A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Sample Dissection
2.2. TRICOL Decellularization Protocol
2.3. DNA Content
2.4. Histological Evaluation
2.5. Immunofluorescence Staining
2.6. Scanning Electron Microscopy (SEM)
2.7. Multiphoton Microscopy
2.8. Attenuated Total Reflectance FTIR analysis (ATR-FTIR)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Biochemical Analyses for Hydroxyproline (HYP), Denatured HYP, Sulfated Glycosaminoglycans (sGAGs), Elastin and Water Quantification
2.11. Uniaxial Tensile Testing
2.12. In Vitro Cytotoxicity Assays
2.13. Statistical Analysis
3. Results
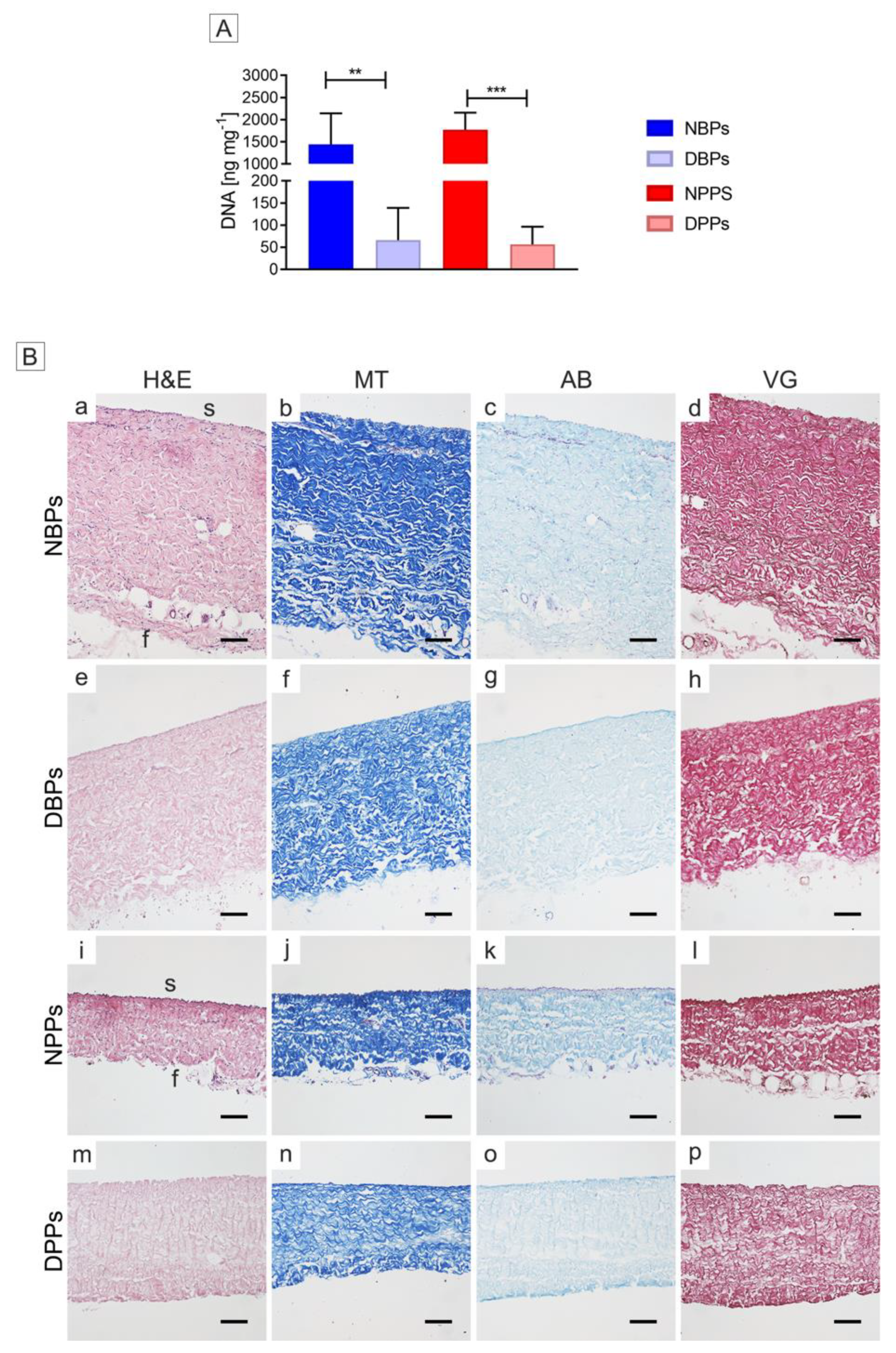
3.1. Cell Removal from Bovine and Porcine Pericardia after TRICOL Decellularization
3.2. Gross Structure of Decellularized Bovine and Porcine Pericardia
3.3. ECM Architecture (Basal Lamina Included) of Bovine and Porcine Pericardia after TRICOL Decellularization
3.4. Differences of Bovine and Porcine Pericardia after TRICOL Decellularization
3.5. Overall Protein Sequence and Denaturation Profile of Bovine and Porcine Pericardia after TRICOL Decellularization
3.6. Biomechanical Properties of Bovine and Porcine Pericardia after TRICOL Decellularization
3.7. Bioactive Properties of Bovine and Porcine Pericardia after TRICOL Decellularization
3.8. Differences of TRICOL Decellularized Bovine and Porcine Pericardia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yakirevich, V.S.; Abdulali, S.A.; Abbott, C.R.; Ionescu, M.I. Reconstruction of the pericardial sac with glutaraldehyde-preserved bovine pericardium. Tex Hear. Inst. J. 1984, 11, 238–242. [Google Scholar]
- David, T.E.; Feindel, C.M.; Ropchan, G.V. Reconstruction of the left ventricle with autologous pericardium. J. Thorac. Cardiovasc. Surg. 1987, 94, 710–714. [Google Scholar] [CrossRef]
- Temeck, B.K.; Katz, N.M.; Wallace, R.B. An approach to reoperative median sternotomy. J. Card. Surg. 1990, 5, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Grillo, H.C. Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann. Thorac. Surg. 1994, 58, 613–620. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Gérard, J.M.; Gersdorff, M. The Tutopatch graft for transcanal myringoplasty. B-ENT 2006, 2, 177–179. [Google Scholar]
- Stavropoulos, A.; Chiantella, G.; Costa, D.; Steigmann, M.; Windisch, P.; Sculean, A. Clinical and Histologic Evaluation of a Granular Bovine Bone Biomaterial Used as an Adjunct to GTR With a Bioresorbable Bovine Pericardium Collagen Membrane in the Treatment of Intrabony Defects. J. Periodontol. 2011, 82, 462–470. [Google Scholar] [CrossRef]
- Merli, M.; Moscatelli, M.; Mariotti, G.; Pagliaro, U.; Raffaelli, E.; Nieri, M. Comparing membranes and bone substitutes in a one-stage procedure for horizontal bone augmentation. A double-blind randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 271–281. [Google Scholar]
- Ionescu, M.I.; Pakrashi, B.C.; Holden, M.P.; Mary, D.A.; Wooler, G.H. Results of aortic valve replacement with frame-supported fascia lata and pericardial grafts. J. Thorac. Cardiovasc. Surg. 1972, 64, 340–353. [Google Scholar] [CrossRef]
- Manji, R.A.; Zhu, L.F.; Nijjar, N.K.; Rayner, D.C.; Korbutt, G.S.; Churchill, T.A.; Rajotte, R.V.; Koshal, A.; Ross, D.B. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation 2006, 114, 318–327. [Google Scholar] [CrossRef]
- Iop, L.; Palmosi, T.; Dal Sasso, E.; Gerosa, G. Bioengineered tissue solutions for repair, correction and reconstruction in cardiovascular surgery. J. Thorac. Dis. 2018, 10, S2390–S2411. [Google Scholar] [CrossRef] [PubMed]
- Courtman, D.W.; Pereira, C.A.; Kashef, V.; McComb, D.; Lee, J.M.; Wilson, G.J. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J. Biomed. Mater. Res. 1994, 28, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Mirsadraee, S.; Wilcox, H.E.; Korossis, S.A.; Kearney, J.N.; Watterson, K.G.; Fisher, J.; Ingham, E. Development and Characterization of an Acellular Human Pericardial Matrix for Tissue Engineering. Tissue Eng. 2006, 12, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Hülsmann, J.; Grün, K.; El Amouri, S.; Barth, M.; Hornung, K.; Holzfuß, C.; Lichtenberg, A.; Akhyari, P. Transplantation material bovine pericardium: Biomechanical and immunogenic characteristics after decellularization vs. glutaraldehyde-fixing. Xenotransplantation 2012, 19, 286–297. [Google Scholar] [CrossRef]
- Cigliano, A.; Gandaglia, A.; Lepedda, A.J.; Zinellu, E.; Naso, F.; Gastaldello, A.; Aguiari, P.; De Muro, P.; Gerosa, G.; Spina, M.; et al. Fine Structure of Glycosaminoglycans from Fresh and Decellularized Porcine Cardiac Valves and Pericardium. Biochem. Res. Int. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aguiari, P.; Iop, L.; Favaretto, F.; Fidalgo, C.M.L.; Naso, F.; Milan, G.; Vindigni, V.; Spina, M.; Bassetto, F.; Bagno, A.; et al. In vitro comparative assessment of decellularized bovine pericardial patches and commercial bioprosthetic heart valves. Biomed. Mater. 2017, 12, 015021. [Google Scholar] [CrossRef]
- Etnel, J.R.G.; Suss, P.H.; Schnorr, G.M.; Veloso, M.; Colatusso, D.F.; Balbi Filho, E.M.; da Costa, F.D.A. Fresh decellularized versus standard cryopreserved pulmonary allografts for right ventricular outflow tract reconstruction during the Ross procedure: A propensity-matched study. Eur. J. Cardio-Thoracic Surg. 2018, 54, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Tutopatch Tutopatch TM and Tutomesh TM Bovine Pericardium Implant Overview. Available online: http://www.lifehealthcare.com.au/wp-content/uploads/2017/07/Tutomesh_and_Tutopatch_Comprehensive_Implant_Overview.pdf (accessed on 20 January 2020).
- COVA+ABDO COVATM+ ABDO. Available online: www.biomup.com/en/cova-plus/cova-plus-abdo/3/ (accessed on 20 January 2020).
- INTEGRA. Flexibility in Your Hands INTEGRA BP Flexible Performance Integra BP: Sutured Dural Graft Easily Conforms to Protect Against CSF Leaks Integra ® Bovine Pericardium (BP) Dural Graft. Available online: www.integralife.com/file/general/1478293848.pdf (accessed on 20 January 2020).
- OSTEOKOR Osteokor Pericardium. Available online: https://www.surgikorimplants.com/regeneration/osteokor-pericardium (accessed on 20 January 2020).
- Filippi, R.; Schwarz, M.; Voth, D.; Reisch, R.; Grunert, P.; Perneczky, A. Bovine pericardium for duraplasty: clinical results in 32 patients. Neurosurg. Rev. 2001, 24, 103–107. [Google Scholar] [CrossRef]
- Steigmann, M. Pericardium Membrane and Xenograft Particulate Grafting Materials for Horizontal Alveolar Ridge Defects. Implant Dent. 2006, 15, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Hoell, T.; Hohaus, C.; Huschak, G.; Beier, A.; Meisel, H.-J. Total dura substitute in the spine: double layer dural substitute made from polylactide layer and bovine pericardium. Acta Neurochir. 2007, 149, 1259–1262. [Google Scholar] [CrossRef]
- Albera, R.; Dagna, F.; Lacilla, M.; Canale, A. Equine versus Bovine Pericardium in Transmeatal Underlay Myringoplasty. Ann. Otol. Rhinol. Laryngol. 2009, 118, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Bel, A.; Kachatryan, L.; Bruneval, P.; Peyrard, S.; Gagnieu, C.; Fabiani, J.-N.; Menasché, P. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubitosi, A.; Docimo, G.; Parmeggiani, D.; Pirozzi, R.; Vitiello, C.; Schettino, P.; Avellino, M.; Casalino, G.; Amato, M.; Ruggiero, R.; et al. Acellular bovine pericardium dermal matrix in immediate breast reconstruction after Skin Sparing Mastectomy. Int. J. Surg. 2014, 12, S205–S208. [Google Scholar] [CrossRef] [Green Version]
- de Dorlodot, C.; De Bie, G.; Deggouj, N.; Decat, M.; Gérard, J.-M. Are bovine pericardium underlay xenograft and butterfly inlay autograft efficient for transcanal tympanoplasty? Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 327–331. [Google Scholar] [CrossRef]
- Le, B.; Borzabadi-Farahani, A.; Nielsen, B. Treatment of Labial Soft Tissue Recession Around Dental Implants in the Esthetic Zone Using Guided Bone Regeneration With Mineralized Allograft: A Retrospective Clinical Case Series. J. Oral Maxillofac. Surg. 2016, 74, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- PhotoFix PhotoFix Decellularized Bovine Pericardium. Available online: www.cryolife.com (accessed on 20 January 2020).
- Majeed, A.; Baird, C.; Borisuk, M.J.; Sanders, S.P.; Padera, R.F. Histology of Pericardial Tissue Substitutes Used in Congenital Heart Surgery. Pediatr. Dev. Pathol. 2016, 19, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Matrix Patch. The Equine Matrix Patch TM is a Cell-Free Pericardial Patch for Use in Cardiac Surgery. Available online: www.autotissue.de/fileadmin/user_upload/Flyer_neu_Homepage.pdf (accessed on 20 January 2020).
- EACTS. Satellite symposium 2017 5 Years Experience with the Decellularized Matrix Patch—Auto Tissue Satellite Symposium-Monday-EACTS. Available online: www.eacts.org/educational-events/eacts-annual-meeting/scientific-programme/auto-tissue-satellite-symposium-monday/ (accessed on 20 January 2020).
- Gauvin, R.; Marinov, G.; Mehri, Y.; Klein, J.; Li, B.; Larouche, D.; Guzman, R.; Zhang, Z.; Germain, L.; Guidoin, R. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J. Biomater. Appl. 2013, 28, 552–565. [Google Scholar] [CrossRef]
- Bagno, A.; Aguiari, P.; Fiorese, M.; Iop, L.; Spina, M.; Gerosa, G. Native Bovine and Porcine Pericardia Respond to Load With Additive Recruitment of Collagen Fibers. Artif. Organs 2017, 42, 540–548. [Google Scholar] [CrossRef]
- Iop, L.; Renier, V.; Naso, F.; Piccoli, M.; Bonetti, A.; Gandaglia, A.; Pozzobon, M.; Paolin, A.; Ortolani, F.; Marchini, M.; et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials 2009, 30, 4104–4116. [Google Scholar] [CrossRef]
- Gallo, M.; Naso, F.; Poser, H.; Rossi, A.; Franci, P.; Bianco, R.; Micciolo, M.; Zanella, F.; Cucchini, U.; Aresu, L.; et al. Physiological Performance of a Detergent Decellularized Heart Valve Implanted for 15 Months in Vietnamese Pigs: Surgical Procedure, Follow-up, and Explant Inspection. Artif. Organs 2012, 36, E138–E150. [Google Scholar] [CrossRef]
- Iop, L.; Bonetti, A.; Naso, F.; Rizzo, S.; Cagnin, S.; Bianco, R.; Dal Lin, C.; Martini, P.; Poser, H.; Franci, P.; et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS One 2014, 9, e99593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iop, L.; Paolin, A.; Aguiari, P.; Trojan, D.; Cogliati, E.; Gerosa, G. Decellularized Cryopreserved Allografts as Off-the-Shelf Allogeneic Alternative for Heart Valve Replacement: In Vitro Assessment Before Clinical Translation. J. Cardiovasc. Transl. Res. 2017, 10, 93–103. [Google Scholar] [CrossRef]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Chuong, C.J.C.; More, R. Collagen fiber architecture of bovine pericardium. ASAIO J. 1994, 40, M632–M637. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Dal Sasso, E.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; Borile, G. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23, 1–9. [Google Scholar] [PubMed]
- OrientationJ. Available online: http://bigwww.epfl.ch/demo/orientation/ (accessed on 20 January 2020).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.; Griffa, A.; Sage, D.; Bouten, C.V.; van de Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef] [Green Version]
- NeuronJ. Available online: https://omictools.com/neuronj-tool (accessed on 20 January 2020).
- Bank, R.A.; Krikken, M.; Beekman, B.; Stoop, R.; Maroudas, A.; Lafebers, F.P.J.G.; Te Koppele, J.M. A simplified measurement of degraded collagen in tissues: Application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biol. 1997, 16, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.A.; O’Brien, W.D. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin. Chim. Acta 1980, 104, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Korossis, S.A.; Booth, C.; Wilcox, H.E.; Watterson, K.G.; Kearney, J.N.; Fisher, J.; Ingham, E. Tissue engineering of cardiac valve prostheses II: biomechanical characterization of decellularized porcine aortic heart valves. J. Heart Valve Dis. 2002, 11, 463–471. [Google Scholar]
- ISO 10993-5 ISO 10993-5 Biological Evaluation of Medical Devices - Part 5: Tests for In Vitro Cytotoxicity. Available online: www.iso.org/standard/68936.html (accessed on 20 January 2020).
- Cebotari, S.; Tudorache, I.; Jaekel, T.; Hilfiker, A.; Dorfman, S.; Ternes, W.; Haverich, A.; Lichtenberg, A. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs 2010, 34, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Holzapfel, G.A. Structural and numerical models for the (visco)elastic response of arterial walls with residual stresses. In Biomechanics of Soft Tissue in Cardiovascular Systems; Springer: Wien, Austria, 2003; pp. 109–184. ISBN 3211004556. [Google Scholar]
- Choe, J.A.; Jana, S.; Tefft, B.J.; Hennessy, R.S.; Go, J.; Morse, D.; Lerman, A.; Young, M.D. Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications. J. Tissue Eng. Regen. Med. 2018, 12, 1608–1620. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Novelo, B.; Avila, E.E.; Cauich-Rodríguez, J.V.; Jorge-Herrero, E.; Rojo, F.J.; Guinea, G.V.; Mata-Mata, J.L. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater. 2011, 7, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Morticelli, L.; Thomas, D.; Ingham, E.; Korossis, S. Investigation of the Suitability of Decellularised Porcine Pericardium for Mitral Valve Reconstruction. QScience Proc. 2012, 2012, 39. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Raman, R.; Prabhakar, V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu. Rev. Biomed. Eng. 2006, 8, 181–231. [Google Scholar] [CrossRef] [PubMed]
- Gesslbauer, B.; Rek, A.; Falsone, F.; Rajkovic, E.; Kungl, A.J. Proteoglycanomics: tools to unravel the biological function of glycosaminoglycans. Proteomics 2007, 7, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, E.O.; Pavao, M.S.G.; Borsig, L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P-selectin. J. Thromb. Haemost. 2011, 9, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Sobczak, A.I.S.; Pitt, S.J.; Stewart, A.J. Glycosaminoglycan Neutralization in Coagulation Control. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of immune responses. Ann. N. Y. Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Langford-Smith, A.; Day, A.J.; Bishop, P.N.; Clark, S.J. Complementing the Sugar Code: Role of GAGs and Sialic Acid in Complement Regulation. Front. Immunol. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Raspanti, M.; Viola, M.; Forlino, A.; Tenni, R.; Gruppi, C.; Tira, M.E. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Biol. 2008, 164, 134–139. [Google Scholar] [CrossRef]
- Mizumoto, S.; Kosho, T.; Yamada, S.; Sugahara, K. Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders. Pharmaceuticals 2017, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Zhang, T.; Ling, T.; Zhou, Z.; Xie, H.; Zhang, W.; Hu, G.; Jiang, C.; Li, M.; Feng, B.; et al. Guided bone regeneration using acellular bovine pericardium in a rabbit mandibular model: in-vitro and in-vivo studies. J. Periodontal Res. 2014, 49, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Gong, D.; Xia, C.; Liu, X.; Xu, Z. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 768–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, T.; Kitagawa, H. Sulfated glycosaminoglycans: their distinct roles in stem cell biology. Glycoconj. J. 2017, 34, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Duval, J.L.; Frei, V.; Abdul-Malak, N.; Sigot-Luizard, M.F.; Herbage, D. Cytocompatibility of calf pericardium treated by glutaraldehyde and by the acyl azide methods in an organotypic culture model. Biomaterials 1995, 16, 1003–1008. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Zouhair, S.; Aguiari, P.; Iop, L.; Vásquez-Rivera, A.; Filippi, A.; Romanato, F.; Korossis, S.; Wolkers, W.F.; Gerosa, G. Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability. Acta Biomater. 2018, 84, 208–221. [Google Scholar] [CrossRef]
- Neethling, W.M.L.; Strange, G.; Firth, L.; Smit, F.E. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: Initial experience with the ADAPT-treated CardioCel® patch. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 698–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobieraj, M.; Cudak, E.; Mrówczyński, W.; Nałęcz, T.K.; Westerski, P.; Wojtalik, M. Application of the CardioCel bovine pericardial patch - a preliminary report. Kardiochirurgia i torakochirurgia Pol. = Polish J. cardio-thoracic Surg. 2016, 13, 210–212. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Milano, A.D.; Bortolotti, U. Use of Pericardium for Cardiac Reconstruction Procedures in Acquired Heart Diseases—A Comprehensive Review. Thorac. Cardiovasc. Surg. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.J.; Marshall, M.; Daniels, I.R. Biological meshes: A review of their use in abdominal wall hernia repairs. Surgeon 2012, 10, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, J.; Li, J.; Shen, Y. Randomized and Comparative Clinical Trial of Bovine Mesh Versus Polypropylene Mesh in the Repair of Inguinal Hernias. Surg. Laparosc. Endosc. Percutaneous Tech. 2019, 30, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Adamo, S.; Gossetti, F.; D’Amore, L.; Ceci, F.; Negro, P.; Bruzzone, P. Biological scaffolds for abdominal wall repair: Future in clinical application? Materials 2019, 12, 2375. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Dávalos Herrera, D.A.; Woodmass, J.M.; Boorman, R.S.; Thornton, G.M.; Lo, I.K.Y. Can Grafts Provide Superior Tendon Healing and Clinical Outcomes After Rotator Cuff Repairs? Orthop. J. Sport. Med. 2016, 4, 232596711667419. [Google Scholar] [CrossRef]
- Gayre, G.S.; Debacker, C.; Lipham, W.; Tawfik, H.A.; Holck, D.; Dutton, J.J. Bovine pericardium as a wrapping for orbital implants. Ophthal. Plast. Reconstr. Surg. 2001, 17, 381–387. [Google Scholar] [CrossRef]
- Straumann Jason. Available online: https://www.straumann.com/en/dental-professionals/products-and-solutions/biomaterials/membranes.html (accessed on 20 January 2020).
- Giesenhagen, B.; Martin, N.; Jung, O.; Barbeck, M. Bone augmentation and simultaneous implant placement with allogenic bone rings and analysis of its purification success. Materials 2019, 12, 1291. [Google Scholar] [CrossRef] [Green Version]
- Mofid, M.M.; Meininger, M.S.; Lacey, M.S. Veritas® bovine pericardium for immediate breast reconstruction: a xenograft alternative to acellular dermal matrix products. Eur. J. Plast. Surg. 2012, 35, 717–722. [Google Scholar] [CrossRef] [Green Version]







| NBPs vs. NPPs | NBPs vs. DBPs | NPPs vs. DPPs | DBPs vs. DPPs | ||
|---|---|---|---|---|---|
| ECM architecture | |||||
| Serosal collagen C | ↓↓↓↓ | ↓ | ↓ | ↓↓↓ | |
| Serosal elastin C | NS | NS | ↓↓↓ | ↓ | |
| Serosal Ps | NS | NS | NS | ↓↓ | |
| Fibrosal collagen C | ↓ | NS | ↓↓↓ | ↓↓ | |
| Fibrosal elastin C | NS | NS | NS | NS | |
| Fibrosal Ps | NS | ↑ | NS | ↓↓↓↓ | |
| ECM biochemical composition | |||||
| Collagen | NS | ↑↑ | NS | NS | |
| Denatured collagen | ↓↓↓ | NS | NS | ↓↓↓↓ | |
| sGAGs | NS | ↓↓ | NS | NS | |
| Elastin | NS | NS | NS | NS | |
| Water | ↓↓ | ↑↑↑↑ | ↓↓ | ↓↓↓↓ | |
| DNA | NS | ↑↑ | ↑↑↑ | NS | |
| ECM structure | |||||
| R | NS | NS | NS | NS | |
| Tonset | NS | NS | ↓ | ↓↓↓↓ | |
| ECM biomechanical properties | |||||
| Thickness | ↓↓↓↓ | NS | ↑↑↑ | ↓ | |
| Elastin-phase modulus | NS | NS | NS | NS | |
| Collagen-phase modulus | ↑ | NS | ↓ | NS | |
| Failure strain | ↓↓↓ | NS | NS | ↓ | |
| UTS | NS | NS | NS | NS | |
| ECM bioactive properties | |||||
| hBM-MSCs MTS 24 h | / | / | / | NS | |
| hBM-MSCs MTS 72 h | / | / | / | NS | |
| HUVECs MTS 24 h | / | / | / | NS | |
| HUVECs MTS 72 h | / | / | / | NS | |
| Currently Applied Commercial Acellular Animal Pericardia | DBPs | DPPs | ||
|---|---|---|---|---|
| Cardiovascular surgery | ||||
| Heart valve reconstruction, aortic repair, intracardiac defect corrections, annulus repair | Matrix P (E), Photofix (B), CardioCel (B), COVA (B) [26,30,31,32,72,73,74] | + | + | |
| Gastrointestinal surgery | ||||
| Anterior abdominal wall reconstruction, hernia repair | Tutopatch (B), Tutomesh (B), COVA (B), Integra (B) [18,19,20,75,76,77] | +* | + | |
| Maxillofacial, bone and tendon surgeries | ||||
| Bone reconstruction, Dental reconstruction, Orbital wrapping, Dura mater plastics, Rotor cuff repair, Myringoplasty | Tutopatch (B), Tutoplast (B), Tutodent; CopiOs Pericardium (B); Osteokor pericardium (B); Lyoplant (B); Jason (P) [21,22,23,25,28,29,78,79,80,81] | +** | + | |
| Plastic surgery | ||||
| Facial aesthetics, Breast aesthetics | Veritas (B) [27,82] | + | + | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouhair, S.; Dal Sasso, E.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. https://doi.org/10.3390/biom10030371
Zouhair S, Dal Sasso E, Tuladhar SR, Fidalgo C, Vedovelli L, Filippi A, Borile G, Bagno A, Marchesan M, De Rossi G, et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules. 2020; 10(3):371. https://doi.org/10.3390/biom10030371
Chicago/Turabian StyleZouhair, Sabra, Eleonora Dal Sasso, Sugat R. Tuladhar, Catia Fidalgo, Luca Vedovelli, Andrea Filippi, Giulia Borile, Andrea Bagno, Massimo Marchesan, Giorgio De Rossi, and et al. 2020. "A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications" Biomolecules 10, no. 3: 371. https://doi.org/10.3390/biom10030371
APA StyleZouhair, S., Dal Sasso, E., Tuladhar, S. R., Fidalgo, C., Vedovelli, L., Filippi, A., Borile, G., Bagno, A., Marchesan, M., De Rossi, G., Gregori, D., Wolkers, W. F., Romanato, F., Korossis, S., Gerosa, G., & Iop, L. (2020). A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules, 10(3), 371. https://doi.org/10.3390/biom10030371












