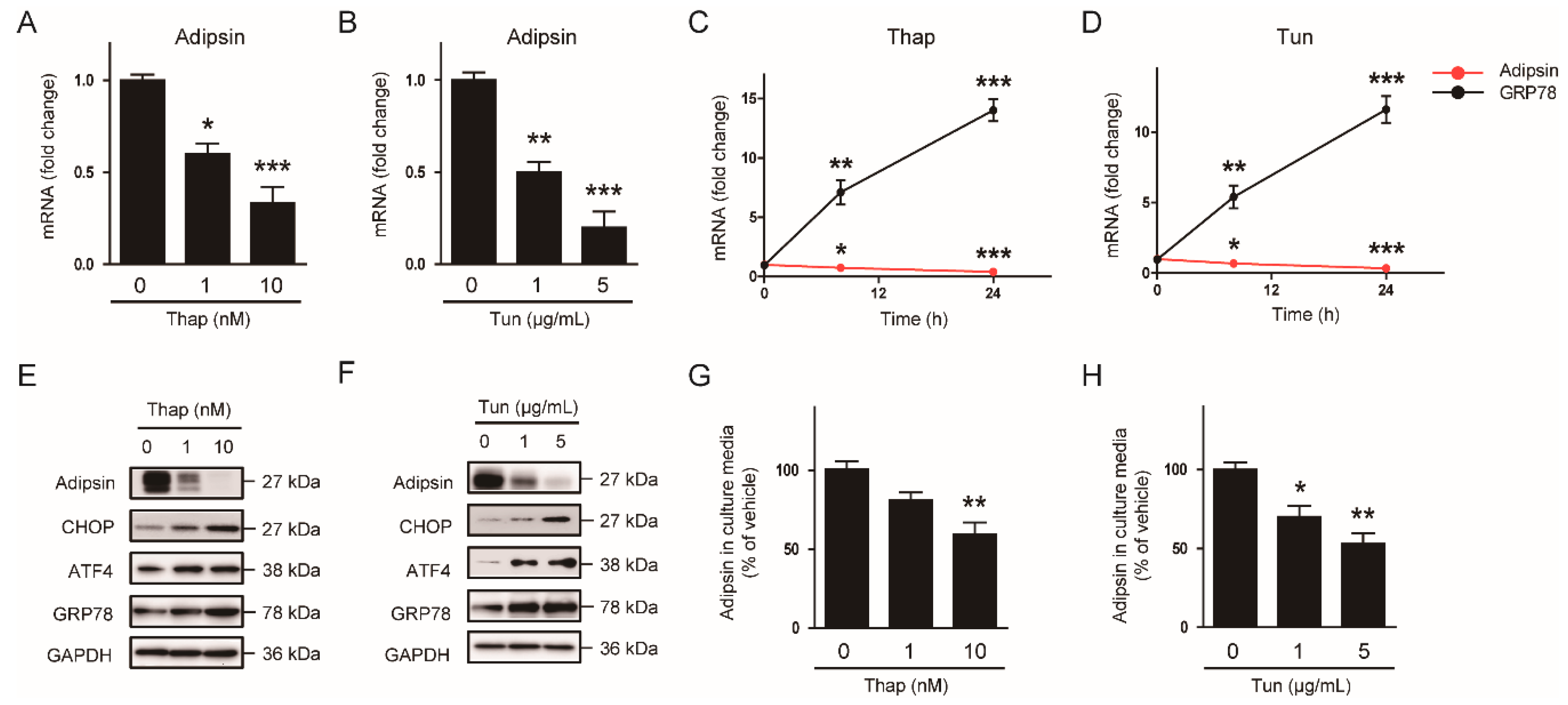
3.3. The ER Stress-Mediated Downregulation of Adipsin is Executed via Intrinsic Mechanisms in Adipocytes
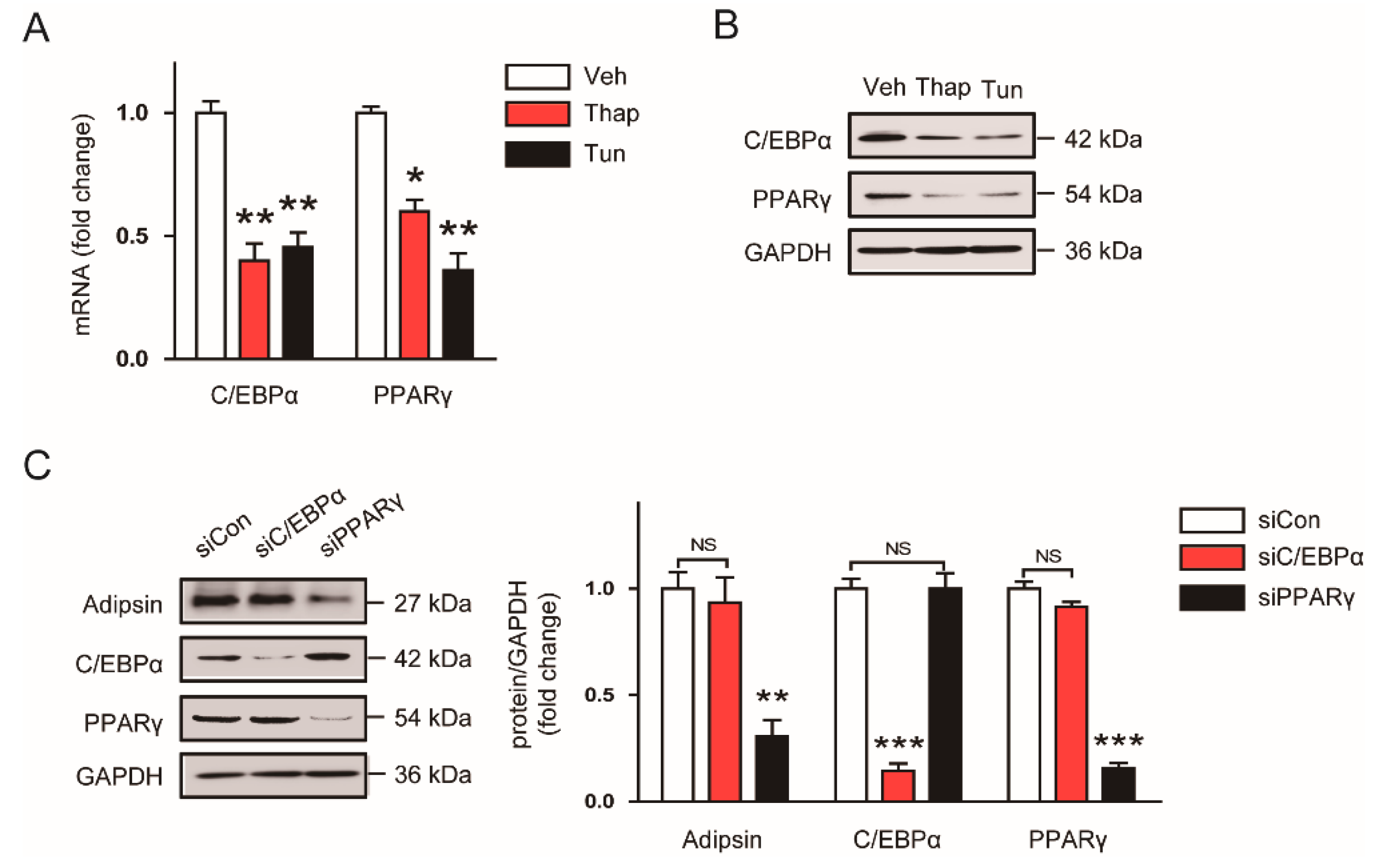
3.4. Decreased Expression of PPARγ Contributes to the ER Stress-Mediated Downregulation of Adipsin
3.5. Chemical Chaperone Recovers the ER Stress-Mediated Downregulation of Adipsin in Vitro and in Vivo
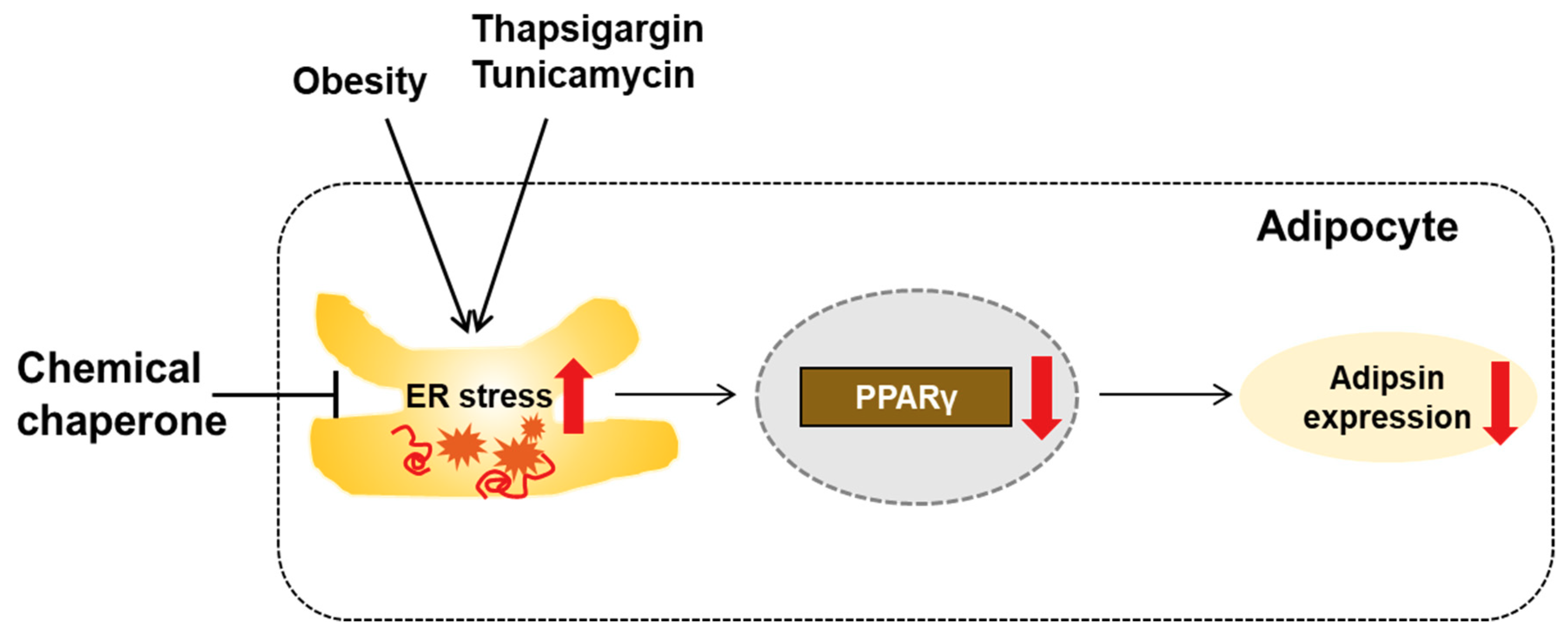
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaems, W.P. WHO recognition of the global obesity epidemic. Int. J. Obes. (Lond.) 2008, 32, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne) 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M.; Frank, M.; Green, H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J. Biol. Chem. 1983, 258, 10083–10089. [Google Scholar] [PubMed]
- Rosen, B.S.; Cook, K.S.; Yaglom, J.; Groves, D.L.; Volanakis, J.E.; Damm, D.; White, T.; Spiegelman, B.M. Adipsin and complement factor D: an immune-related defect in obesity. Science 1989, 244, 1483–1487. [Google Scholar] [CrossRef]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Chatterjee Bhowmick, D.; Murano, I.; Cohen, P.; et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef]
- Flier, J.S.; Cook, K.S.; Usher, P.; Spiegelman, R.M. Severely impaired adipsin expression in genetic and acquired obesity. Science 1987, 237, 405–408. [Google Scholar] [CrossRef]
- Gómez-Banoy, N.; Guseh, J.S.; Li, G.; Rubio-Navarro, A.; Chen, T.; Poirier, B.; Putzel, G.; Rosselot, C.; Pabón, M.A.; Camporez, J.P.; et al. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat. Med. 2019, 25, 1739–1747. [Google Scholar] [CrossRef]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, Q.H.; Lu, Y.J.; Ren, K.; Yi, G.H. Involvement of the IRE1α-XBP1 pathway and XBP1s-dependent transcriptional reprogramming in metabolic diseases. DNA Cell Biol. 2015, 34, 6–18. [Google Scholar] [CrossRef]
- Mota de Sá, P.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional regulation of adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar] [PubMed]
- Tomaru, T.; Steger, D.J.; Lefterova, M.I.; Schupp, M.; Lazar, M.A. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding proteins. J. Biol. Chem. 2009, 284, 6116–6125. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Oh, S.Y.; Lee, M.Y.; Yoon, S.; Kim, K.S.; Kim, J.W. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes 2004, 53, 2757–2766. [Google Scholar] [CrossRef]
- Maeda, N.; Takahashi, M.; Funahashi, T.; Kihara, S.; Nishizawa, H.; Kishida, K.; Nagaretani, H.; Matsuda, M.; Komuro, R.; Ouchi, N.; et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001, 50, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Mullican, S.E.; Tomaru, T.; Qatanani, M.; Schupp, M.; Lazar, M.A. Endoplasmic reticulum stress regulates adipocyte resistin expression. Diabetes 2009, 58, 1879–1886. [Google Scholar] [CrossRef][Green Version]
- Takahashi, N.; Yoshizaki, T.; Hiranaka, N.; Suzuki, T.; Yui, T.; Akanuma, M.; Kanazawa, K.; Yoshida, M.; Naito, S.; Fujiya, M.; et al. Endoplasmic reticulum stress suppresses lipin-1 expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 431, 25–30. [Google Scholar] [CrossRef][Green Version]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.Y.; Jun, H.S. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front.Endocrinol. (Lausanne) 2018, 9, 384. [Google Scholar] [CrossRef]
- Sharma, N.K.; Das, S.K.; Mondal, A.K.; Hackney, O.G.; Chu, W.S.; Kern, P.A.; Rasouli, N.; Spencer, H.J.; Yao-Borengasser, A.; Elbein, S.C. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 2008, 93, 4532–4541. [Google Scholar] [CrossRef]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef]
- Gregor, M.F.; Yang, L.; Fabbrini, E.; Mohammed, B.S.; Eagon, J.C.; Hotamisligil, G.S.; Klein, S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 2009, 58, 693–700. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, W.; Li, S.; Li, C.; Feng, Y.; Geng, D. Gastric sleeve surgery alleviates obesity-associated insulin resistance and suppresses endoplasmic reticulum stress in adipose tissue of db/db mice. Obes. Surg. 2019, 29, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, M.; Zhang, J.; Chen, H.; Dong, L.Q.; Liu, F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes 2010, 59, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Torre-Villalvazo, I.; Bunt, A.E.; Alemán, G.; Marquez-Mota, C.C.; Diaz-Villaseñor, A.; Noriega, L.G.; Estrada, I.; Figueroa-Juárez, E.; Tovar-Palacio, C.; Rodriguez-López, L.A. Adiponectin synthesis and secretion by subcutaneous adipose tissue is impaired during obesity by endoplasmic reticulum stress. J. Cell. Biochem. 2018, 119, 5970–5984. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Lee, Y.J.; Jang, H.J.; Lee, K.W.; Kim, Y.S.; Jun, H.S.; Kang, S.S.; Chun, J.; Kang, Y. A chemical chaperone 4-PBA ameliorates palmitate-induced inhibition of glucose-stimulated insulin secretion (GSIS). Arch. Biochem. Biophys. 2008, 475, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Engin, F.; Yermalovich, A.; Nguyen, T.; Hummasti, S.; Fu, W.; Eizirik, D.L.; Mathis, D.; Hotamisligil, G.S. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci. Transl. Med. 2013, 5, 211ra156. [Google Scholar] [CrossRef]
- Bronczek, G.A.; Vettorazzi, J.F.; Soares, G.M.; Kurauti, M.A.; Santos, C.; Bonfim, M.F.; Carneiro, E.M.; Balbo, S.L.; Boschero, A.C.; Costa Júnior, J.M. The bile acid TUDCA improves beta-cell mass and reduces insulin degradation in mice with early-stage of type-1 diabetes. Front. Physiol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Vettorazzi, J.F.; Ribeiro, R.A.; Borck, P.C.; Branco, R.C.; Soriano, S.; Merino, B.; Boschero, A.C.; Nadal, A.; Quesada, I.; Carneiro, E.M. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism 2016, 65, 54–63. [Google Scholar] [CrossRef]
- Kusaczuk, M. Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef]
- Tanabe, K.; Amo-Shiinoki, K.; Hatanaka, M.; Tanizawa, Y. Interorgan Crosstalk Contributing to β-Cell Dysfunction. J. Diabetes Res. 2017, 2017, 3605178. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5’→3’) |
|---|---|
| Adipsin | Forward: CAT GCT CGGCCC TAC ATGG Reverse: CACAGAGTCGTCATCCGTCAC |
| PPARγ 1 | Forward: TCGCTGATGCACTGCCTATG Reverse: GAGAGGTCCACAGAGCTGATT |
| C/EBPα 2 | Forward: CAAGAACAGCAACGAGTACCG Reverse: GTCACTGGTCAACTCCAGCAC |
| ATF4 3 | Forward: CCTGAACAGCGAAGTGTTGG Reverse: TGGAGAACCCATGAGGTTTCA |
| CHOP 4 | Forward: CTGCCTTTCACCTTGGAGAC Reverse: CGTTTCCTGGGGATGAGATA |
| GRP78 5 | Forward: CATGGTTCTCACTAAAATGAAGG Reverse: GCTGGTACAGTAACAACTG |
| GAPDH 6 | Forward: ACTCCACTCACGGCAAATTC Reverse: TCTCCATGGTGGTGAAGACA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, K.-Y.; Jeon, E.J.; Leem, J.; Park, J.-H.; Cho, H. Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes. Biomolecules 2020, 10, 314. https://doi.org/10.3390/biom10020314
Ryu K-Y, Jeon EJ, Leem J, Park J-H, Cho H. Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes. Biomolecules. 2020; 10(2):314. https://doi.org/10.3390/biom10020314
Chicago/Turabian StyleRyu, Ka-Young, Eon Ju Jeon, Jaechan Leem, Jae-Hyung Park, and Hochan Cho. 2020. "Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes" Biomolecules 10, no. 2: 314. https://doi.org/10.3390/biom10020314
APA StyleRyu, K.-Y., Jeon, E. J., Leem, J., Park, J.-H., & Cho, H. (2020). Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes. Biomolecules, 10(2), 314. https://doi.org/10.3390/biom10020314





