Site-Specific Fluorogenic Protein Labelling Agent for Bioconjugation
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Test Protein
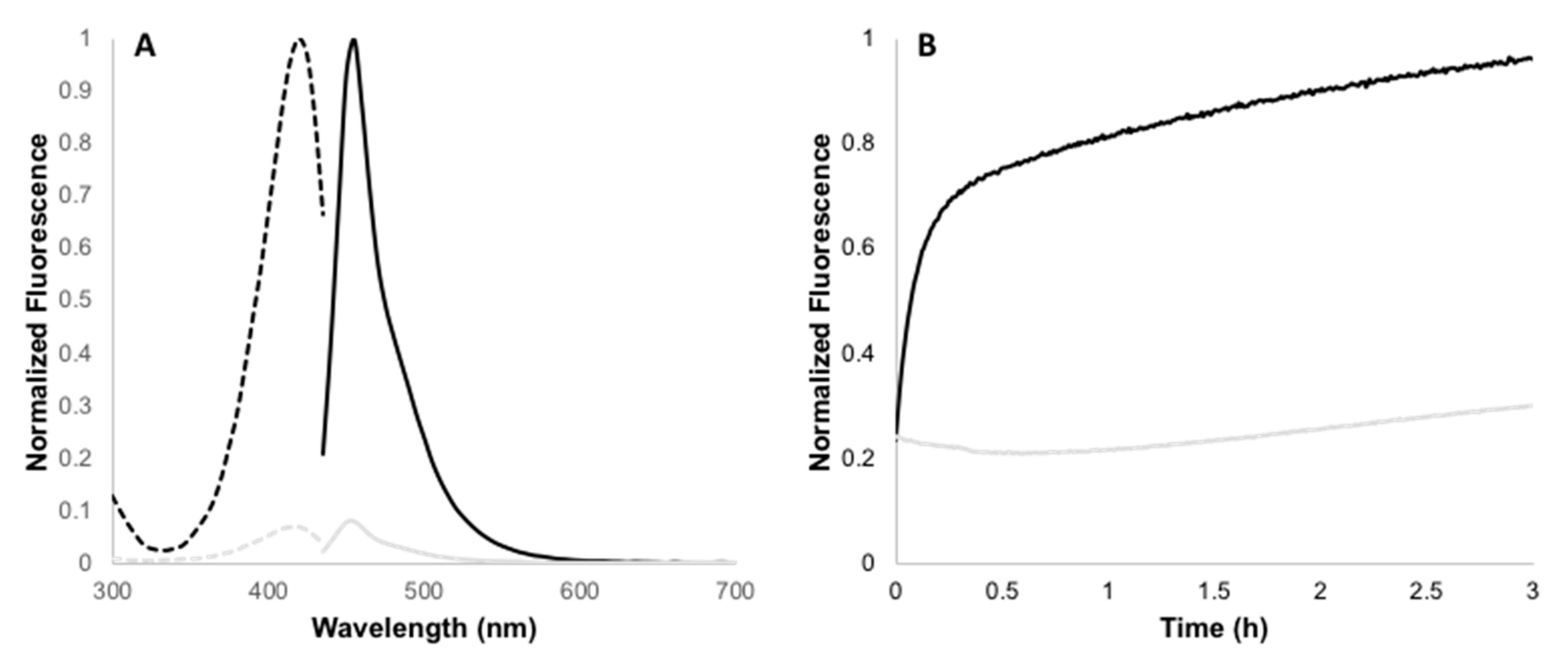
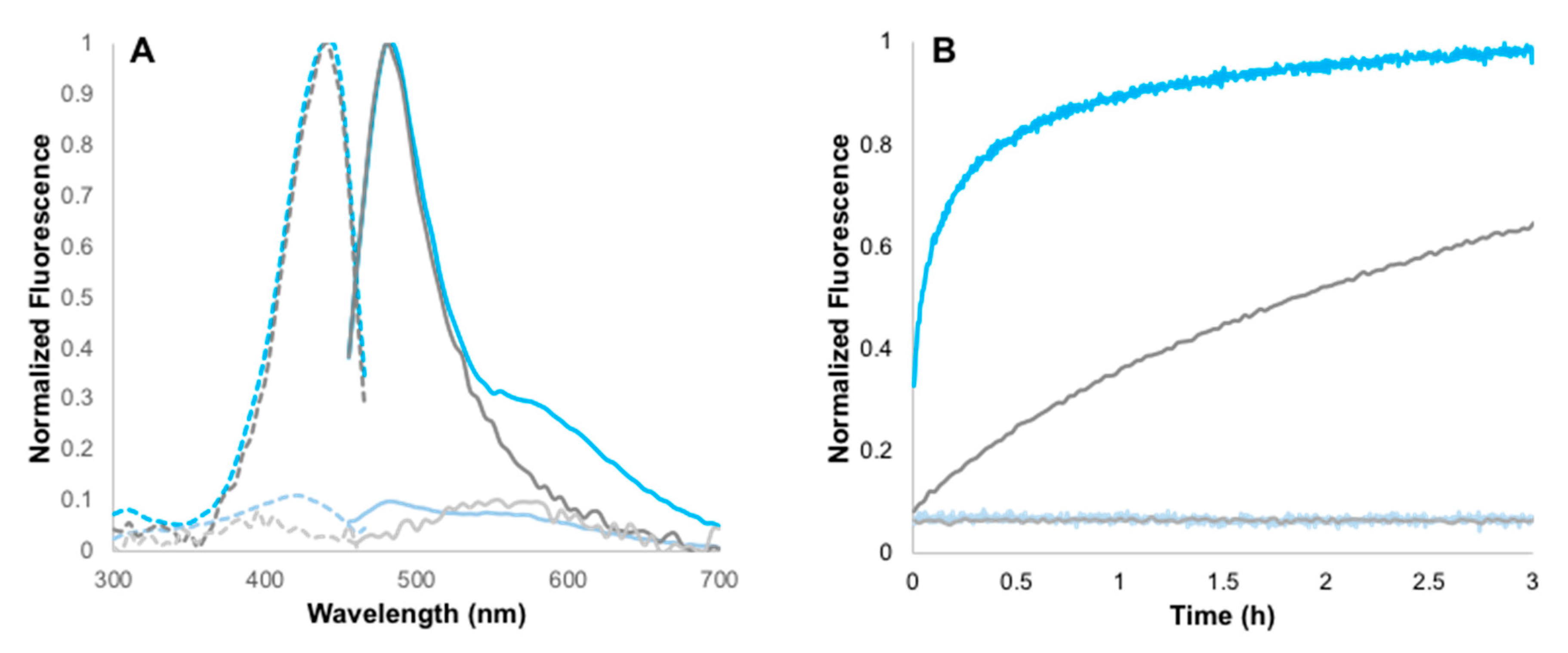
2.2. Determination of Fluorescence Enhancement Ratios
2.3. Kinetic Studies
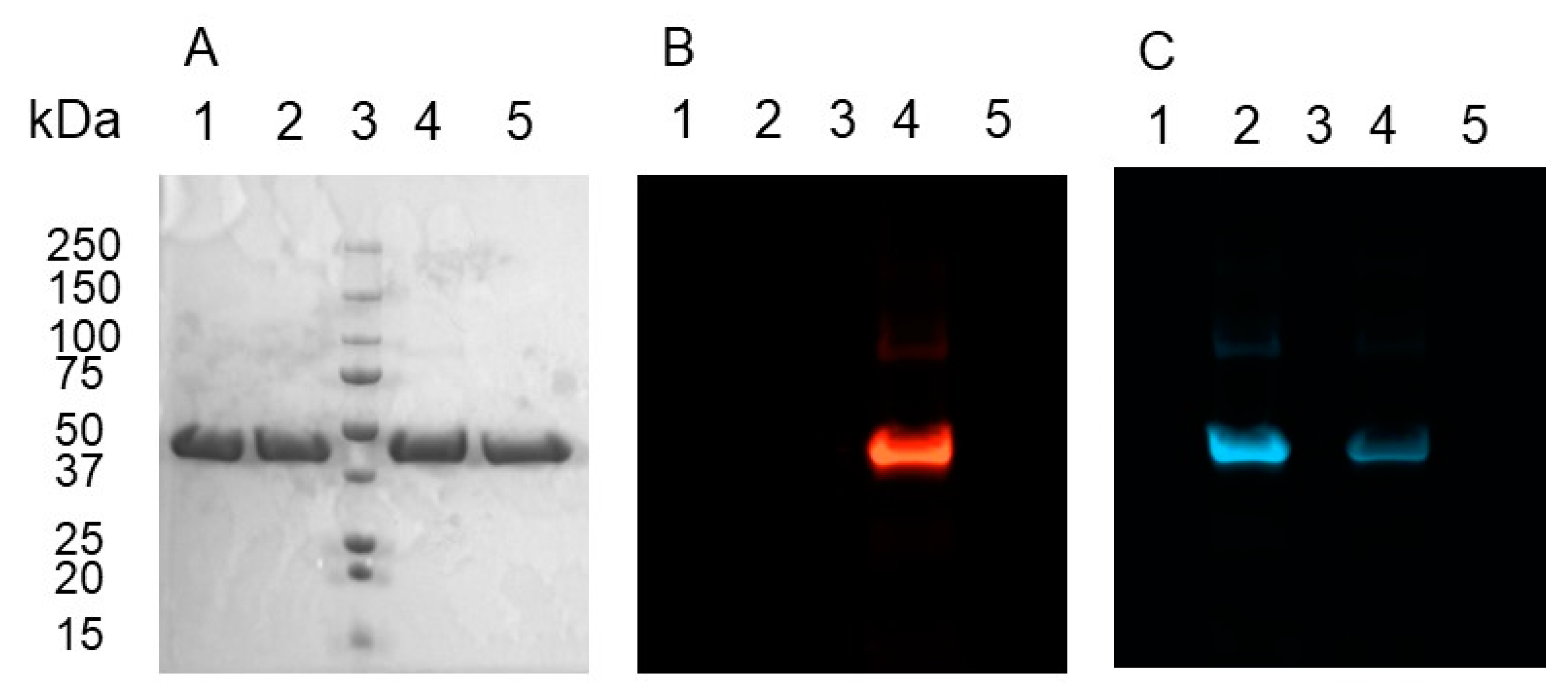
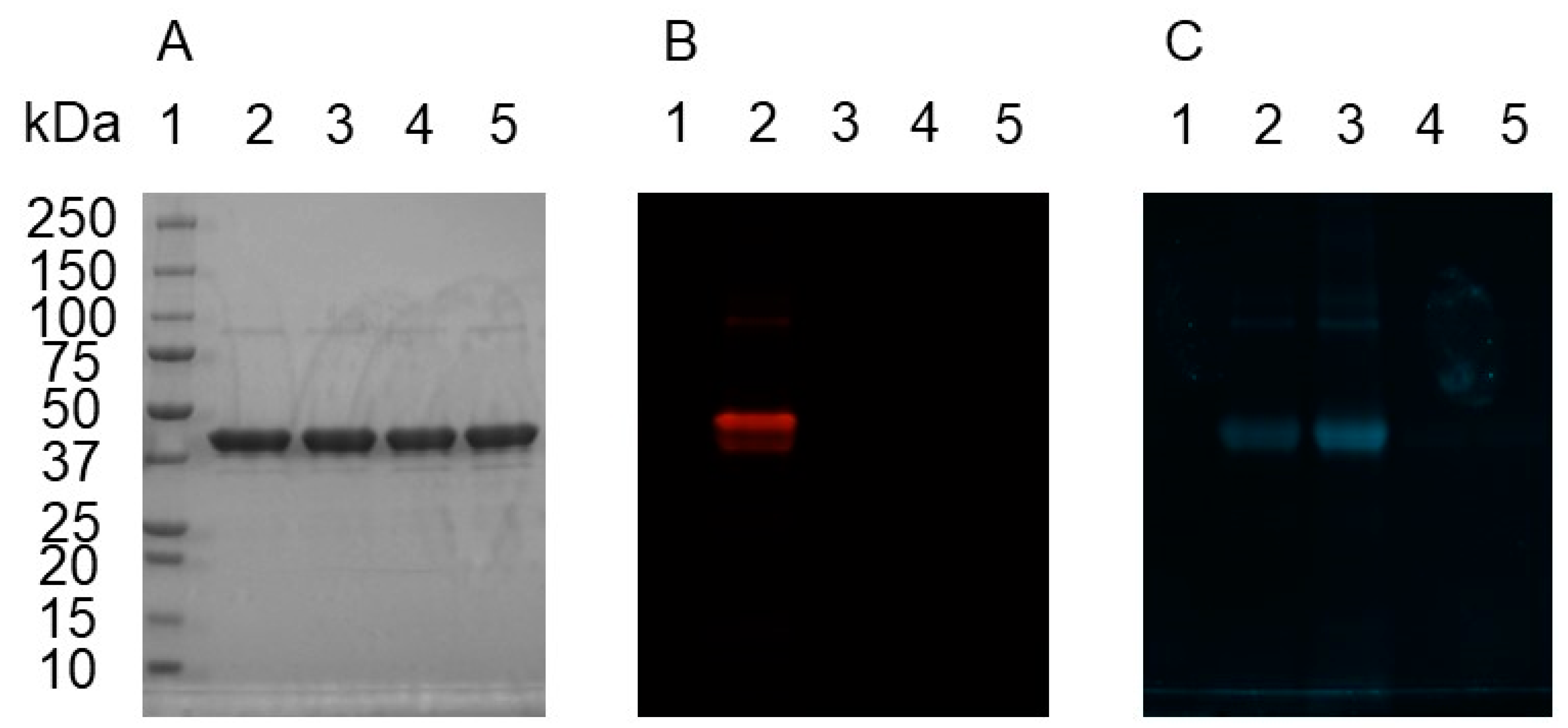
2.4. Bioconjugation of Rhodamine B Azide to MBP-dC10*-15 or 6 Adduct
2.5. Bioconjugation of Rh-B-N3-15 Adduct to MBP-dC10*
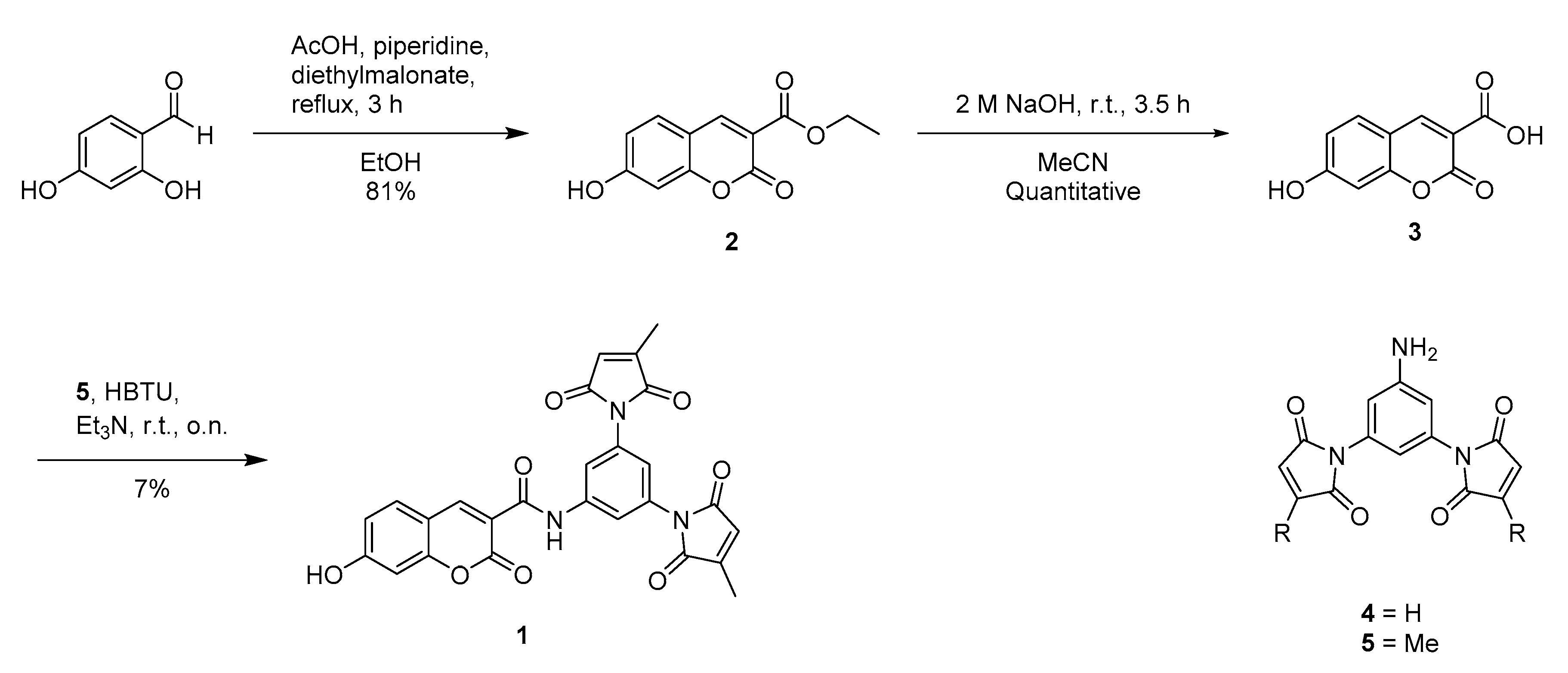
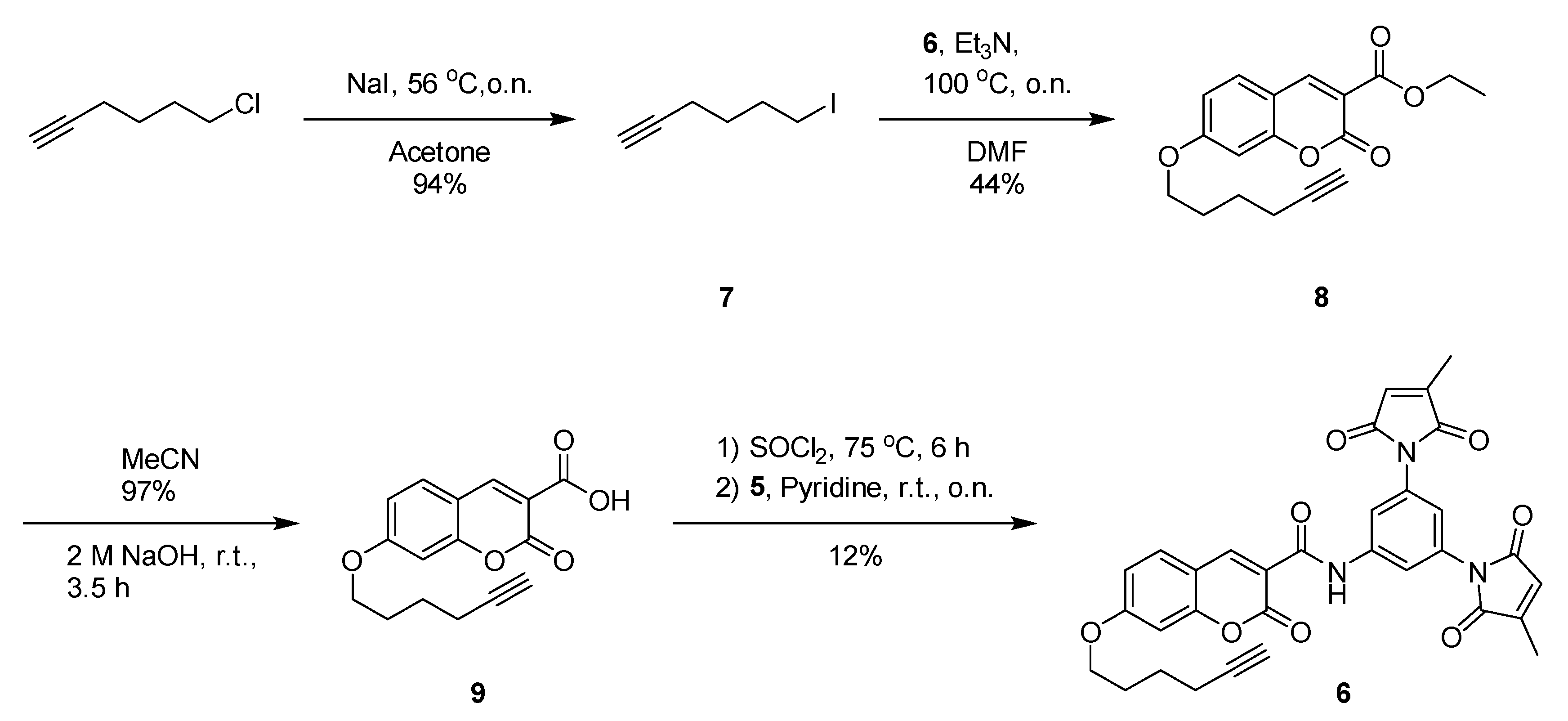
2.6. Synthesis
3. Results and Discussion
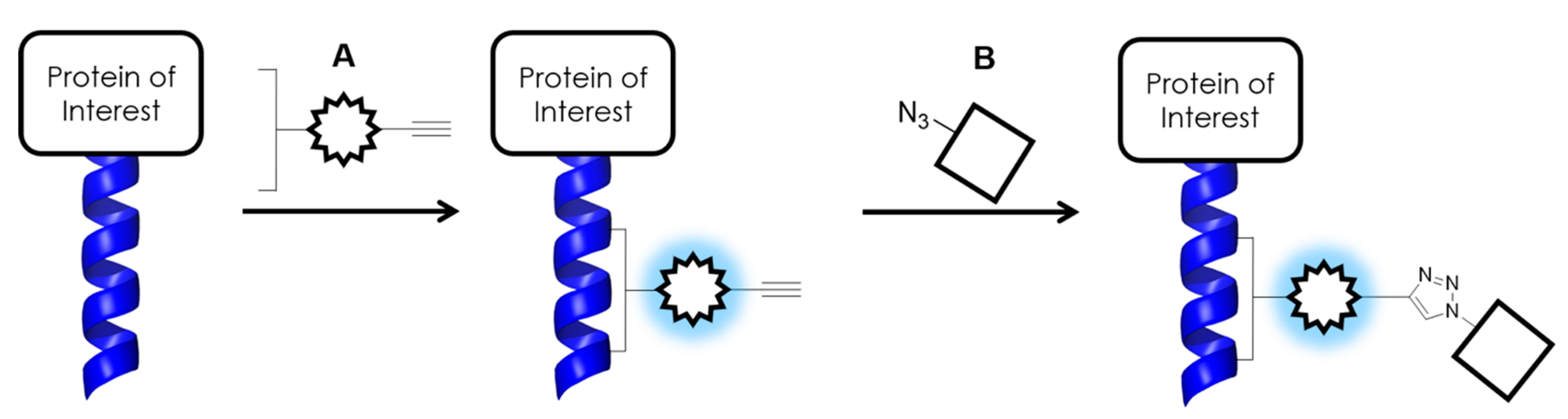
3.1. Initial Design
3.2. Improved Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pasut, G.; Veronese, F.M. PEG conjugates in clinical development or use as anticancer agents: An overview. Adv. Drug Deliv. Rev. 2009, 61, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Sievers, E.L.; Senter, P.D. Antibody-Drug Conjugates in Cancer Therapy. Annu. Rev. Med. 2013, 64, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Canalle, L.A.; Löwik, D.W.P.M.; Van Hest, J.C.M. Polypeptide-polymer bioconjugates. Chem. Soc. Rev. 2010, 39, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Deonarain, M.P.; Yahioglu, G.; Stamati, I.; Marklew, J. Emerging formats for next-generation antibody drug conjugates. Expert Opin. Drug Discov. 2015, 10, 463–481. [Google Scholar] [CrossRef]
- Chudasama, V.; Maruani, A.; Caddick, S. Recent advances in the construction of antibody-drug conjugates. Nat. Chem. 2016, 8, 114–119. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Yamada, K.; Ito, Y. Recent Chemical Approaches for Site-Specific Conjugation of Native Antibodies: Technologies toward Next-Generation Antibody–Drug Conjugates. ChemBioChem 2019, 20, 2729–2737. [Google Scholar] [CrossRef]
- Kim, J.S.; Sirois, A.R.; Vazquez Cegla, A.J.; Jumai’An, E.; Murata, N.; Buck, M.E.; Moore, S.J. Protein-Polymer Conjugates Synthesized Using Water-Soluble Azlactone-Functionalized Polymers Enable Receptor-Specific Cellular Uptake toward Targeted Drug Delivery. Bioconjug. Chem. 2019, 30, 1220–1231. [Google Scholar] [CrossRef]
- Klok, H.A. Peptide/protein-synthetic polymer conjugates: Quo vadis. Macromolecules 2009, 42, 7990–8000. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Pelegri-Oday, E.M.; Lin, E.W.; Maynard, H.D. Therapeutic protein-polymer conjugates: Advancing beyond pegylation. J. Am. Chem. Soc. 2014, 136, 14323–14332. [Google Scholar] [CrossRef] [PubMed]
- Ducry, L.; Stump, B. Antibody-drug conjugates: Linking cytotoxic payloads to monoclonal antibodies. Bioconjug. Chem. 2010, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Bertozzi, C.R. Site-specific antibody-drug conjugates: The nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug. Chem. 2015, 26, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, J.; Liu, S. Emerging Applications of Fluorogenic and Non-fluorogenic Bifunctional Linkers. Chemistry. 2018, 24, 16484–16505. [Google Scholar] [CrossRef]
- Liu, G.; Shi, G.; Sheng, H.; Jiang, Y.; Liang, H.; Liu, S. Doubly Caged Linker for AND-Type Fluorogenic Construction of Protein/Antibody Bioconjugates and In Situ Quantification. Angew. Chem. Int. Ed. Engl. 2017, 56, 8686–8691. [Google Scholar] [CrossRef]
- Robin, M.P.; Wilson, P.; Mabire, A.B.; Kiviaho, J.K.; Raymond, J.E.; Haddleton, D.M.; O’Reilly, R.K. Conjugation-induced fluorescent labeling of proteins and polymers using dithiomaleimides. J. Am. Chem. Soc. 2013, 135, 2875–2878. [Google Scholar] [CrossRef]
- Dirks, A.T.J.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Monitoring Protein- Polymer Conjugation by a Fluorogenic Cu (I)-Catalyzed Azide- Alkyne 1, 3-Dipolar Cycloaddition. Bioconjug. Chem. 2009, 20, 1129–1138. [Google Scholar] [CrossRef]
- Chen, Y.; Tsao, K.; Acton, S.L.; Keillor, J.W. A Green BODIPY-Based, Super-Fluorogenic, Protein-Specific Labelling Agent. Angew. Chemie Int. Ed. 2018, 57, 12390–12394. [Google Scholar] [CrossRef]
- Chen, Y.; Clouthier, C.M.; Tsao, K.; Strmiskova, M.; Lachance, H.; Keillor, J.W. Coumarin-based fluorogenic probes for no-wash protein labeling. Angew. Chem. Int. Ed. Engl. 2014, 53, 13785–13788. [Google Scholar] [CrossRef]
- Chen, Y.; Tsao, K.; Keillor, J.W. Fluorogenic protein labelling: a review of photophysical quench mechanisms and principles of fluorogen design. Can. J. Chem. 2014, 398, 1–10. [Google Scholar] [CrossRef]
- Guy, J.; Caron, K.; Dufresne, S.; Michnick, S.W.; Skene, W.G.; Keillor, J.W. Convergent preparation and photophysical characterization of dimaleimide dansyl fluorogens: elucidation of the maleimide fluorescence quenching mechanism. J. Am. Chem. Soc. 2007, 129, 11969–11977. [Google Scholar] [CrossRef] [PubMed]
- Strmiskova, M.; Tsao, K.; Keillor, J.W. Rational design of a highly reactive dicysteine peptide tag for fluorogenic protein labelling. Org. Biomol. Chem. 2018, 16, 6332–6340. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Castonguay, R.; Campos-Reales Pineda, N.B.; Jacquier, V.; Caron, K.; Michnick, S.W.; Keillor, J.W. De novo helical peptides as target sequences for a specific, fluorogenic protein labelling strategy. Mol. Biosyst. 2010, 6, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Nasheri, N.; Joyce, M.; Rouleau, Y.; Yang, P.; Yao, S.; Tyrrell, D.L.; Pezacki, J.P. Modulation of fatty acid synthase enzyme activity and expression during hepatitis C virus replication. Chem. Biol. 2013, 20, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Liu, K.; Ngai, M.H.; Lear, M.J.; Wenk, M.R.; Yao, S.Q. Activity-based proteome profiling of potential cellular targets of orlistat - An FDA-approved drug with anti-tumor activities. J. Am. Chem. Soc. 2010, 132, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Abdizadeh, T.; Kalani, M.R.; Abnous, K.; Tayarani-Najaran, Z.; Khashyarmanesh, B.Z.; Abdizadeh, R.; Ghodsi, R.; Hadizadeh, F. Design, synthesis and biological evaluation of novel coumarin-based benzamides as potent histone deacetylase inhibitors and anticancer agents. Eur. J. Med. Chem. 2017, 132, 42–62. [Google Scholar] [CrossRef]
- Jiang, X.R.; Wang, P.; Smith, C.L.; Zhu, B.T. Synthesis of novel estrogen receptor antagonists using metal-catalyzed coupling reactions and characterization of their biological activity. J. Med. Chem. 2013, 56, 2779–2790. [Google Scholar] [CrossRef]
- Takakura, H.; Sasakura, K.; Ueno, T.; Urano, Y.; Terai, T.; Hanaoka, K.; Tsuboi, T.; Nagano, T. Development of luciferin analogues bearing an amino group and their application as BRET donors. Chem. Asian J. 2010, 5, 2053–2061. [Google Scholar] [CrossRef]
- Chen, Y.; Tsao, K.; De Francesco, É.; Keillor, J.W. Ring Substituent Effects on the Thiol Addition and Hydrolysis Reactions of N-Arylmaleimides. J. Org. Chem. 2015, 80, 12182–12192. [Google Scholar] [CrossRef]
- Jewett, J.C.; Bertozzi, C.R. Synthesis of a fluorogenic cyclooctyne activated by Cu-free click chemistry. Org. Lett. 2011, 13, 5937–5939. [Google Scholar] [CrossRef]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Kwan, D.H.; Ernst, S.; Kötzler, M.P.; Withers, S.G. Chemoenzymatic Synthesis of a Type 2 Blood Group A Tetrasaccharide and Development of High-throughput Assays Enables a Platform for Screening Blood Group Antigen-cleaving Enzymes. Glycobiology 2015, 25, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Petrella, A.; Chacón-Huete, F.; Covone, J.; Tsai, T.W.; Yu, C.C.; Forgione, P.; Kwan, D.H. A High-Throughput Glycosyltransferase Inhibition Assay for Identifying Molecules Targeting Fucosylation in Cancer Cell-Surface Modification. ACS Chem. Biol. 2019, 14, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Zhang, T.; Shen, S.-L.; Miao, J.-Y.; Zhao, B.-X. A ratiometric lysosomal pH probe based on the coumarin–rhodamine FRET system. RSC Adv. 2015, 5, 49115–49121. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, K.K.; Lee, A.C.; Racine, K.É.; Keillor, J.W. Site-Specific Fluorogenic Protein Labelling Agent for Bioconjugation. Biomolecules 2020, 10, 369. https://doi.org/10.3390/biom10030369
Tsao KK, Lee AC, Racine KÉ, Keillor JW. Site-Specific Fluorogenic Protein Labelling Agent for Bioconjugation. Biomolecules. 2020; 10(3):369. https://doi.org/10.3390/biom10030369
Chicago/Turabian StyleTsao, Kelvin K., Ann C. Lee, Karl É. Racine, and Jeffrey W. Keillor. 2020. "Site-Specific Fluorogenic Protein Labelling Agent for Bioconjugation" Biomolecules 10, no. 3: 369. https://doi.org/10.3390/biom10030369
APA StyleTsao, K. K., Lee, A. C., Racine, K. É., & Keillor, J. W. (2020). Site-Specific Fluorogenic Protein Labelling Agent for Bioconjugation. Biomolecules, 10(3), 369. https://doi.org/10.3390/biom10030369





