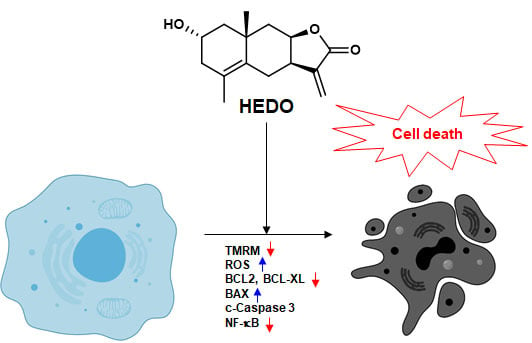
2α-Hydroxyeudesma-4,11(13)-Dien-8β,12-Olide Isolated from Inula britannica Induces Apoptosis in Diffuse Large B-cell Lymphoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation
2.3. Cell Culture
2.4. Antiproliferation Assay
2.5. Microscopy
2.6. Tetramethylrhodamine Methyl Ester Perchlorate (TMRM) Assay
2.7. Measurement of Reactive Oxygen Species (ROS) and Mitochondrial Membrane Potential (Δψm)
2.8. Cell Cycle Progression
2.9. Apoptosis Analysis
2.10. Western Blot Analysis
2.11. RT-qPCR
2.12. Confocal Imaging
2.13. Live/Dead Fluorescence Microscopy Assay
2.14. Statistical Analysis
3. Results
3.1. Extraction and Isolation of HEDO
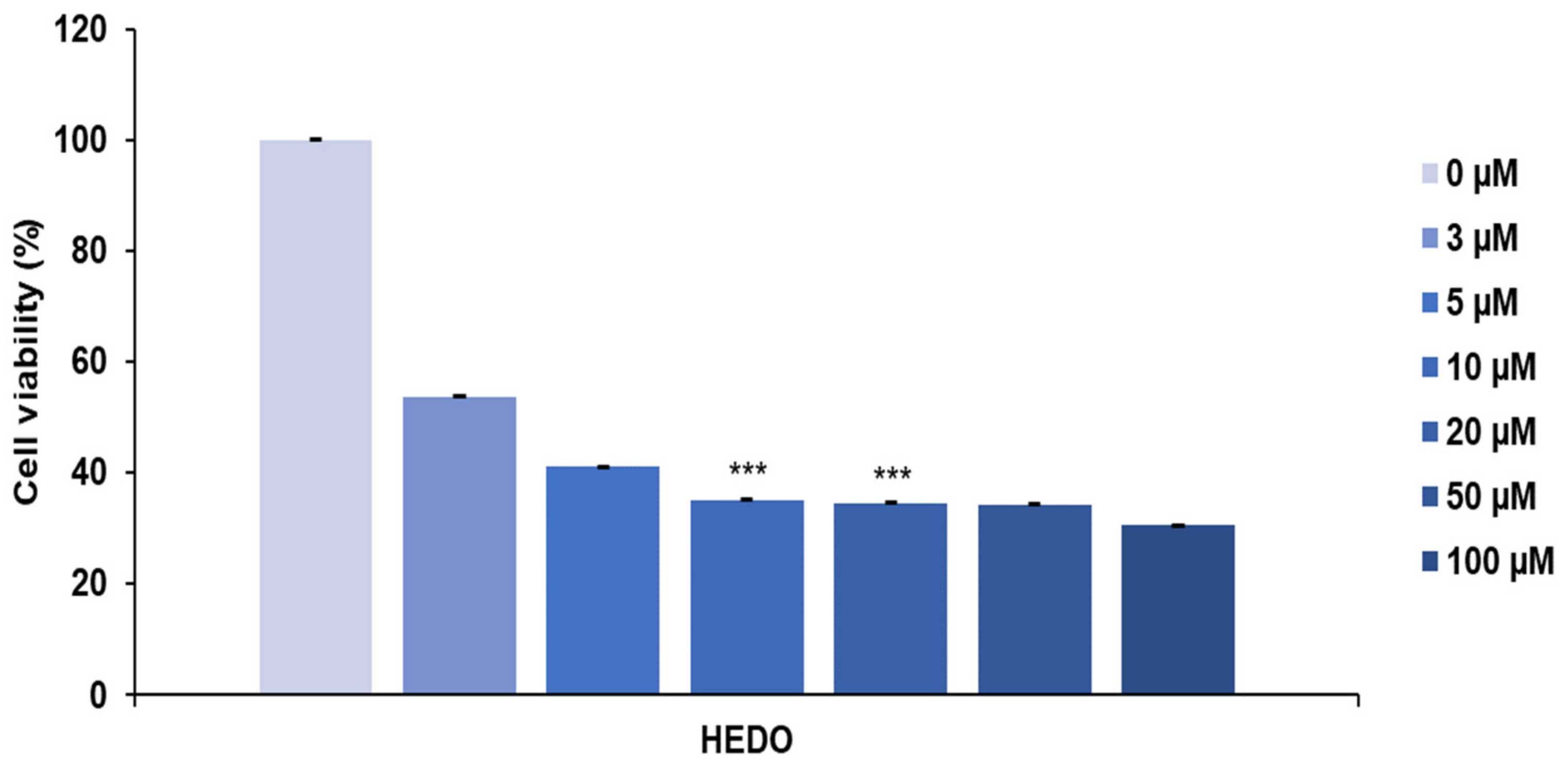
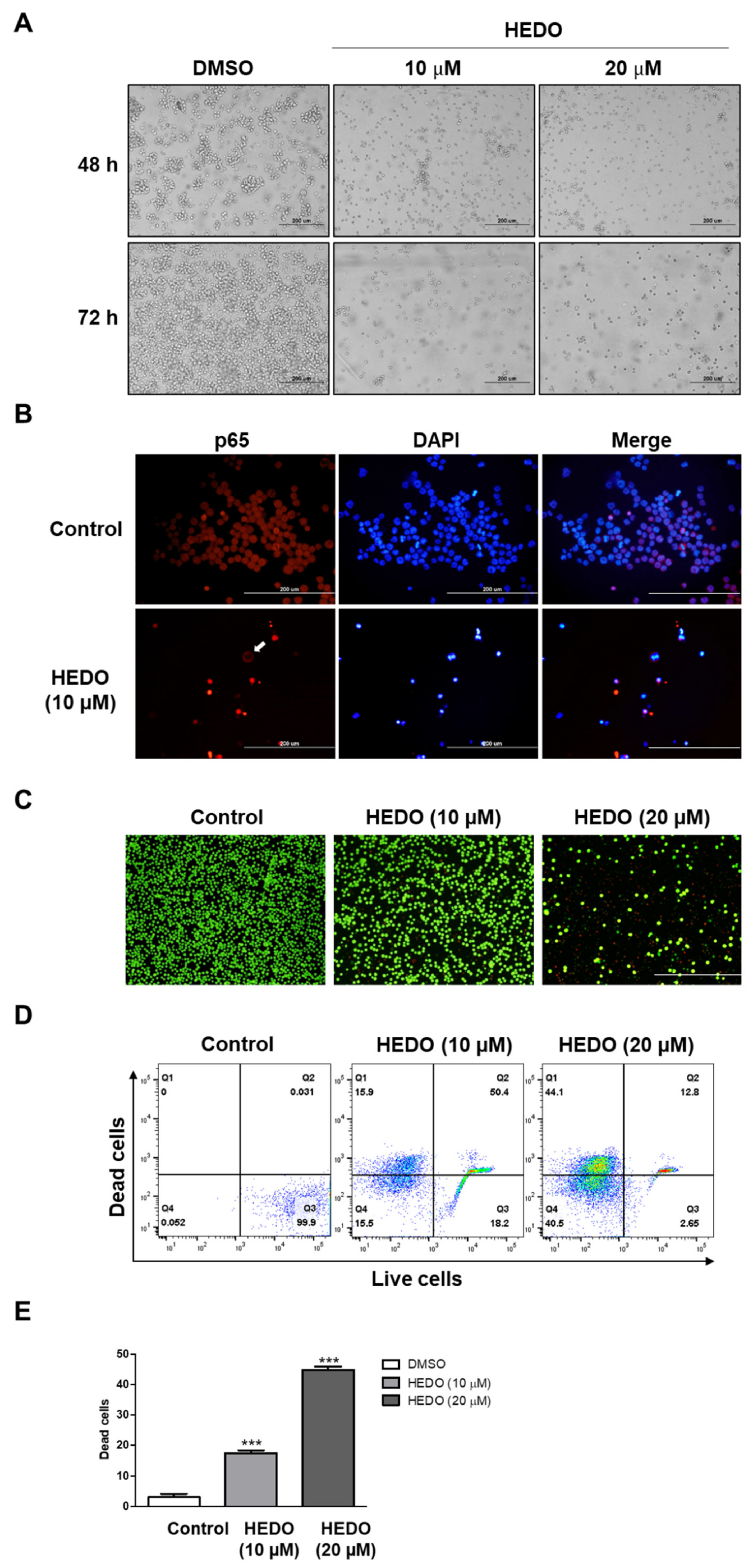
3.2. HEDO-Induced Antiproliferative Effect
3.3. HEDO Effects on Mitochondrial Membrane Potential
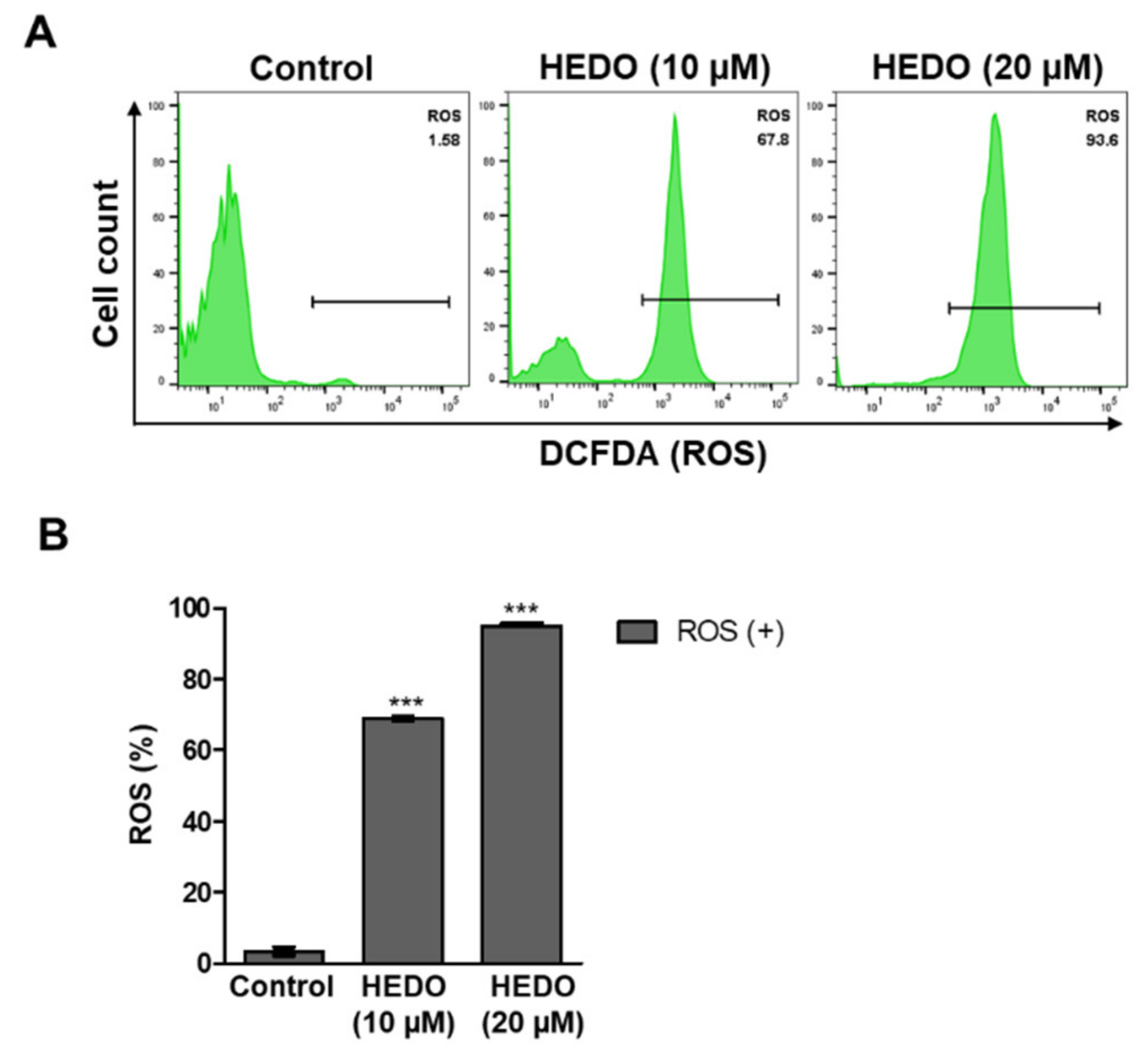
3.4. HEDO-Induced Reactive Oxygen Species (ROS)
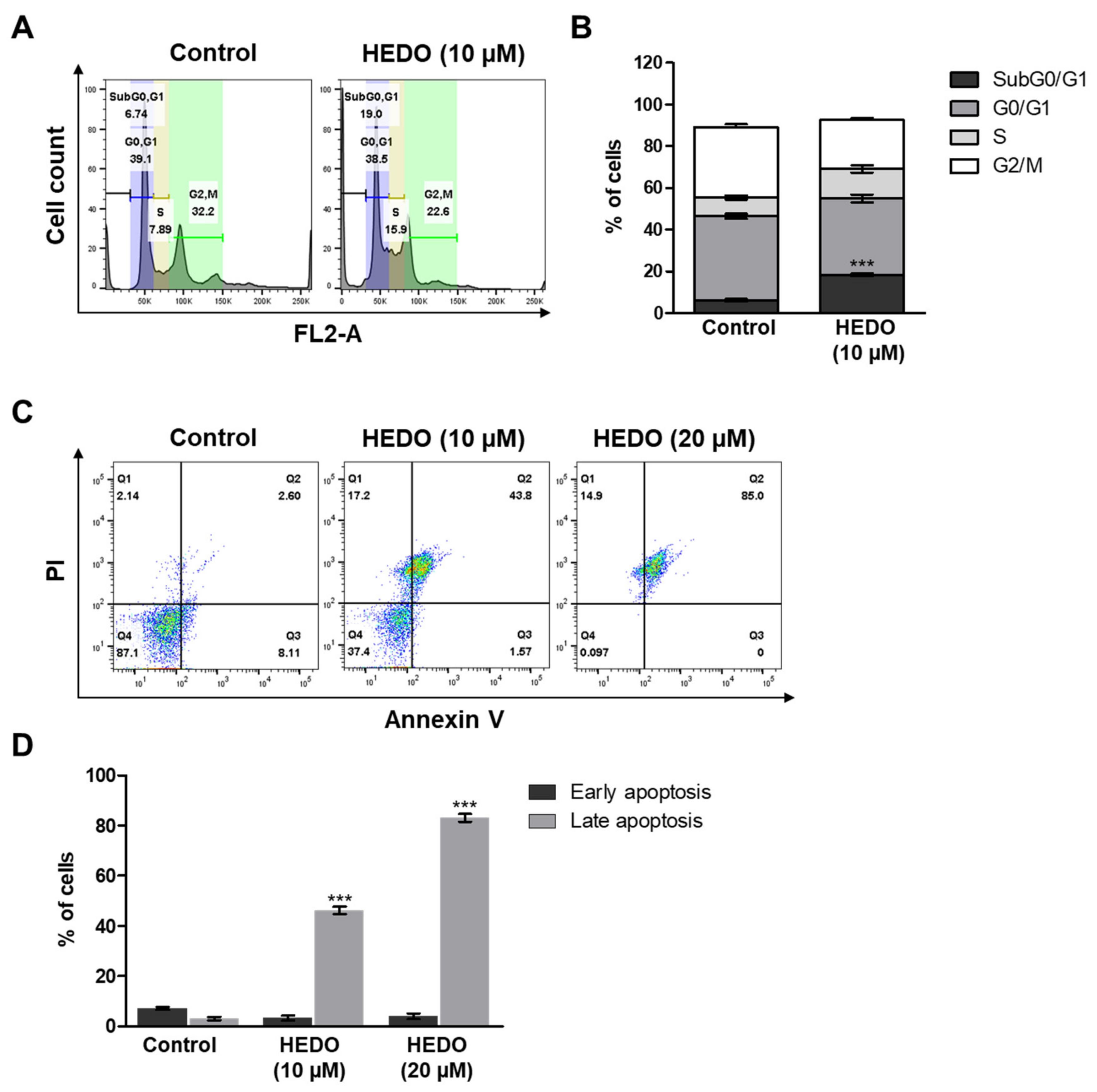
3.5. HEDO-Induced Cell Cycle Arrest
3.6. HEDO-Induced Apoptosis
3.7. Inhibition Analysis of NF-κB
3.8. Live/Dead Assay in OCI-LY3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaffer, A.L.; Young, R.M.; Staudt, L.M. Pathogenesis of Human B Cell Lymphomas. Annu. Rev. Immunol. 2012, 30, 565–610. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenwald, A.; Wright, G.; Leroy, K.; Yu, X.; Gaulard, P.; Gascoyne, R.D.; Chan, W.C.; Zhao, T.; Haioun, C.; Greiner, T.C.; et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J. Exp. Med. 2003, 198, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Elsen, M.B.; Davis, R.E.; Ma, C.L.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Miao, Y.; Medeiros, L.J.; Li, Y.; Li, J.; Young, K.H. Genetic alterations and their clinical implications in DLBCL. Nat. Rev. Clin. Oncol. 2019, 16, 634–652. [Google Scholar] [CrossRef]
- Lees, C.; Keane, C.; Gandhi, M.K.; Gunawardana, J. Biology and therapy of primary mediastinal B-cell lymphoma: Current status and future directions. Br. J. Haematol. 2019, 185, 25–41. [Google Scholar] [CrossRef]
- Young, R.M.; Phelan, J.D.; Wilson, W.H.; Staudt, L.M. Pathogenic B-cell receptor signaling in lymphoid malignancies: New insights to improve treatment. Immunol. Rev. 2019, 291, 190–213. [Google Scholar] [CrossRef]
- Compagno, M.; Lim, W.K.; Grunn, A.; Nandula, S.V.; Brahmachary, M.; Shen, Q.; Bertoni, F.; Ponzoni, M.; Scandurra, M.; Califano, A.; et al. Mutations of multiple genes cause deregulation of NF-B in diffuse large B-cell lymphoma. Nature 2009, 459, 717–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, K.; Karin, M. NF-B, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.M.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical constituents of plants from the Genus Inula. Chem. Biodivers. 2006, 3, 371–384. [Google Scholar] [CrossRef]
- Bai, N.; Zhou, Z.; Zhu, N.; Zhang, L.; Quan, Z.; He, K.; Zheng, Q.Y.; Ho, C.-T. Antioxidative flavonoids from the flower of Inula britannica. J. Food Lipids 2005, 12, 141–149. [Google Scholar] [CrossRef]
- Bai, N.; Lai, C.-S.; He, K.; Zhou, Z.; Zhang, L.; Quan, Z.; Zhu, N.; Zheng, Q.Y.; Pan, M.-H.; Ho, C.-T. Sesquiterpene Lactones from Inula b ritannica and Their Cytotoxic and Apoptotic Effects on Human Cancer Cell Lines. J. Nat. Prod. 2006, 69, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Hamayun, M.; Gilani, S.A.; Ahmad, S.; Rehman, G.; Kim, Y.H.; Kang, S.M.; Lee, I.J. Secondary metabolites from Inula britannica L. and their biological activities. Molecules 2010, 15, 1562–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.W.; Qin, J.J.; Cheng, X.R.; Shen, Y.H.; Shan, L.; Jin, H.Z.; Zhang, W.D. Inula sesquiterpenoids: Structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs 2014, 23, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Habibi, Z.; Saberi, M.; Jakupovic, J. A nor-guaianolide and A glaucolide-like eudesmanolide from Pulicaria undulata. Phytochemistry 1991, 30, 2405–2406. [Google Scholar] [CrossRef]
- Thuy, N.T.T.; Lee, J.-E.; Yoo, H.M.; Cho, N. Antiproliferative Pterocarpans and Coumestans from Lespedeza bicolor. J. Nat. Prod. 2019, 82, 3025–3032. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xu-Monette, Z.Y.; Li, L.; Manyam, G.C.; Visco, C.; Tzankov, A.; Wang, J.; Montes-Moreno, S.; Dybkaer, K.; Chiu, A.; et al. RelA NF-kB subunit activation as a therapeutic target in diffuse large B-cell lymphoma. Aging (Albany. N. Y.) 2016, 8, 3321–3340. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.R.; Uddin, S.; Ahmed, M.; Al-Dayel, F.; Bavi, P.P.; Al-Kuraya, K.S. Phosphorylated IκBα Predicts Poor Prognosis in Activated B-Cell Lymphoma and Its Inhibition with Thymoquinone Induces Apoptosis via ROS Release. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A. Chimeric antigen receptor T cell therapy for non-Hodgkin lymphoma. Curr. Res. Transl. Med. 2018, 66, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.W. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am. Soc. Hematol. Educ. Program. 2011, 2011, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991, 2, 325–357. [Google Scholar] [CrossRef]
- Chuang, S.E. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 2000, 21, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Metabolomic Profile of the Genus Inula. Chem. Biodivers. 2015, 12, 859–906. [Google Scholar] [CrossRef]
- Zhang, S.; Won, Y.K.; Ong, C.N.; Shen, H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. - Anti-Cancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef]
- Tang, S.A.; Zhu, H.; Qin, N.; Zhou, J.Y.; Lee, E.; Kong, D.X.; Jin, M.H.; Duan, H.Q. Anti-inflammatory terpenes from flowers of Inula japonica. Planta Med. 2014, 80, 583–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.D.; Qin, J.J.; Jin, H.Z.; Yin, Y.H.; Li, H.L.; Yang, X.W.; Li, X.; Shan, L.; Zhang, W.D. Sesquiterpenoids from Inula racemosa Hookf. Inhibit nitric oxide production. Planta Med. 2012, 78, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural product target network reveals potential for cancer combination therapies. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins (Basel). 2010, 2, 517–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Sachs, J.R.; Mayawala, K.; Gadamsetty, S.; Kang, S.P.; De Alwis, D.P. Optimal dosing for targeted therapies in oncology: Drug development cases leading by example. Clin. Cancer Res. 2016, 22, 1318–1324. [Google Scholar] [CrossRef] [Green Version]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Schulze, A.; Harris, A.L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012, 491, 364–373. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta - Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.Y.; Ou, N.; Lu, Q. Bin Antioxidant Induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, M.; Kauffman, M.; Traore, K.; Zhu, H.; Trush, M.; Jia, Z.; Li, Y. MitoSOX-Based Flow Cytometry for Detecting Mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, H.; Sato, M.; Kezuka, K.; Sato, I.; Feng, X.; Okumura, S.; Fujita, T.; Yokoyama, U.; Eguchi, H.; Ishikawa, Y.; et al. Effect of ascorbic acid on reactive oxygen species production in chemotherapy and hyperthermia in prostate cancer cells. J. Physiol. Sci. 2012, 62, 251–257. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Yang, Y.; Xu, C.; Gao, S. A flow cytometry-based assay for measuring mitochondrial membrane potential in cardiac myocytes after hypoxia/reoxygenation. J. Vis. Exp. 2018, 2018, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Feng, L.; Oldham, E.A.; Keating, M.J.; Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000, 407, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cordero, C.; Jose Leon-Gonzalez, A.; Manuel Calderon-Montano, J.; Burgos-Moron, E.; Lopez-Lazaro, M. Pro-Oxidant Natural Products as Anticancer Agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Mignotte, B.; Vayssière, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Hongmei, Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. Apoptosis Med. 2012, 3–22. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science (80-.) 2004, 305, 626–629. [Google Scholar] [CrossRef]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; Chin, H.S.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science (80-.) 2018, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep. 2018, 25, 2339–2353.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-κB signaling: Balancing life and death - A new approach to cancer therapy. J. Clin. Invest. 2005, 115, 2625–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Kucharczak, J.; Simmons, M.J.; Fan, Y.; Gélinas, C. To be, or not to be: NF-κB is the answer - Role of Rel/NF-κB in the regulation of apoptosis. Oncogene 2003, 22, 8961–8982. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.L.; Liao, Z.D.; Chen, F.F.; Zhang, W.; Ren, Y.S.; Wang, C.C.; Chen, X.X.; Peng, D.Y.; Kong, L.Y. Benzophenones from Anemarrhena asphodeloides Bge. Exhibit anticancer activity in HepG2 cells via the NF-κB signaling pathway. Molecules 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, D.K.; Lee, I.-S.; Shin, H.-S.; Yoo, H.M. 2α-Hydroxyeudesma-4,11(13)-Dien-8β,12-Olide Isolated from Inula britannica Induces Apoptosis in Diffuse Large B-cell Lymphoma Cells. Biomolecules 2020, 10, 324. https://doi.org/10.3390/biom10020324
Jang DK, Lee I-S, Shin H-S, Yoo HM. 2α-Hydroxyeudesma-4,11(13)-Dien-8β,12-Olide Isolated from Inula britannica Induces Apoptosis in Diffuse Large B-cell Lymphoma Cells. Biomolecules. 2020; 10(2):324. https://doi.org/10.3390/biom10020324
Chicago/Turabian StyleJang, Dae Kil, Ik-Soo Lee, Han-Seung Shin, and Hee Min Yoo. 2020. "2α-Hydroxyeudesma-4,11(13)-Dien-8β,12-Olide Isolated from Inula britannica Induces Apoptosis in Diffuse Large B-cell Lymphoma Cells" Biomolecules 10, no. 2: 324. https://doi.org/10.3390/biom10020324
APA StyleJang, D. K., Lee, I. -S., Shin, H. -S., & Yoo, H. M. (2020). 2α-Hydroxyeudesma-4,11(13)-Dien-8β,12-Olide Isolated from Inula britannica Induces Apoptosis in Diffuse Large B-cell Lymphoma Cells. Biomolecules, 10(2), 324. https://doi.org/10.3390/biom10020324