Current and Novel Therapies Against Helminthic Infections: The Potential of Antioxidants Combined with Drugs
Abstract
:1. Helminthic Infections: An Overview
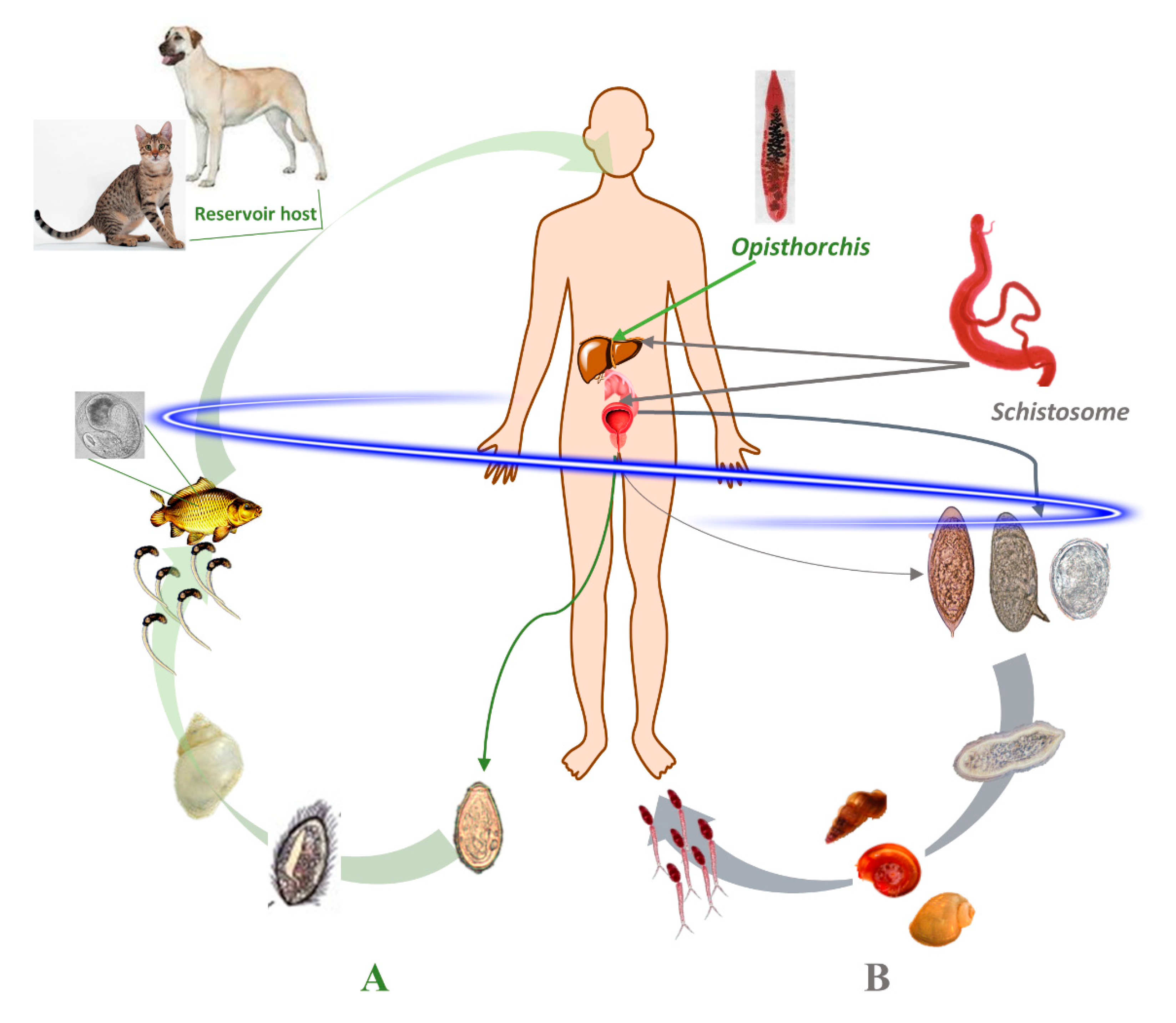
1.1. Schistosomes: Geographical Distribution, Life Cycle and Infection
1.2. Opisthorchis: Geographical Distribution, Life Cycle and Infection
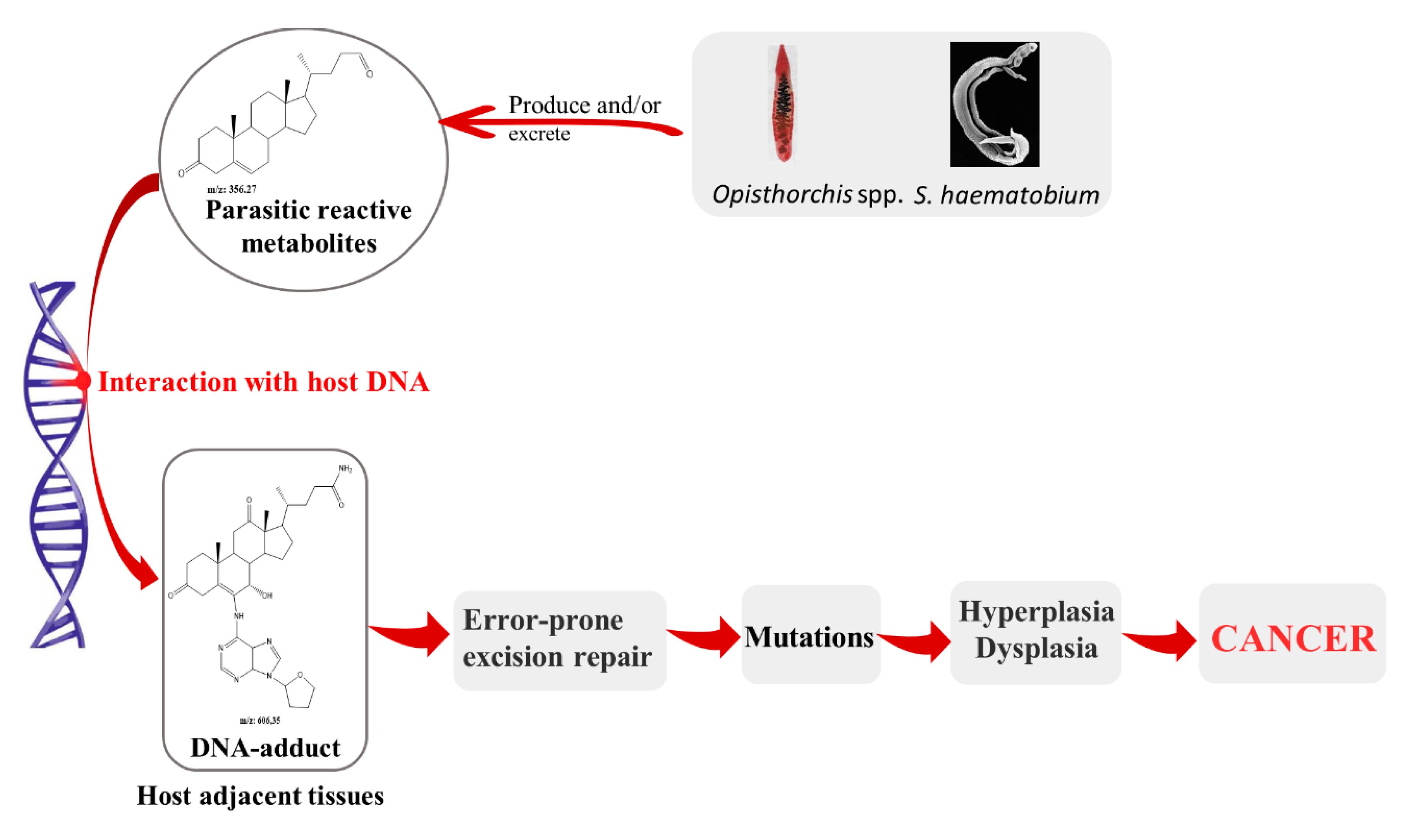
2. Parasites and Its Metabolites: Their Role on Pathogenesis and Carcinogenesis Associated to Infection
3. Chemotherapy Against Schistosomiasis and Opisthorchiasis
3.1. Drug Repurposing and Combine Treatments for Opisthorchiasis and Schistosomiasis
3.1.1. Schistosomiasis
3.1.2. Opisthorchiasis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- King, C.H. Lifting the burden of schistosomiasis-defining elements of infection-associated disease and the benefits of antiparasite treatment. J. Infect. Dis. 2007, 196, 653–655. [Google Scholar] [CrossRef]
- Budke, C.M.; Jiamin, Q.; Qian, W.; Torgerson, P.R. Economic effects of echinococcosis in a disease-endemic region of the Tibetan Plateau. Am. J. Trop. Med. Hyg. 2005, 73, 2–10. [Google Scholar] [CrossRef]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [Green Version]
- van der Werf, M.J.; de Vlas, S.J.; Brooker, S.; Looman, C.W.; Nagelkerke, N.J.; Habbema, J.D.; Engles, D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003, 86, 125–139. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Biological Agents. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans. World Health Organization. Int. Agency Res. Cancer 2012, 100B, 1–441. [Google Scholar]
- Ross, A.G.P.; Bartley, P.B.; Sleigh, A.C.; Olds, R.; Li, Y.; Williams, G.M.; McManus, P. Schistosomiasis. N. Engl. J. Med. 2002, 346, 1212–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissier, J.; Moné, H.; Mitta, G.; Bargues, M.D.; Molyneux, D.; Mas-Coma, S. Schistosomiasis reaches Europe. Lancet. Infect. Dis 2015, 15, 757–758. [Google Scholar] [CrossRef]
- Berry, A.; Moné, H.; Iriart, X.; Mouahid, G.; Aboo, O.; Boissier, J.; Fillaux, J.; Cassaing, S.; Debuisson, C.; Valentin, A.; et al. Schistosomiasis haematobium, Corsica, France. Emerg. Infect. Dis. 2014, 20, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Noël, H.; Ruello, M.; Maccary, A.; Pelat, C.; Sommen, C.; Boissier, J.; Barré-Cardi, H.; Fillaux, J.; Termignon, J.-L.; Debruyne, M.; et al. Large outbreak of urogenital schistosomiasis acquired in Southern Corsica, France: Monitoring early signs of endemicization? Clin. Microbiol. Infect. 2018, 24, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moné, H.; Holtfreter, M.C.; Allienne, J.-F.; Mintsa-Nguéma, R.; Ibikounlé, M.; Boissier, J.; Berry, A.; Mitta, G.; Ritcher, J.; Mouhadi, G. Introgressive hybridizations of Schistosoma haematobium by Schistosoma bovis at the origin of the first case report of schistosomiasis in Corsica (France, Europe). Parasitol. Res. 2015, 114, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Dickman, K.; Tisch, D.J. Reassessment of the cost of chronic helminth infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005, 365, 1561–1569. [Google Scholar] [CrossRef]
- Murare, H.M.; Taylor, P. Haematuria and proteinuria during Schistosoma haematobium infection: Relationship to intensity of infection and the value of chemical reagent strips for pre- and post-treatment diagnosis. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 426–430. [Google Scholar] [CrossRef]
- Hicks, R.M.; Ismail, M.M.; Walters, C.L.; Beecham, P.T.; Rabie, M.F.; El Alamy, M.A. Association of bacteriuria and urinary nitrosamine formation with Schistosoma haematobium infection in the Qalyub area of Egypt. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 519–527. [Google Scholar] [CrossRef]
- Hodder, S.L.; Mahmoud, A.A.; Sorenson, K.; Weinert, D.M.; Stein, R.L.; Ouma, J.H.; Koech, D.; King, C.H. Predisposition to urinary tract epithelial metaplasia in Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 2000, 63, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.H.; Sheweita, S.A.; O´Connor, P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microb. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Porta, C.; Riboldi, E.; Sica, A. Mechanism linking pathogens-associated inflammation and cancer. Cancer Lett. 2011, 305, 250–262. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J. Foodborne trematodiasis. Clin. Microbiol. Rev. 2009, 22, 466–483. [Google Scholar] [CrossRef] [Green Version]
- Petney, T.N.; Andrews, R.H.; Saijuntha, W.; Wenz-Mücke, A.; Sithithaworn, P. The zoonotic, fishborne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int. J. Parasitol. 2013, 43, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Sithithaworn, P.; Andrews, R.H.; Nguyen, V.D.; Wongsaroj, T.; Sinuon, M.; Odermatt, P.; Nawa, Y.; Liang, S.; Brindley, P.J.; Sripa, B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol. Int. 2012, 61, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, M.J.; Pakharukova, M.Y.; Laha, T.; Sripa, B.; Maksimova, G.A.; Rinaldi, G.; Brindley, P.J.; Mordvinov, V.A.; Amaro, T.; Santos, L.L.; et al. Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model. Carcinogenesis 2017, 38, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Mordvinov, V.A. The liver fluke Opisthorchis felineus: Biology, epidemiology and carcinogenic potential. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksimova, G.A.; Pakharukova, M.Y.; Kashina, E.V.; Zhukova, N.A.; Kovner, A.V.; Lvova, M.N.; Katokhin, A.V.; Tolstikova, T.G.; Sripa, B.; Mordvinov, V.A. Effect of Opisthorchis felineus infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian hamster. Parasitol. Int. 2017, 66, 458–463. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini-multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Harinasuta, T.; Riganti, M.; Bunnag, D. Opisthorchis viverrini infection: Pathogenesis and clinical features. Arzeimittelforschung 1984, 34, 1167–1169. [Google Scholar]
- Mairiang, E.; Mairiang, P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003, 88, 221–227. [Google Scholar] [CrossRef]
- Ogodorova, L.M.; Fedorova, O.S.; Sripa, B.; Mordvinovm, V.A.; Katokhinm, A.V.; Keiser, J.; Odermatt, P.; Brindley, P.J.; Mayboroda, O.A.; Velavan, T.P.; et al. Opisthorchiasis: An overlooked danger. PLoS Negl. Trop. Dis. 2015, 9, e0003563. [Google Scholar]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-R.; Oh, J.K.; Masuyer, E.; Curado, M.P.; Bouvard, V.; Fang, Y.Y.; Wiangnon, S.; Sripa, B.; Hong, S.T. Epidemiology of cholangiocarcinoma: An update focusing on risk factors. Cancer Sci. 2010, 101, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Thunyaharn, N.; Promthet, S.; Wiangnon, S.; Suwanrungruang, K.; Kamsa-ard, S. Survival of cholangiocarcinoma patients in northeastern Thailand after supportive treatment. Asian Pac. J. Cancer Prev. 2013, 14, 7029–7032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honeycutt, J.; Hammam, O.; Fu, C.L.; Hsieh, M.H. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol. 2014, 30, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale, N.; Gouveia, M.J.; Botelho, M.C.; Sripa, B.; Suttiprapa, S.; Rinaldi, G.; Gomes, P.; Brindley, P.J.; Correia da Costa, J.M. Carcinogenic liver fluke Opisthorchis viverrini oxysterols detected by LC-MS/MS survey of soluble fraction parasite extract. Parasitol. Int. 2013, 62, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, M.J.; Santos, J.; Brindley, P.J.; Rinaldi, G.; Lopes, C.; Santos, L.L.; da Costa, J.M.C.; Vale, N. Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett. 2015, 359, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Santos, J.; Santos, L.L.; Brindley, P.J.; da Costa, J.M.C. The role of estradiol metabolism in urogenital induced bladder cancer. Tumor Biol. 2017, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Plieskatt, J.L.; Deenonpoe, R.; Mulvenna, J.P.; Krause, L.; Sripa, B.; Bethony, J.M.; Brindley, P.J. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013, 27, 4572–4584. [Google Scholar] [CrossRef] [Green Version]
- Pairojkul, C.; Shirai, T.; Hirohashi, S.; Thamavit, W.; Bhudhisawat, W.; Uttaravicien, T.; Itoh, M.; Ito, N. Multistage Carcinogenesis of Liver-Fluke-Associated Cholangiocarcinoma in Thailand. Princess Takamatsu Symp. 1991, 22, 77–86. [Google Scholar]
- Moore, M.A.; Thamavit, W.; Tiwawech, D.; Ito, N. Cell death and proliferation in Opisthorchis viverrini-DHPN induced carcinogenesis in the Syrian Hamster hepato-pancreatic axis. In Chemical Carcinogenesis 2; Columbano, A., Feo, F., Pascale, R., Pani, P., Eds.; Springer: Boston, MA, USA, 1991. [Google Scholar]
- Thamavit, W.; Moore, M.A.; Hiasa, Y.; Ito, N. Enhancement of DHPN-induced hepatocellular cholangiocellular and pancreatic carcinogenesis by Opisthorchis viverrini infestation in Syrian golder hamsters. Carcinogenesis 1988, 9, 1095–1098. [Google Scholar] [CrossRef]
- Thamavit, W.; Pairojkul, C.; Tiwawech, D.; Itoh, M.; Shirai, T.; Ito, N. Promotion of cholangiocarcinogenesis in the hamster liver by bile duct ligation after dimethylnitrosamine initiation. Carcinogenesis 1993, 14, 2415–2417. [Google Scholar] [CrossRef]
- Brindley, P.J.; Correia da Costa, J.M.; Sripa, B. Why does infection with some helminths cause cancer? Trends Parasitol. 2015, 3, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.C.; Miller, J.A. Mechanism of chemical carcinogenesis. Cancer 1981, 47, 1055–1064. [Google Scholar] [CrossRef]
- Cavalieri, E.; Rogan, E. The molecular etiology and prevention of estrogen-initiated cancers. Ockham´s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol. Asp. Med. 2014, 36, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, E.; Rogan, E. Depurinating estrogen-DNA adducts, generators of cancer initiation: Their minimization leads to cancer prevention. Clin. Trans. Med. 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalieri, E.; Chakravarti, D.; Guttenplan, J.; Hart, E.; Ingle, J.; Jankowiak, R.; Muti, P.; Rogan, E.; Russo, J.; Santen, R.; et al. Catechol estrogen quinones as initiators of breast and other human cancers: Implication for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta 2006, 1766, 67–78. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Rogan, E.G. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Botelho, M.; Vale, N.; Gouveia, M.J.; Rinaldi, G.; Santos, J.; Santos, L.L.; Gomes, P.; Brindley, P.J.; Correia da Costa, J.M. Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: An oestrogen-DNA adducts mediated pathway? Int. J. Parasitol. 2013, 43, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.; Fernandes, E.; Ferreira, J.A.; Lima, L.; Tavares, A.; Peixoto, A.; Parreira, B.; Correia da Costa, J.M.; Brindley, P.J.; Lopes, C.; et al. P53 and cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with Schistosoma haematobium infection. PLoS Negl. Trop. Dis. 2015, 8, e3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia da Costa, J.M.; Vale, N.; Gouveia, M.J.; Botelho, M.C.; Sripa, B.; Santos, L.L.; Santos, J.H.; Rinaldi, G.; Brindley, P.J. Schistosome and liver fluke derived catechol-estrogens and helminth associated cancers. Front. Genet. 2014, 5, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olliaro, P.; Delgado-Romero, P.; Keiser, J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J. Antimicrob. Chemother. 2014, 69, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Correia da Costa, J.M. Praziquantel for Schistosomiasis: Single drug metabolism revisited, mode of action, and resistance. Antimicrob. Agent. Chemother. 2017, 61, e02582–e02616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzinger, J.; Keiser, J. Schistosomiasis and soil-transmitted helminthiasis: Common drugs for treatment and control. Exp. Opin. Pharmacother. 2004, 5, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Wegner, D.H. Multicentre trials of praziquantel in human schistosomiasis: Design and techniques. Bull. World Health Organ. 1979, 57, 767–771. [Google Scholar]
- Keiser, J.; Utzinger, J. Chemotherapy for major food-borne trematodes: A review. Exp. Opin. Pharmacother. 2004, 5, 1711–1726. [Google Scholar] [CrossRef]
- Bunnang, D.; Harinasuta, T. Studies on the chemotherapy of human opisthorchiasis: III. Minimum effective dose of praziquantel. Southeast. Asian J. Trop. Med. Public Health 1981, 12, 413–417. [Google Scholar]
- Gurarie, D.; Yoon, N.; Li, E.; Ndeffo-Mbah, M.; Durham, D.; Phillips, A.Q.; Osvaldo Aurelio, H.; Ferro, J.; Galvani, A.P.; King, C.H. Modelling control of Schistosoma haematobium infection: Prediction of the long-term impact of mass drug administration in Africa. Parasit. Vectors 2015, 8, 529. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Sustaining the drive to overcome the global impact of neglected tropical diseases. In Second WHO Report on Neglected Tropical Diseases; Crompton, D.W.T. (Ed.) WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Sayasone, S.; Meister, I.; Andrews, J.R.; Odermatt, P.; Vonghachack, Y.; Xayavong, S.; Senggnam, K.; Phongluxa, K.; Hattendorf, J.; Bogoch, I.I.; et al. Efficacy and safety of praziquantel against light infections of Opisthorchis viverrini; a randomized parallel single-blind dose-ranging trial. Clin. Infect. Dis. 2017, 64, 451–458. [Google Scholar]
- Andrews, R.H.; Sithithaworn, P.; Petey, T.N. Opisthorchis viverrini: An underestimated parasite in world health. Trends Parasitol. 2008, 24, 497–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, T.; O’Connor, T.; Techasen, A.; Namwat, N.; Loilome, W.; Andrews, R.H.; Khuntikeop, N.; Yongvanit, P.; Sithithaworn, P.; Taylor-Robinson, S.D. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: An unresolved problem. Int. J. Gen. Med. 2017, 10, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Model Prescribing Information. Drugs Used in Parasitic Diseases, 2nd ed.; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Pakharukova, M.Y.; Shilov, A.G.; Pirozhkova, D.S.; Katokhin, A.V.; Mordvinov, V.A. The first comprehensive study of praziquantel effects in vivo and in vitro on European liver fluke Opisthorchis felineus (Trematoda). Int. J. Antimicrob. Agent 2015, 46, 94–100. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Bertino, A.M. Asymmetries of poverty: Why global burden of disease valuations underestimates the burden of neglected tropical diseases. PLoS Negl. Trop. Dis. 2008, 2, e209. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, M.J.; Brindley, P.J.; Gärtner, F.; Correia da Costa, J.M.; Vale, N. Drug repurposing for schistosomiasis: Combinations of drugs or biomolecules. Pharmaceuticals 2018, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Day, T.A.; Botros, S. Drug resistance in schistosomes. In Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology; Maule, A.G., Marks, N.J., Eds.; CABI: London, UK, 2006; pp. 256–265. [Google Scholar]
- Pinlaor, S.; Ma, N.; Hiraku, Y.; Yongvanit, P.; Semba, R.; Oikawa, S.; Murata, M.; Sripa, B.; Sithithaworn, P.; Kawanishi, S. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanine in the bile duct of hamsters via inducible nitric synthase. Carcinogenesis 2004, 25, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Pinlaor, S.; Prakobwong, S.; Boonmars, T. Effect of praziquantel treatment on the expression of matrix metalloproteinases in relation to tissue resorption during fibrosis in hamsters with acute and chronic infection. Acta Trop. 2009, 111, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kamsa-ard, S.; Laopaiboon, M.; Luvira, V.; Bhudhisawasdi, V. Association between praziquantel and cholangiocarcinoma in patients infected with Opisthorchis viverrini: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 7011–7016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, C.; Troiani, A.R.; Festucci, A.; Pica-Mattocia, L.; Liberti, P.; Wolstenholme, A.; Franclow, K.; Doenhoff, M.J.; Cioli, D. Sequence and level of endogenous expression of calcium channel beta subunits in Schistosoma mansoni displaying different susceptibilities to praziquantel. Mol. Biochem. Parasitol. 2003, 130, 111–115. [Google Scholar] [CrossRef]
- Greenberg, R.M. Are Ca2+ channels targets of praziquantel action? Int. J. Parasitol. 2005, 35, 1–9. [Google Scholar] [CrossRef]
- Angelucci, F.; Basso, A.; Belielli, A.; Brunori, M.; Pica-Mattocia, L.; Valle, C. The anti-schistosomal drug praziquantel is an adenosine antagonist. Parasitology 2007, 134, 1215–1221. [Google Scholar] [CrossRef]
- Tallina, H.; El Ridi, R. Praziquantel binds Schistosoma mansoni adult worm actin. Int. J. Antimicrob. Agent 2007, 29, 570–575. [Google Scholar] [CrossRef]
- Chan, J.D.; Zarowiecki, M.; Marchant, J.S. Ca2+ channels and praziquantel: A view from the free world. Parasitol. Int. 2013, 62, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Troiani, A.R.; Pica-Mattocia, L.; Valle, C.; Cioli, D.; Mignogna, G.; Ronketti, F.; Todd, M. Is actin a praziquantel receptor? Int. J. Antimicrob. Agent 2007, 30, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.D.; Cupit, P.M.; Gunaratne, G.S.; McCorvy, J.D.; Yang, Y.; Stolz, K.; Webb, T.R.; Dosa, P.I.; Roth, B.L.; Abagyan, R.; et al. The anthelmintic praziquantel is a human serotoninergic G-protein-coupled receptor ligand. Nat. Commun. 2017, 8, 1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trainor-Moss, S.; Mutapi, F. Schistosomiasis therapeutics: What is in pipeline? Exp. Rev. Clin. Pharmacol. 2016, 9, 157–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrifield, M.; Hotez, P.J.; Beaumier, C.M.; Gillspie, P.; Strychm, U.; Hayward, T.; Bottazzi, M.E. Advancing a vaccine to prevent human schistosomiasis. Vaccine 2016, 34, 2988–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Fallon, P.G. Schistosome resistance to praziquantel. Drug Resist. Update 1998, 1, 236–241. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Liang, Y. Susceptibility or resistance of praziquantel in human schistosome: A review. Parasitol. Res. 2012, 111, 1871–1877. [Google Scholar] [CrossRef]
- Cioli, D. Is there any real resistance and are there alternatives? Curr. Opin. Infect. Dis. 2000, 13, 659–663. [Google Scholar] [CrossRef]
- Tinga, N.; De, N.; Vien, H.V.; Chau, L.; Toan, N.D.; Kager, P.A.; Vries, P.J. Little effect of praziquantel or artemisinin on clonorchiasis in Northern Vietnam. A pilot study. Trop. Med. Int. Health 1999, 4, 814–818. [Google Scholar] [CrossRef]
- Soukhathammavong, P.; Odermatt, P.; Sayasone, S.; Vonghachack, Y.; Vounatsou, P.; Hatz, C.; Akkhavong, K.; Keiser, J. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, tribendimidine, and praziquantel in patients with Opisthorchis viverrini: A randomised, exploratory, open-label, phase 2 trial. Lancet. Infect. Dis. 2011, 11, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, A.A.; Siddiqui, B.A.; Gangley-Leal, L. Schistosomiasis vacines. Hum. Vaccin. 2011, 7, 1192–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaramonte, M.G.; Donaldson, D.D.; Cheever, A.W.; Wynn, T.A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 1999, 104, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; William, S.; Herdtweck, E.; Botros, S.; Dömling, A. MCR synthesis of praziquantel derivatives. Chem. Biol. Drug Des. 2012, 79, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef] [Green Version]
- Padhy, B.M.; Gupta, Y.K. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef]
- Kerantzas, C.A.; Jacobs, W.R., Jr. Origins of combination therapy for tuberculosis: Lessons for future antimicrobial development and application. mBio 2017, 8, e01686–e01716. [Google Scholar] [CrossRef] [Green Version]
- Maenza, J.; Flexner, C. Combination antiretroviral therapy for HIV infection. Am. Fam. Physician 1998, 57, 2789–2798. [Google Scholar]
- Wells, T.N.C.; van Huijsduijnen, R.H.; van Voortris, W.C. Malaria medicines: A glass half full? Nat. Rev. Drug Dis. 2015, 14, 424–442. [Google Scholar] [CrossRef]
- Richards, H.C.; Foster, R. A new series of 2-aminomethyltetrahydroquinoline derivatives displaying schistosomicidal activity in rodents and primates. Nature 1969, 222, 581–582. [Google Scholar] [CrossRef]
- Fewick, A.; Savioli, L.; Engles, D.; Robert Bergquist, N.; Todd, M.H. Drugs for the control of parasitic diseases: Current status and development in schistosomiasis. Trends Parasitol. 2003, 19, 509–515. [Google Scholar] [CrossRef]
- Shaw, J.R.; Brammer, K.W. The treatment of experimental schistosomiasis with a combination of oxamniquine and praziquantel. Trans. R. Soc. Med. Trop. Hyg. 1983, 77, 39–40. [Google Scholar] [CrossRef]
- Botros, S.; Soliman, A.; El-Gawhary, N.; Selim, N.; Guirguis, N. Effect of combined low dose of praziquantel and oxamniquine on different stages of schistosome maturity. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 86–89. [Google Scholar] [CrossRef]
- Delgado, V.S.; Suárez, D.P.; Cesar, I.M.; Incani, R.N. Experimental chemotherapy of Schistosoma mansoni with praziquantel and oxamniquine: Differential effect on single of combined formulations of drugs on various strains and on both sexes of the parasite. Parasitol. Res. 1992, 78, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Teesdale, C.H. Synergy of concurrent low dose of oxamniquine and praziquantel in schistosomiasis. Br. Med. J. 1983, 287, 877–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creasey, A.M.; Taylor, P.; Thomas, J.E. Dosage trial of a combination of oxamniquine and praziquantel in the treatment of schistosomiasis in Zimbabwean schoolchildren. Cent. Afr. J. Med. 1986, 32, 165–167. [Google Scholar] [PubMed]
- Ashley, E.A.; White, N.J. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 2005, 18, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez del Villar, L.; Burguillo, F.J.; López-Abán, J.; Muro, A. Systematic review and meta-analysis of artemisinin-based therapies for the treatment and prevention of schistosomiasis. PLoS ONE 2012, 7, e45867. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Zoheiry, M.M.K.; Nosseir, M.M.F. Effect of combined chemotherapy and anti-inflammatory drugs on murine schistosomiasis. Arzneim-Forsch Drug Res. 2002, 52, 294–301. [Google Scholar] [CrossRef]
- Hassan, S.I.; Ali, I.; Nessim, N.G.; Amer, N.M.; Abd el Kader el Khafif, M.; Ashour, A.; el Mohandes, M. Treatment of acute schistosomiasis mansoni with praziquantel and an antifibrotic agent in mice. Arzneim-Forsch Drug Res. 2003, 53, 440–444. [Google Scholar] [CrossRef]
- Giboda, M.; Zenka, J.; Julis, I.; Vítovec, J. Experimental schistosomiasis mansoni: Modulation of granulomas by inhibition of collagen cross-link formation. Preliminary report. Ann. Trop. Med. Parasitol. 1992, 86, 631–636. [Google Scholar] [CrossRef]
- Soliman, M.F.M.; Ibrahim, M.M. Antischistosomal action of atorvastatin alone and concurrently with medroxyprogesterone acetate on Schistosoma haematobium harboured in hamster: Surface ultrastructure and parasitological study. Acta Trop. 2005, 93, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yepes, E.; Varela, M.R.E.; López-Abán, J.; Rojas-Caraballo, J.; Muro, A.; Mollinedo, F. Inhibition of granulomatous inflammation and prophylactic treatment of schistosomiasis with a combination of edelfosine and praziquantel. PLoS Negl. Trop. Dis. 2015, 9, e0003893. [Google Scholar] [CrossRef]
- Rojo-Arreola, L.; Long, T.; Asarnow, D.; Suzuk, B.M.; Singh, R.; Caffrey, C.R. Chemical and genetic validation of the statin drug target to treat the helminth disease, schistosomiasis. PLoS ONE 2014, 9, e87594. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Ibhraim, N.; El-Rigal, N. Comparative in vivo antioxidant levels in Schistosoma mansoni mice treated with praziquantel or the essential oil Melaleuca armillaris leaves. Pak. J. Biol. Sci 2012, 15, 971–978. [Google Scholar] [CrossRef]
- Soliman, R.H.; Ismail, O.A.; Badr, M.S.; Nasr, S.M. Resveratrol ameliorates oxidative stress and organ dysfunction in Schistosoma mansoni infected mice. Exp. Parasitol. 2017, 174, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mata-Santos, H.A.; Lino, F.G.; Rocha, C.C.; Paiva, C.N.; Castelo Branco, M.T.; Pyrrho Ados, S. Silymarin treatment reduces granuloma and hepatic fibrosis in experimental schistosomiasis. Parasitol. Res. 2010, 107, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.O.A.; El-Shinnawy, N.A. Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice. Asian Pac. J. Trop. Med. 2015, 8, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantawy, M.M.; Ay, H.F.; Rizk, M.Z. Therapeutic effects of Allium sativum and Allium cepa in Schistosoma mansoni experimental infection. Rev. Inst. Med. Trop. 2011, 53, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantawy, M.M.; Aly, H.F.; Zayed, N.; Fahmy, Z.H. Antioxidant and schistosomicidal effect of Allium sativum and Allium cepa against Schistosoma mansoni different stages. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 69–80. [Google Scholar]
- Mahmoud, M.R.; El-Abhar, H.S.; Saleh, S. The effect of Nigella sativa oil against the liver damage induced by S. mansoni infection in mice. J. Enthopharmacol. 2002, 79, 1–11. [Google Scholar] [CrossRef]
- El-Shennawy, N.S.; Soliman, M.F.M.; Reyad, S.I. The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in mice. Rev. Inst. Med. Trop. 2008, 50, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ansary, A.K.; Ahmed, S.A.; Aly, S.A. Antischistosomal and liver protective effects of curcuma longa extract in Schistosoma mansoni infected mice. Indian J. Exp. Biol. 2007, 45, 791–801. [Google Scholar] [PubMed]
- Eraky, M.A.; El-Kholy, A.A.E.; Rashed, G.A.E.; Hammam, O.A.; Moharam, A.F.; Abou-Ouf, E.A.; Aly, N.S.; Kishik, S.M.; Abdallah, K.F.; Hamdan, D.I. Dose-response relationship in Schistosoma mansoni juvenile and adult stages following limonin treatment in experimentally infected mice. Parasitol. Res. 2016, 115, 4045–4054. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.N.; Rehder, V.L.G.; Oliveira, A.S.S.; Jeraldo, V.D.L.S.; Linhares, A.X.; Allegretti, S.M. Antihelmintic activity in vitro and in vivo of Baccharis trímera (less) DC against immature and adult worms of Schistosoma mansoni. Exp. Parasitol. 2014, 139, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.M.; Metwally, N.M.; Mahmoud, S.S. Sativa seeds against Schistosoma mansoni different stages. Mem. Inst. Oswaldo Cruz 2005, 100, 205–211. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, R.N.; Rehder, V.L.G.; Oliveira, A.S.S.; Júnior, Í.M.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Jeraldo, V.d.L.S.; Linhares, A.X.; Allegretti, S.M. Schistosoma mansoni: In vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp. Parasitol. 2012, 132, 135–143. [Google Scholar] [CrossRef]
- Patocka, N.; Sharma, N.; Rashid, R.; Ribeiro, P. Serotonin signaling in Schistosoma mansoni: A serotonin-activated G protein-coupled receptor controls parasite movement. PLoS Pathog. 2014, 10, e1003878. [Google Scholar] [CrossRef]
- Ribeiro, R.; Patocka, N. Neurotransmitter transporters in schistosomes: Structure, function and prospects for drug discovery. Parasitol. Int. 2013, 62, 629–638. [Google Scholar] [CrossRef]
- Han, Z.-G.; Brindley, P.J.; Wang, S.-Y.; Chen, Z. Schistosoma genomics: New perspectives on schistosome biology and host-parasite interaction. Annu. Rev. Genom. Hum. Genet. 2009, 10, 211–240. [Google Scholar] [CrossRef]
- de Paula Aguiar, D.; Brunetto, M.M.M.; Rezende, M.E.; Graciano de Paula, R.; Ferreira, P.M.; Afonso, A.; Belo, S.; Tomie Ouchida, A.; Curti, C.; Cunha, W.R.; et al. Curcumin generates oxidative stress and induces apoptosis in adult Schistosoma mansoni worms. PLoS ONE 2016, 11, e0167135. [Google Scholar] [CrossRef]
- Soliman, M.F.M.; El Shenawy, N.S.; El Arabi, S.E. Schistosoma mansoni: Melatonin enhances efficacy of cercarial and soluble antigen in the induction of protective immunity against infection in the hamster. Exp. Parasitol. 2008, 119, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, J.I.; Mohammed, A.R.; Hussein, N.A.; El-Shennawy, A.; Noshy, M.; Abbas, M. In vivo antioxidant and antigenotoxic evaluation of an enaminone derivative BDHQ combined with praziquantel in uninfected and Schistosoma mansoni infected mice. J. Appl. Pharm. Sci. 2014, 4, 25–33. [Google Scholar]
- Metawally, N.S. Potency of Allium sativum and Allium cepa oils against Schistosoma mansoni infection in mice. Egypt J. Hosp. Med. 2006, 23, 319–322. [Google Scholar]
- Erasmus, D.A. A comparative study of the reproductive system of mature, immature and “unisexual” female Schistosoma mansoni. Parasitology 1973, 67, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafeez, E.H.; Ahmad, A.K.; Abdulla, A.M.; Aabdel-Wahab, S.; Mosalem, F.A. Therapeutic effect of alpha lipoic acid combined with praziquantel on liver fibrosis induced by Schistosoma mansoni challenged mice. Parasitol. Res. 2012, 111, 577–586. [Google Scholar] [CrossRef]
- El-Sokkary, G.H.; Omar, H.M.; Hassanein, A.M.M.; Cuzzocrea, S.; Reiter, R.J. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Rad. Biol. Med. 2002, 32, 319–332. [Google Scholar] [CrossRef]
- El-Lakkany, N.M.; Hammam, O.A.; El-Maadawy, W.H.; Badawy, A.A.; Ain-Shoka, A.A.; Ebeid, F.A. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit. Vectors 2012, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Soliman, M.F.M.; El-Shennawy, W.S. Evaluation of the protective effect of two antioxidative agents in mice experimentally infected with Schistosoma mansoni: Haematological and histopathological aspects. Pak. J. Biol. Sci. 2003, 6, 887–897. [Google Scholar]
- Seif el-Din, S.H.; Al-Hroob, A.M.; Ebeid, F.A. Schistosoma mansoni: N-acetylcysteine downregulates oxidative stress and enhances the antischistosomal activity of artemether in mice. Exp. Parasitol. 2011, 128, 230–235. [Google Scholar] [CrossRef]
- El-Shennawy, A.M.; Mohamed, A.H.; Abass, M. Studies on parasitologic and haematologic activities of an enaminone derivative of 4-hydroxyquinolin-2(1H)-one against murine schistosomiasis mansoni. MedGenMed 2007, 9, 15. [Google Scholar]
- Wan, K.; Wang, P.; Zhang, L. In vivo and in vitro activity of oil extract of garlic (Allium sativum Linnaeus) against Schistosoma japonicum cercariae. Rev. Soc. Bras. Med. Trop. 2017, 50, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Sheir, S.K.; Maghraby, A.M.; Mohamed, A.H.; Osman, G.Y.; Al-Qormuti, S.A. Immunomodulatory and ameliorative role of Nigella sativa oil on Schistosoma mansoni infected mice. Can. J. Pure Appl. Sci. 2015, 9, 3345–3355. [Google Scholar]
- Allam, G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 2009, 214, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.L.; Alburquerque, M.C.P.A.; Silva, R.A.; Schirato, G.V.; de Pontes Filho, N.T.; de Araújo, S.B.; Souza, V.M.; Costa, V.M.; Malagueño, E. Immunohistopathological changes in murine schistosomiasis mansoni under the influence of N-acetyl-L-cysteine. Parasitol. Res. 2012, 111, 1569–1578. [Google Scholar] [CrossRef]
- Seif el-Din, S.H.; Ebeid, F.A.; Badawy, A.A.; Ezzat, A.R. Protective effects of β-carotene; N-acetylcysteine with or without praziquantel treatment in Schistosoma mansoni-infected mice. Egypt J. Schistosomiasis Infect. Endem. Dis. 2006, 28, 67–90. [Google Scholar]
- Gouveia, M.J.; Brindley, P.; Azevedo, C.; Gärtner, F.; Costa, J.M.C.; Vale, N. The antioxidants resveratrol and N-acetylcysteine enhance anthelmintic activity of praziquantel and artesunate against Schistosoma mansoni. Parasit. Vectors 2019, 12, 309. [Google Scholar] [CrossRef] [Green Version]
- Khandelwal, N.; Shaw, J.; Jain, M.K. Biliary parasites: Diagnostic and therapeutic strategies. Curr. Treat. Options Gastrenterol. 2008, 11, 85–95. [Google Scholar] [CrossRef]
- Meister, I.; Assawasuwannakit, P.; Vanobberghen, F.; Penny, M.A.; Odermatt, P.; Savasone, S.; Huwyler, J.; Tarning, J.; Keiser, J. Pooled population pharmacokinetic analysis of tribendimidine for the treatment of Opisthorchis viverrini infections. Antimicrob. Agents Chemother. 2019, 63, e01391-18. [Google Scholar] [CrossRef] [Green Version]
- Mordvinov, V.A.; Furman, D.P. The digenea parasite Opisthorchis felineus: A target for the discovery and development of novel drugs. Infect. Disord. Drugs Targets 2010, 10, 385–401. [Google Scholar] [CrossRef]
- Pungpark, S.; Bunnang, D.; Harinasuta, T. Albendazole in the treatment of opisthorchiasis and concomitant intestinal helminthic infections. Southeast. Asian J. Trop. Med. Public Health 1984, 15, 44–50. [Google Scholar]
- Chistyachenko, Y.S.; Meteleva, E.S.; Pakharukova, M.Y.; Katokhin, A.V.; Khvostov, M.V.; Varlamova, A.I.; Glamazdin, I.I.; Khalikov, S.S.; Polyakov, N.E.; Arhipov, I.A.; et al. A physicochemical and pharmacological study of the newly synthesized complex of albendazole and the polysaccharide arabinogalactan from Larch Wood. Curr. Drug Deliv. 2015, 12, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Shu-Hua, X.; Jian, X.; Zhen-San, C.; Odermatt, P.; Tesana, S.; Tanner, M.; Utzinger, J. Effect of artesunate and artemether against Clonorchis sinensis and Opisthorchis viverrini in rodent models. Int. J. Antimicrob. Agents 2006, 28, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Odermatt, P.; Tesana, S. Dose-response relationships and tegumental surface alterations in following treatment with mefloquine in vivo and in vitro. Parasitol. Res. 2009, 105, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.H.; Wu, H.M.; Tanner, M.; Utzinger, J.; Wang, C. Tribendimidine: A promising, safe and broad-spectrum anthelminthic agent from China. Acta Tropica 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J.; Xiao, S.; Odermatt, P.; Tesana, S. Opisthorchis viverrini: Efficacy and tegumental alterations following administration of tribendimine in vivo and in vitro. Parasitol. Res. 2008, 102, 771–776. [Google Scholar] [CrossRef]
- Sayasone, S.; Odermatt, P.; Vonghachack, Y.; Xayavong, S.; Senggnam, K.; Duthaler, U.; Akkhavong, K.; Hattendorf, J.; Keiser, J. Efficacy and safety of tribendimine against Opisthorchis viverrini: Two randomized, parallel-group, single-blind, dose-ranging, phase 2 trials. Lancet. Infect. Dis. 2016, 16, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Keiser, J.; Adelfio, R.; Vargas, M.; Odermatt, P.; Tesana, S. Activity of tribendimidine and praziquantel combination against the liver fluke Opisthorchis viverrini in vitro and in vivo. J. Helminthol. 2013, 87, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Pakharukova, M.Y.; Ershov, N.I.; Vorontsova, E.V.; Katkhin, A.V.; Merkulova, T.I.; Modvinov, V.A. Cytochrome P450 in fluke Opisthorchis felineus; identification and characterization. Mol. Biochem. Parasitol. 2012, 181, 190–194. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Vavilin, V.A.; Sripa, B.; Laha, T.; Brindley, P.J.; Mordvinov, V.A. Functional analysis of the unique cytochrome P450 of the liver fluke Opisthorchis felineus. PLoS Negl. Trop. Dis. 2015, 9, e0004258. [Google Scholar] [CrossRef]
- Mordvinov, V.A.; Shilov, A.G.; Pakharukova, M.Y. Anthelmintic activity of cytochrome P450 inhibitors miconazole and clotrimazole: In vitro effect on the liver fluke Opisthorchis felineus. Int. J. Antimicrob. Agents 2017, 50, 87–100. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Pakharukov, Y.V.; Mordvinov, V.A. Effects of miconazole/clotrimazole and praziquantel combinations against the liver fluke Opisthorchis felineus in vivo and in vitro. Parasitol Res. 2018, 117, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Laothong, U.; Pinlaor, P.; Hiraku, Y.; Boonsiri, P.; Prakobwong, S.; Khoontawad, J.; Pinlaor, S. Protective effect of melatonin against Opisthorchis viverrini-induced oxidative and nitrosative DNA damage and liver injury in hamsters. J. Pineal Res. 2010, 49, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Laothong, U.; Pinlaor, P.; Boonsiri, P.; Pairojkul, C.; Priprem, A.; Johns, N.P.; Charoensuk, L.; Intuyod, K.; Pinlaor, S. Melatonin inhibits cholangiocarcinoma and reduces liver injury in Opisthorchis viverrini-infected and N-nitrosodimethylamine-treated hamsters. J. Pineal Res. 2013, 55, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wongsena, W.; Charoensuk, L.; Dangtakot, R.; Pinlaor, P.; Intuyod, K.; Pinlaor, S. Melatonin suppresses eosinophils and Th17 cells in hamsters treated with a combination of human liver fluke infection and a chemical carcinogen. Pharmacol. Rep. 2018, 70, 98–105. [Google Scholar] [CrossRef]
- Pinlaor, S.; Yongvanit, P.; Prakobwong, S.; Kaewsamut, B.; Khootaward, J.; Pinlaor, P.; Hiraku, Y. Curcumin reduces oxidative and nitrative DNA damage balancing of oxidant-antioxidant status in hamsters infected with Opisthorchis viverrini. Mol. Nutr. Food Res. 2009, 53, 1316–1328. [Google Scholar] [CrossRef]
- Pinlaor, S.; Prakobwong, S.; Hiraku, Y.; Pinlaor, P.; Laothong, U.; Yongvanit, P. Reduction of periductal fibrosis in liver fluke-infected hamsters after long-term curcumin treatment. Eur. J. Pharmacol. 2010, 638, 134–141. [Google Scholar] [CrossRef]
- Charoensuk, L.; Pinlaor, P.; Prakobwong, S.; Hiraku, Y.; Laothong, U.; Ruangjirachuporn, W.; Yongvanit, P.; Pinlaor, S. Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters. Int. J. Parasitol. 2011, 41, 615–626. [Google Scholar] [CrossRef]
- Charoensuk, L.; Pinlaor, P.; Wanichwecharungruang, S.; Intuyod, K.; Vaeteewoottacharn, K.; Chaidee, A.; Yongvanit, Y.; Pairojkul, C.; Suwannateep, N.; Pinlaor, S. Nanoencapsulated curcumin and praziquantel treatment reduces periductal fibrosis and attenuates bile canalicular abnormalities in Opisthorchis viverrini-infected hamsters. Nanomedicine 2016, 12, 21–32. [Google Scholar] [CrossRef]
- Wonkchalee, O.; Boonmars, T.; Aromdee, C.; Laummaunwai, P.; Khunkiti, W.; Vaeteewoottacharn, K.; Sriraj, P.; Aukkanimart, R.; Loilome, W.; Chamgramol, Y.; et al. Anti-inflammatory, antioxidant and hepatoprotective effects of Thunbergia laurifolia Linn. on experimental opisthorchiasis. Parasitol. Res. 2012, 111, 353–359. [Google Scholar] [CrossRef]
- Wonchalee, N.; Boonmars, T.; Laummaunwai, P.; Aromdee, C.; Hahnvajanawong, C.; Wu, Z.; Sriraj, P.; Aukkanimart, R.; Chamgramol, Y.; Pairojkul, C.; et al. A combination of praziquantel and the traditional medicinal plant Thunbergia laurifolia on Opisthorchis viverrini infection and cholangiocarcinoma in a hamster model. Parasitol. Res. 2013, 112, 4211–4219. [Google Scholar] [CrossRef]
- Jamnongkan, W.; Thanee, M.; Yongvanit, P.; Loilome, W.; Thanan, R.; Kimawaha, P.; Boonmars, T.; Silakit, R.; Namwat, N.; Techasen, A. Antifibrotic effect of xanthohumol in combination with praziquantel is associated with altered redox status and reduced iron accumulation during liver fluke-associated cholangiocarcinogenesis. Peer J. 2018, 6, e4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Drugs/AntiOx | Model | Treatment | Main Findings | Ref. |
|---|---|---|---|---|
| Oxamniquine (OXA) | S. mansoni-infected mice | OXA plus PZQ | The combinations of the two drugs were markedly superior than those alone. | [95] |
| 1/3 the curative dose of PZQ plus 1/3 the curative dose of OXA | A potentiating effect was observed in animals receiving combination therapy; Reduction of worm burden and tissue egg load. | [96] | ||
| schistosomiasis mansoni (different parasitic strains: two Venezuelan (YT and SM) and one Brazilian (BH) strain in vivo | Single oral doses of PZQ (250 or 500 mg/kg), oxamniquine (OXA; 40, 60 or 100 mg/kg) or to low-dose combinations of both drugs (33 mg/kg PZQ and 25 mg/kg OXA; 66 mg/kg Pz and 12.5 mg/kg OXA; 250 mg/kg PZQ and 40 mg/kg OXA), | At lower doses of either drug, adult worms of the SM isolate were less susceptible than those of the BH and YT isolates; Lower doses, PZQ more effective in reducing liver or intestinal egg counts than OXA; Males more susceptible to OXA than females. | [97] | |
| schistosomiasis mansoni and hematobia clinical trial | OXA (4–10 mg/kg) plus PZQ (10-20 mg/kg), | High efficacy of combined regimen in low single doses of 7.5 and 15.0 mg/kg of OXA and PZQ, respectively. | [98] | |
| Artemisinin’s | schistosomiasis mansoni and hematobia In vitro, in vivo and clinical | Alone or combine with PZQ. (review in [65,100,101]) | Higher worm burden reductions following treatment with combined regimen compared to PZQ or Artemether alone in vivo; Artemisinin’s highly active against juvenile stage of parasites; Antimalarials used in combination with PZQ exhibited the increased cure rates for schistosomiasis. | [100] [101] |
| opisthorchiasis viverrini in vivo | ART and AS were administered at a dose of 400 mg/kg and 600 mg/kg | Worm burden infections of 78% and 66%; complete elimination of the parasite was not achieved at higher dose; Showed toxicity above 400 mg/kg. | [147] | |
| ibuprofen and naproxen | S. mansoni- infected mice | alone (200 mg/kg for two weeks) or combine same dosage + PZQ (2 × 500 mg/kg) | Alone did not significantly reduce the worm distribution, egg load or change the program pattern; However, was reduced the granuloma size; Combination ibuprofen and naproxen with PZQ caused a slight increase of percentage of dead ova; marked reduction in the mean granuloma diameter and circulating antigen which was more pronounced than with anti-inflammatory alone. | [102] |
| β-aminopropionitrile- -monofumara-te salt β-aminopropi-onitrile | S. mansoni- infected mice | Alone (5 mg powder of salts in 0.5 mL saline) or combined with PZQ (500 mg/kg b. w.) | Reduced sizes of granulomas and alleviated the host resistance to challenge infection; Decreased liver and spleen weights and a significant reduction in the number of eggs trapped in both liver (86%) and the intestine (99.1%) in comparison to PZQ alone. | [103] [104] |
| atorvastatin (AV) and medroxy- -proges- -terone acetate (MPA) | S. haematobium- infected hamsters | MPA was administered intramuscularly (0.1 mg/kg) at days 7 and 35 p.i. followed by AV treatment regimen (0.9 mg/kg for 49 consecutive days) | Drugs induced tegumental damage and reduced the total number of worms recovered from infected hamsters; Female worms were less susceptible to either drugs alone or combined in comparison to males; Combined regimen decreased the number of eggs in tissue. | [105] |
| Edelfosine (EDLF) | S. mansoni in vitro and in vivo | In vitro: 10 and 20 μM EDLF; In vivo: PZQ (100 mg/kg/day) plus EDLF (45 mg/kg/day) daily 3 days prior to infection until eight days p.i. | In vitro: activity against schistosomula induced interruption of oviposition; In vivo: combination with PZQ resulted not only in the elimination of developmental stages and reduced granuloma size and hepatomegaly; favor resistance to re-infection. | [106] |
| Albendazole (ABZ) | Opisthorchiasis viverini In vivo | Alone (400 mg twice daily for 3 days) | Moderate cure rates but with egg reduction rates of >92% | [144] |
| Arabino- .galactan-ABZ complex | Opisthorchiasis felinea in vitro | Anthelmintic activity at 10-fold lower doses than parent drug alone; Lower acute toxicity and hepatotoxicity. | [145] | |
| Mefloquine | Opisthorchiasis viverini In vitro and vivo | Alone (200–400 mg/kg) | High worm burdens not only against juvenile but also against adult worms; Severe tegumental alterations. | [147] |
| Tribendimidine (TBD) | Opisthorchiasis viverini In vitro, in vivo and clinical trials | In vitro: 0.001, 0.01, 0.1 and 1mg/mL TBD or PZQ. In vivo: Alone (single 400 mg/kg dose) or combined with PZQ (100 and 200 mg/kg) | In vitro: lower drug concentrations lead to its rapid contraction and consequently to death In vivo: high worm burden reduction Combined with PZQ: low to moderate worm burden reductions suggesting antagonistic effects. Clinical trials: excellent efficacy and tolerability at doses of 100 mg/kg and above. | [149] [150] [151] |
| Miconazole (MCZ) and Clotrimazole (CTZ) | Opisthorchiasis felinea In vitro and in vivo | In vitro: 0.001, 0.01, 0.1, 1, 10, 100 and 500 μM. In vivo: MCZ and CTZ (100 or 200 mg/kg) combined with PZQ (131 or 400 mg/kg b.w.) | In vitro: reduce not only CYP activity and decrease parasites viability; Combined with PZQ: PZQ–CTZ and PZQ–MCZ acts synergistically in vitro but antagonist in vivo. | [154] [155] |
| M. armillaris | S. mansoni- infected mice | M. armillaris 150 mg/kg orally from 2nd week p.i. twice a week for 6 weeks plus PZQ at 600 mg/kg, orally for 2 consecutive days after 8 weeks p.i.. | Combined regimen ameliorated antioxidant enzymes activity and lipid peroxides; Oil enhanced antioxidant system defense ameliorated pathologies associated with infection. | [107] |
| Resveratrol | S. mansoni- infected mice | 20 mg/kg once daily for 2 weeks | Ameliorated antioxidant system and lipid metabolism. Significant improvement of specific biomarkers of lung and brain homeostasis. | [109] |
| S. mansoni in vitro | Alone (100 μM) or combined with PZQ at constant ratio 1:1. | Alone presented moderate activity against schistosomula but combined with PZQ enhanced anthelmintic activity of drug. | [140] | |
| Sylimarin | S. mansoni- infected mice | 10, 20 or 25 doses of 10 mg/kg Syl at 55 days p.i. | Did not present antischistosomal activity; Diminished the granuloma and fibrosis. | [110] |
| S. mansoni- infected mice | Alone (750 mg/kg/day) or combine with PZQ (1000 mg/kg) | Alone: Moderate worm burden reduction and ameliorated egg load in liver; Modulation of granuloma size and conservation of hepatic GSH. Combined regime: Improvement of liver function and histopathology. Did not interfere or affect the antischistosomal activity of PZQ. Almost eradicated the presence of adult worms. | [131] | |
| Limonin | S. mansoni In vitro and in vivo | Alone in a single dose of 50 or 100 mg/kg on day 21 p.i.; Same dose given on 56 p.i. | In vitro: Antischistosomal activity more pronounced against immature worms than adult; induced tegument alterations; In vivo: Reduction of worm burden more effective at day 21 p.i. than on day 56 p.i. Significant reduction in the hepatic and intestinal tissue egg load; Ameliorated hepatic pathologies. | [117] |
| α-Lipoic acid (ALA) | S. mansoni- infected mice | ALA (single dose 30 mg/kg) combined with PZQ (500 mg/kg) divided into 2 doses 9 weeks p.i. | Combined regimen results in reduction in the worm burden more pronounced in combined regimen, egg count and granuloma size. Recovered the level serum of hepatic enzymes and increased the tissue level of biomarkers of antioxidant function and stress oxidative. | [129] |
| B. trimera | S. mansoni- infected mice | 24, 48, 91 and 130 µg/mL | Highest concentration presented better antischistosomal activity, reducing motility; Ceased oviposition at sub-lethal concentrations and induced decoupling. | [120] |
| 4-Hydroxyquinolin- -2(1H)-one (BDHQ) | S. mansoni- infected mice | Alone at lower or higher dose or for consecutive days; | Active against larval and mature worms; Affected genital systems either males and females. | [126] |
| Alone (600 mg/kg) or combine with PZQ (BDHQ 300 mg/kg + PZQ 250 mg/kg) | BDHQ alone or combined resulted in highly significant reduction in total worm burden; reduction of granuloma size more pronounced with combined regimen. | [134] | ||
| A. sativum | S. japonicum In vitro and in vivo | In vitro: 10−2 to 10−6 (v/v) concentration. In vivo: Mice pre-treated with garlic and then infected. | Antischistosomal activity against S. japonicum against cercariae; Pre-treated with highest concentration lead to total inhibition of infection. | [135] |
| S. mansoni- infected mice | 100 mg/kg body weight from 1 to 7 days p.i., 14 to 21 or 1 to 42 days p.i. | Affected parasite tegument; induced significant worm burden reduction, hepatic and intestinal ova count. Decreased granuloma number and size; Improved immunological parameters. | [111] | |
| A. sativum + A. cepa | S. mansoni- infected mice | A. sativum or A. Cepa: 2 g/100 g body weight daily for 45 consecutive days. PZQ: 500 mg/kg bw on 2 successive days 45 days p.i. | Almost completely eradicated worms, egg load tissue and presence of granulomas. Ameliorated liver architecture and its functions. | [112] |
| In vitro: 0.5–5 ppm In vivo: Same regimen as in vitro. | Highly active against all developmental stages of parasites; Induced decoupling; Enhanced host antioxidant system. | [113] | ||
| N. sativa | S. mansoni- infected mice | Alone (2.5 and 5 mL/kg orally) or in combination with PZQ (500 mg/kg for 2 consecutive days) | Alone: Decreased the number and ova of parasites in liver and also reduced number of granulomas. Combined with PZQ: Improved most parameters with most prominent effect was further lowered in dead ova number over that produced by PZQ. | [114] |
| S. mansoni- infected mice | Alone (0.2 mg/kg alone) or combined with garlic oil (125 mg/kg p.i.) for successive 28 days, starting 1st day p.i. | Compounds alone resulted reduced number of mature eggs while combined regimen resulted in increase of percentage of dead eggs. Combined regimen had more significant effect on serum enzymes (AST and ALP). | [115] | |
| Alone (0.2 mg/kg of body weight) for 4 weeks starting from 1st day p.i. or combine with Arthemether (single dose 300 mg/kg b.w. follow 49 days p.i) or PZQ (500 mg/kg) for consecutive days. | N. sativa either alone or combined with Arthemether or PZQ resulted in improvement of host immunological response stimulating cytokines. Additionally, ameliorated healing process of granulomas lesion. | [136] | ||
| N-acetyl- -cysteine | S. mansoni- infected mice | Alone (200 mg/kg/day on 1st day after infection for acute phase; On 45th for the intermediate; 59 and 75th for chronic stages) or combined with PZQ (100 mg/kg) from 45th to 49th day p.i.). | Antioxidant alone did not present antischistosomal activity; Combined with PZQ: reduced granulomas size and alone NAC was capable to improve liver fibrosis reducing liver damage. | [132] |
| Alone (300 mg/kg 5 days a week for 4 weeks) or combine with PZQ (300 mg/kg 7 weeks p.i.) | Combined regimen improved levels of serum enzymes and decreased the total number of worms and consequently decreased liver egg load. | [133] | ||
| S. mansoni in vitro | Alone (100 μM) or combined with PZQ at constant ratio 1:1. | NAC did not present significant activity against schistosomula of S. mansoni in vitro. When combined with PZQ, slightly improved its antischistosomal activity, was observed. | [140] | |
| Curcumin | S. mansoni in vitro | 1.56 to 100 μM | Induced decoupling and affected viability of parasite; Affected parasite´s mitochondria and altered oxidative stress parameters increasing oxidative stress that leads to parasite death. | [124] |
| S. mansoni- infected mice | 300 mg/kg bw after one-month p.i., twice a week for 2 months | Affected the fecundity of adult worms, reducing the number of eggs. | [116] | |
| Total dose 400 mg/kg bw divided into 16 injections | Reduced presence of parasites and eggs on liver; Improved the infection-associated pathologies as granuloma, hepatic enzymes; increased inflammatory response. | [137] | ||
| O. viverrini- infected hamster | Alone administered on normal diet to make the final concentration of 1%(w/w) | Reduced oxidative and nitrative DNA damage; enhanced the expression of antioxidant genes; Decreased inflammatory cell infiltration and periductal fibrosis; | [159] [160] | |
| CCM (37, 75 and 150 mg/kg body weight) combine with PZQ | In combined regimen, curcumin decreased oxidative and nitrative stress derived from PZQ treatment and reduced liver injury. | [161] | ||
| CCM (0.40g) and PZQ (300 mg/kg body weight for two constitutive days) nanocapsulated | More efficient than combined regimen without nanocapsulation in reducing periductal fibrosis; Also prevented alteration of genes in bile acid metabolism. | [162] | ||
| Melatonin | S. mansoni- infected mice | Alone (3.35 mg/kg daily) or combined with cercarial antigen preparation or soluble worm antigen preparation (30 μg/mL) | Mel alone did not decrease worm burden while when combined, almost eliminated parasites completely; Ameliorated oxidative stress. | [125] |
| S. mansoni- infected mice | Alone (10 mg/kg, 2 weeks) following infection | Reduction of granuloma formation and highly protective against pathological changes not only in liver but kidney; Stimulated antioxidative enzymes and mitochondrial oxidative phosphorylation rendered in amelioration of pathologies associated with infection | [130] | |
| O. viverrini- infected hamster | Alone in several doses (5 up to 20 mg/kg body weight) for 30 days | Reduced the formation of oxidative and nitrosative DNA lesions; increased in the expression of antioxidant genes; | [156] | |
| Melatonin | O. viverrini- infected hamster | Alone (50 mg/kg) | Significant reduction in liver/body weight ratios, decreased tumor volumes and maintained tumor dormancy which translated in improvement of animal survival. Exerted an immunomodulatory effect and might act as chemopreventive. | [157] [158] |
| aqueous extract of Thunbergia laurifolia | O. viverrini -infected hamsters | Alone (100 mg/kg/dose) | Did not present any effect against worms, however, lead to reduction of the aggregation of inflammatory cells. | [163] |
| Extract (100 mg/kg/dose) combine with PZQ (400 mg/kg) | Reduced inflammatory cell aggregation and inhibited development of cholangiocarcinoma. | [164] | ||
| Xanthumol | Alone (20 μM or 171 mg/B.W./day) or combined with PZQ (single dose of 400 mg/kg) | Either alone or in combination, xanthumol, presented an effect on DNA damage, ameliorated periductal fibrosis. These effects were more pronounced in combined regimen, leading to suppression of development of cholangiocarcinma. This suppression might be related to antioxidant activity of xanthohumol protecting the cholangiocytes. | [165] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, N.; Gouveia, M.J.; Gärtner, F. Current and Novel Therapies Against Helminthic Infections: The Potential of Antioxidants Combined with Drugs. Biomolecules 2020, 10, 350. https://doi.org/10.3390/biom10030350
Vale N, Gouveia MJ, Gärtner F. Current and Novel Therapies Against Helminthic Infections: The Potential of Antioxidants Combined with Drugs. Biomolecules. 2020; 10(3):350. https://doi.org/10.3390/biom10030350
Chicago/Turabian StyleVale, Nuno, Maria João Gouveia, and Fátima Gärtner. 2020. "Current and Novel Therapies Against Helminthic Infections: The Potential of Antioxidants Combined with Drugs" Biomolecules 10, no. 3: 350. https://doi.org/10.3390/biom10030350






