Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Lipid and Fatty Acid Analyses
2.3. Statistical Analysis
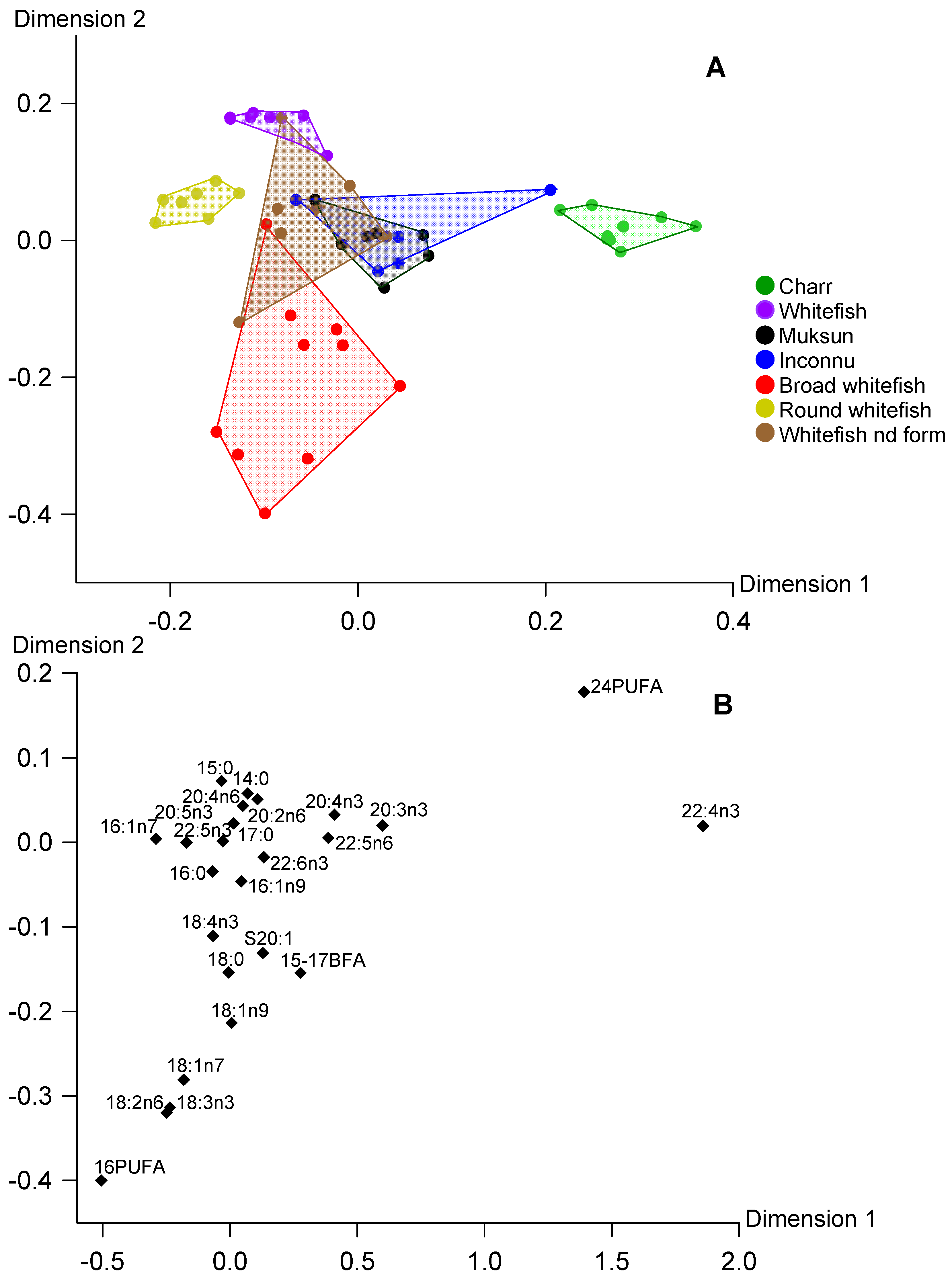
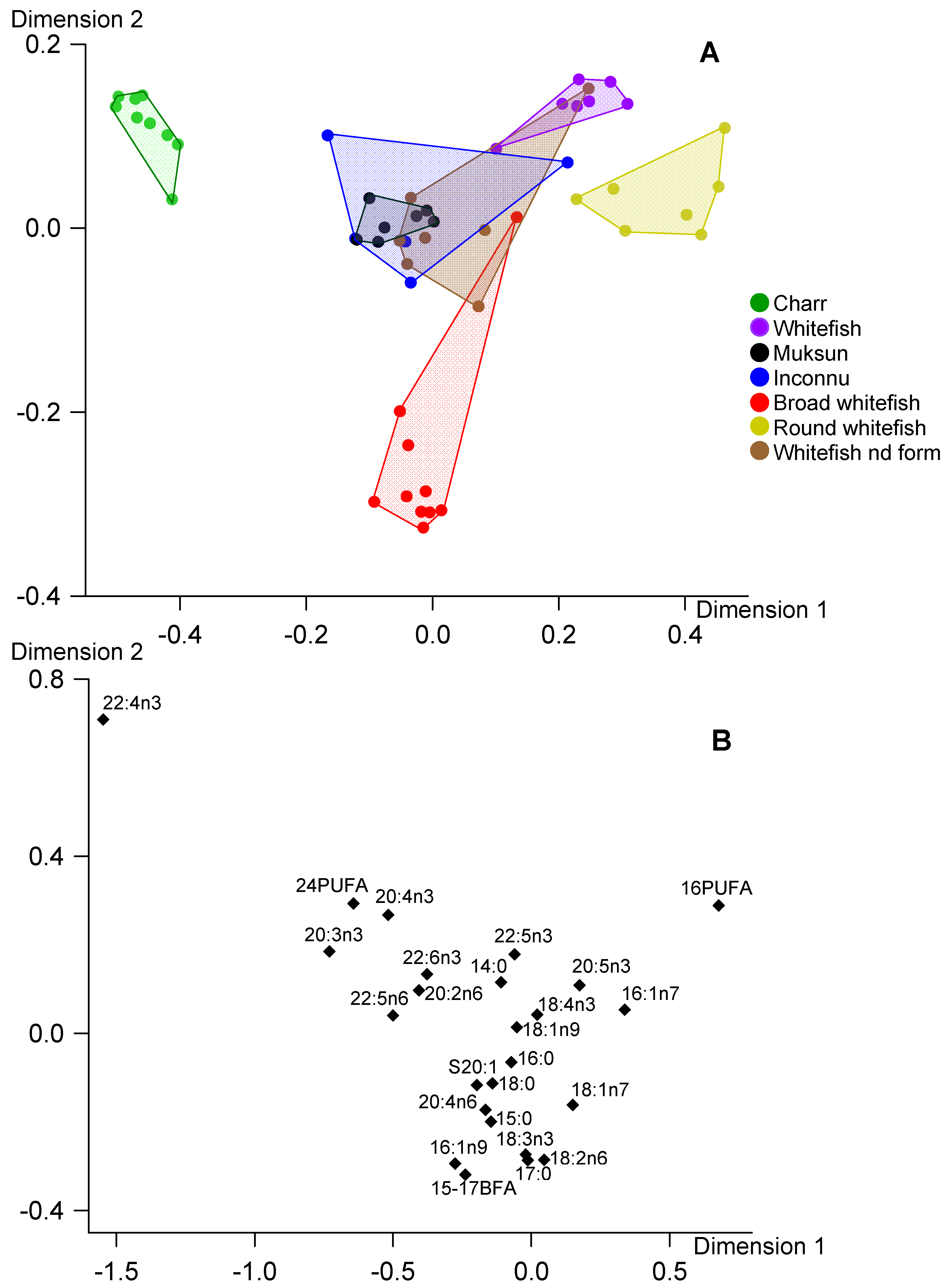
3. Results
4. Discussion
4.1. Main Finding
4.2. Fatty Acid Markers in Fish Total Lipids, TAG and PL
4.3. Content of Essential LC-PUFA in Fish PL and TAG
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Poudyal, H.; Brown, L. Should the pharmacological actions of dietary fatty acids in cardiometabolic disorders be classified based on biological or chemical function? Prog. Lipid Res. 2015, 59, 172–200. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127,477 participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [PubMed]
- Wu, J.H.; Micha, R.; Mozaffarian, D. Dietary fats and cardiometabolic disease: Mechanisms and effects on risk factors and outcomes. Nat. Rev. Cardiol. 2019, 16, 581–601. [Google Scholar] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J. Nutr. 2009, 139, 804S–819S. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease. Circulation 2017, 135, e867. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Tolomeev, A.P.; Dgebuadze, Y.Y. Meta-Analysis of factors associated with omega-3 fatty acid contents of wild fish. Rev. Fish Biol. Fish. 2018, 28, 277–299. [Google Scholar] [CrossRef] [Green Version]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Lipid Mediat. 2013, 107, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hixson, S.M.; Arts, M.T. Climate warming is predicted to reduce omega-3 long-chain, polyunsaturated fatty acid production in phytoplankton. Glob. Chang. Biol. 2016, 22, 2744–2755. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gribble, M.O.; Karimi, R.; Feingold, B.J.; Nyland, J.F.; O’Hara, T.M.; Gladyshev, M.I.; Chen, C.Y. Mercury, selenium and fish oils in marine food webs and implications for human health. J. Mar. Biol. Assoc. UK 2016, 96, 43–59. [Google Scholar] [CrossRef] [Green Version]
- Weil, C.; Lefevre, F.; Bugeon, L. Characteristics and metabolism of different adipose tissues in fish. Rev. Fish Biol. Fish. 2013, 23, 157–173. [Google Scholar] [CrossRef]
- Sargent, J.R. The lipids. In Fish Nutrition; Sargent, J.R., Henderson, R.J., Tocher, D.R., Eds.; Academic Press: New York, NY, USA, 1989; pp. 153–218. [Google Scholar]
- Vance, D.E.; Vance, J.E. (Eds.) Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier Science: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Iverson, S. Tracing aquatic food webs using fatty acids: From qualitative indicators to quantitative determination. In Lipids in Aquatic Ecosystems; Arts, M.T., Kainz, M., Brett, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 281–307. [Google Scholar]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estevez, A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Parrish, C.C. Essential fatty acids in aquatic food webs. In Lipids in Aquatic Ecosystems; Arts, M.T., Kainz, M., Brett, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 309–325. [Google Scholar]
- Mairesse, G.; Thomas, M.; Gardeur, J.-N.; Brun-Bellut, J. Effects of geographic source, rearing system, and season on the nutritional quality of wild and farmed Perca fluviatilis. Lipids 2006, 41, 221–229. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N. Long-Chain omega-3 polyunsaturated fatty acids in natural ecosystems and the human diet: Assumptions and challenges. Biomolecules 2019, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N.; Glushchenko, L.A.; Rudchenko, A.E.; Makhrov, A.A.; Borovikova, E.A.; Dgebuadze, Y.Y. Fatty acid composition and contents of seven commercial fish species of genus Coregonus from Russian Subarctic water bodies. Lipids 2017, 52, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, A.L. Earlier studies, morphology and hydrology of lakes. In Geographic Characteristic of Lakes of Taimyr Peninsula; Adamenko, V.N., Egorov, A.N., Eds.; Nauka: Leningrad, Russia, 1985; pp. 184–193. (In Russian) [Google Scholar]
- Heintz, R.A.; Wipfli, M.S.; Hudson, J.P. Identification of marine-derived lipids in juvenile Coho Salmon and aquatic insects through fatty acid analysis. Trans. Am. Fish. Soc. 2010, 139, 840–854. [Google Scholar] [CrossRef]
- Young, J.W.; Guest, M.A.; Lansdell, M.; Phleger, C.F.; Nichols, P.D. Discrimination of prey species of juvenile swordfish Xiphias gladius (Linnaeus, 1758) using signature fatty acid analyses. Prog. Oceanogr. 2010, 86, 139–151. [Google Scholar] [CrossRef]
- Czesny, S.J.; Rinchard, J.; Hanson, S.D.; Dettmers, J.M.; Dabrowski, K. Fatty acid signatures of Lake Michigan prey fish and invertebrates: Among-Species differences and spatiotemporal variability. Can. J. Fish. Aquat. Sci. 2011, 68, 1211–1230. [Google Scholar] [CrossRef]
- McMeans, B.C.; Arts, M.T.; Lydersen, C.; Kovacs, K.M.; Hop, H.; Falk-Petersen, S.; Fisk, A.T. The role of Greenland sharks (Somniosus microcephalus) in an Arctic ecosystem: Assessed via stable isotopes and fatty acids. Mar. Biol. 2013, 160, 1223–1238. [Google Scholar] [CrossRef]
- Hielscher, N.N.; Malzahn, A.M.; Diekmann, R.; Aberle, N. Trophic niche partitioning of littoral fish species from the rocky intertidal of Helgoland, Germany. Helgol. Mar. Res. 2015, 69, 385–399. [Google Scholar] [CrossRef]
- Strandberg, U.; Hiltunen, M.; Jelkänen, E.; Taipale, S.J.; Kainz, M.J.; Brett, M.T.; Kankaala, P. Selective transfer of polyunsaturated fatty acids from phytoplankton to planktivorous fish in large boreal lakes. Sci. Total Environ. 2015, 536, 858–865. [Google Scholar] [CrossRef]
- Chavarie, L.; Howland, K.; Gallagher, C.; Tonn, W. Fatty acid signatures and stomach contents of four sympatric Lake Trout: Assessment of trophic patterns among morphotypes in Great Bear Lake. Ecol. Freshw. Fish 2016, 25, 109–124. [Google Scholar] [CrossRef]
- Wiegand, M.D.; Johnston, T.A.; Porteous, L.R.; Ballevona, A.J.; Casselman, J.M.; Leggett, W.C. Comparison of ovum lipid provisioning among lake whitefish, walleye and northern pike co-habiting in Bay of Quinte (Lake Ontario, Canada). J. Gt. Lakes Res. 2014, 40, 721–729. [Google Scholar] [CrossRef]
- Napolitano, G.E. Fatty acids as trophic and chemical markers in freshwater ecosystems. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 21–44. [Google Scholar]
- Giri, S.S.; Graham, J.; Hamid, N.K.A.; Donald, J.A.; Turchini, G.M. Dietary micronutrients and In Vivo n-3 LC-PUFA biosynthesis in Atlantic salmon. Aquaculture 2016, 452, 416–425. [Google Scholar] [CrossRef]
- Oboh, A.; Kabeya, N.; Carmona-Antoñanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two alternative pathways for docosahexaenoic acid (DHA, 22:6n-3) biosynthesis are widespread among teleost fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Olsen, Y. Lipids and essential fatty acids in aquatic food webs: What can freshwater ecologists learn from mariculture. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 161–202. [Google Scholar]
- Litzow, M.A.; Bailey, K.M.; Prahl, F.G.; Heintz, R. Climate regime shifts and reorganization of fish communities: The essential fatty acid limitation hypothesis. Mar. Ecol. Prog. Ser. 2006, 315, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ahlgren, G.; Vrede, T.; Goedkoop, W. Fatty acid ratios in freshwater fish, zooplankton and zoobenthos—Are their specific optima? In Lipids in Aquatic Ecosystems; Arts, M.T., Kainz, M., Brett, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 147–178. [Google Scholar]
- Pérez, M.J.; Rodríguez, C.; Cejas, J.R.; Martín, M.V.; Jerez, S.; Lorenzo, A. Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp. Biochem. Physiol. B 2007, 146, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Schregel, W.D.; Wei, Y. Effects of thermal acclimation on tissue fatty acid composition of freshwater alewives (Alosa pseudoharengus). Fish Physiol. Biochem. 2012, 38, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kayhan, H.; Bashan, M.; Kaçar, S. Seasonal variations in the fatty acid composition of phospholipids and triacylglycerols of brown trout. Eur. J. Lipid Sci. Technol. 2015, 117, 738–744. [Google Scholar] [CrossRef]
- Suomela, J.-P.; Lundén, S.; Kaimainen, M.; Mattila, S.; Kallio, H.; Airaksinen, S. Effects of origin and season on the lipids and sensory quality of European whitefish (Coregonus lavaretus). Food Chem. 2016, 197, 1031–1037. [Google Scholar] [CrossRef]
- Sardenne, F.; Kraffe, E.; Amiel, A.; Fouché, E.; Debrauwer, L.; Ménard, F.; Bodin, N. Biological and environmental influence on tissue fatty acid compositions in wild tropical tunas. Comp. Biochem. Physiol. A 2017, 204, 17–27. [Google Scholar] [CrossRef]
- Suito, T.; Nagao, K.; Hatano, M.; Kohashi, K.; Tanabe, A.; Ozaki, H.; Kawamoto, J.; Kurihara, T.; Mioka, T.; Tanaka, K.; et al. Synthesis of omega-3 long-chain polyunsaturated fatty acid-rich triacylglycerols in an endemic goby, Gymnogobius isaza, from Lake Biwa, Japan. J. Biochem. 2018, 164, 127–140. [Google Scholar] [CrossRef]
- Rincon-Cervera, M.A.; Gonzalez-Barriga, V.; Valenzuela, R.; Lopez-Arana, S.; Romero, J.; Valenzuela, A. Profile and distribution of fatty acids in edible parts of commonly consumed marine fishes in Chile. Food Chem. 2019, 274, 123–129. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Bechtel, P.J. Lipid analysis of fillets from giant grenadier (Albatrossia pectoralis), arrow-tooth flounder (Atheresthes stomias), pacific cod (Gadus macrocephalus) and walleye pollock (Theragra chalcogramma). J. Muscle Foods 2006, 17, 20–33. [Google Scholar] [CrossRef]
- He, C.; Cao, J.; Jiang, X.; Wen, C.; Bai, X.; Li, C. Fatty acid profiles of triacylglycerols and phospholipids of sea-cage cultured Trachinotus blochii: A comparative study of head, viscera, skin, bone, and muscle. J. Food Sci. 2019, 84, 650–658. [Google Scholar] [CrossRef]
- Xue, C.; Okabe, M.; Saito, H. Differences in lipid characteristics among populations: Low-Temperature adaptability of ayu, Plecoglossus altivelis. Lipids 2012, 47, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Murzina, S.A.; Nefedova, Z.A.; Pekkoeva, S.N.; Veselov, A.E.; Efremov, D.A.; Nemova, N.N. Age-Specific lipid and fatty acid profiles of Atlantic salmon juveniles in the Varzuga River. Int. J. Mol. Sci. 2016, 17, 1050. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, G.; Blomqvist, P.; Boberg, M.; Gustafsson, I.-B. Fatty acid content of the dorsal muscle—An indicator of fat quality in freshwater fish. J. Fish Biol. 1994, 45, 131–157. [Google Scholar]
- Rojbek, M.C.; Tomkiewicz, J.; Jacobsen, C.; Stottrup, J.G. Forage fish quality: Seasonal lipid dynamics of herring (Clupea harengus L.) and sprat (Sprattus sprattus L.) in the Baltic Sea. ICES J. Mar. Sci. 2014, 71, 56–71. [Google Scholar] [CrossRef] [Green Version]
- Kainz, M.J.; Hager, H.H.; Rasconi, S.; Kahilainen, K.K.; Amundsen, P.-A.; Hayden, B. Polyunsaturated fatty acids in fishes increase with total lipids irrespective of feeding sources and trophic position. Ecosphere 2017, 8, e01753. [Google Scholar] [CrossRef]
- Leaver, M.J.; Taggart, J.B.; Villeneuve, L.; Bron, J.E.; Guy, D.R.; Bishop, S.C.; Houston, R.D.; Matika, O.; Tocher, D.R. Heritability and mechanisms of n-3 long chain polyunsaturated fatty acid deposition in the flesh of Atlantic salmon. Comp. Biochem. Physiol. D 2011, 6, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Ruffle, B.; Burmaster, D.E.; Anderson, P.D.; Gordon, H.D. Lognormal distributions for fish consumption by the general U.S. population. Risk Anal. 1994, 14, 395–404. [Google Scholar] [CrossRef]
- Williams, M.C.W.; Schrank, C.; Anderson, H.A. Fatty acids in thirteen Wisconsin sport fish species. J. Gt. Lakes Res. 2014, 40, 771–777. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Glushchenko, L.A.; Makhutova, O.N.; Rudchenko, A.E.; Shulepina, S.P.; Dubovskaya, O.P.; Zuev, I.V.; Kolmakov, V.I.; Sushchik, N.N. Comparative analysis of content of omega-3 polyunsaturated fatty acids in food and muscle tissue of fish from aquaculture and natural habitats. Contemp. Probl. Ecol. 2018, 11, 297–308. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.M.; Hallarake, H.; Sabo, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Maki, K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins Leukot. Essent. 2016, 111, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Laugero, K.D.; Mackey, B.; Kelley, D.S. Accretion of dietary docosahexaenoic acid in mouse tissues did not differ between its purified phospholipid and triacylglycerol forms. Lipids 2019, 54, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Common and Species Name | Lake | n | L | W | Food |
|---|---|---|---|---|---|
| Charr Salvelinus drjagini | Sobachye | 9 | 608 ± 17 | 2371 ± 271 | Fish (salmonids) |
| Whitefish Coregonus lavaretus | Sobachye | 7 | 480 ± 23 | 1153 ± 167 | Amphipods, mollusks, chironomid larvae |
| Muksun Coregonus muksun | Pyasino | 8 | 492 ± 14 | 1271 ± 160 | Ostracods, mollusks, chironomid larvae, detritus |
| Inconnu Stenodus leucichthys nelma | Pyasino | 5 | 675 ± 86 | 3239 ± 1581 | Fish |
| Broad whitefish Coregonus nasus | Pyasino | 10 | 563 ± 17 | 1916 ± 183 | Gastropods, detritus |
| Round whitefish Prosopium cylindraceum | Sobachye | 7 | 409 ± 7 | 488 ± 27 | Caddisfly and chironomid larvae, filamentous algae |
| Whitefish Coregonus lavaretus non-identified form | Sobachye | 7 | 402 ± 12 | 568 ± 80 | Chironomid and other insect pupa and adults |
| Common and Species Name | Moisture | Lipids | Total Fatty Acids |
|---|---|---|---|
| Charr Salvelinus drjagini | 69.8 ± 1.3 | 155.8 ± 7.4 | 78.8 ± 5.1 D |
| Whitefish Coregonus lavaretus | 69.8 ± 1.4 | 82.0 ± 1.9 | 62.7 ± 7.2 CD |
| Muksun Coregonus muksun | 74.4 ± 1.0 | n.d. | 45.4 ± 8.7 BC |
| Inconnu Stenodus leucichthys nelma | 72.1 ± 2.6 | 68.4 ± 11.4 | 36.5 ± 10.0 ABC |
| Broad whitefish Coregonus nasus | 73.8 ± 1.4 | n.d. | 31.9 ± 5.1 AB |
| Round whitefish Prosopium cylindraceum | 76.1 ± 0.6 | 39.1 ± 3.3 | 13.8 ± 1.3 A |
| Whitefish Coregonus lavaretus non-identified form | 76.0 ± 0.9 | 41.1 ± 2.1 | 11.5 ± 1.9 A |
| Fatty Acid | Charr | Whitefish | Muksun | Inconnu | Broad Whitefish | Round Whitefish | Whitefish nd |
|---|---|---|---|---|---|---|---|
| 14:0 | 4.0 ± 0.1 A | 2.9 ± 0.1 B | 4.2 ± 0.2 A | 3.4 ± 0.1 AC | 2.5 ± 0.2 B | 2.2 ± 0.2 B | 2.3 ± 0.2 B |
| 15:0 | 0.3 ± 0.0 A | 0.2 ± 0.0 CD | 0.5 ± 0.0 B | 0.4 ± 0.0 A | 0.5 ± 0.0 B | 0.2 ± 0.0 D | 0.3 ± 0.0 C |
| 16:0 | 15.4 ± 0.2 C | 14.9 ± 0.2 C | 15.2 ± 0.2 C | 16.3 ± 0.7 AC | 18.2 ± 0.4 AB | 16.6 ± 0.2 AC | 18.9 ± 0.8 B |
| 16:1n-9 | 0.4 ± 0.0 ABD | 0.2 ± 0.0 C | 0.3 ± 0.0 CD | 0.4 ± 0.0 AC | 0.5 ± 0.1 B | 0.2 ± 0.0 C | 0.4 ± 0.1 ABD |
| 16:1n-7 | 6.6 ± 0.2 B | 17.4 ± 0.5 D | 10.1 ± 0.3 AC | 13.4 ± 1.0 ACE | 11.8 ± 0.8 C | 15.2 ± 0.4 DE | 9.7 ± 1.4 BC |
| 15-17BFA | 1.8 ± 0.0 A | 1.0 ± 0.0 CD | 1.8 ± 0.1 A | 1.3 ± 0.0 AC | 2.5 ± 0.2 B | 0.6 ± 0.0 D | 1.0 ± 0.1 CD |
| 16PUFA | 0.2 ± 0.0 B | 4.6 ± 0.2 C | 2.3 ± 0.2 DE | 1.5 ± 0.2 AD | 1.3 ± 0.1 A | 2.9 ± 0.1 E | 1.5 ± 0.4 AD |
| 17:0 | 0.2 ± 0.0 A | 0.3 ± 0.0 D | 0.4 ± 0.0 B | 0.2 ± 0.0 AD | 0.4 ± 0.0 B | 0.1 ± 0.0 C | 0.2 ± 0.0 A |
| 18:0 | 3.2 ± 0.1 C | 1.9 ± 0.0 D | 2.4 ± 0.1 A | 2.5 ± 0.2 AB | 3.0 ± 0.1 BC | 2.8 ± 0.0 AB | 2.5 ± 0.2 A |
| 18:1n-9 | 17.9 ± 0.1 AC | 19.8 ± 0.2 C | 16.1 ± 0.4 A | 16.8 ± 0.8 AC | 16.3 ± 0.7 A | 11.7 ± 0.6 B | 12.3 ± 1.0 B |
| 18:1n-7 | 3.1 ± 0.0 C | 3.9 ± 0.1 AC | 4.0 ± 0.3 AC | 4.3 ± 0.2 AB | 5.1 ± 0.1 B | 4.6 ± 0.1 AB | 3.7 ± 0.5 AC |
| 18:2n-6 | 3.0 ± 0.1 AC | 2.1 ± 0.1 A | 2.8 ± 0.1 AC | 2.7 ± 0.2 AC | 4.7 ± 0.6 B | 3.7 ± 0.1 BC | 2.7 ± 0.3 AC |
| 18:3n-3 | 2.6 ± 0.1 AC | 1.3 ± 0.0 A | 2.9 ± 0.2 BC | 2.2 ± 0.2 AC | 3.8 ± 0.6 B | 2.7 ± 0.2 AB | 1.6 ± 0.1 AC |
| 18:4n-3 | 1.7 ± 0.0 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 |
| ∑20:1 * | 1.6 ± 0.0 AD | 1.2 ± 0.1 BCD | 2.3 ± 0.1 A | 2.1 ± 0.9 ABC | 1.7 ± 0.3 AB | 0.6 ± 0.0 C | 0.8 ± 0.1 BC |
| 20:2n-6 | 1.0 ± 0.0 C | 0.3 ± 0.0 A | 0.6 ± 0.0 B | 0.4 ± 0.0 A | 0.6 ± 0.0 B | 0.3 ± 0.0 A | 0.4 ± 0.1 A |
| 20:4n-6 | 1.9 ± 0.0 AC | 1.6 ± 0.0 CD | 2.4 ± 0.1 B | 2.3 ± 0.3 AB | 2.8 ± 0.1 B | 1.4 ± 0.1 D | 2.6 ± 0.2 B |
| 20:3n-3 | 1.5 ± 0.0 B | 0.2 ± 0.0 C | 0.6 ± 0.0 AB | 0.4 ± 0.0 ABC | 0.3 ± 0.0 AC | 0.2 ± 0.0 C | 0.4 ± 0.1 AC |
| 20:4n-3 | 2.9 ± 0.0 A | 0.7 ± 0.0 BC | 1.2 ± 0.1 AC | 1.1 ± 0.1 AC | 0.6 ± 0.0 B | 0.8 ± 0.0 BC | 0.8 ± 0.1 BC |
| 20:5n-3 | 4.8 ± 0.1 C | 10.4 ± 0.2 A | 9.6 ± 0.2 AB | 7.4 ± 0.7 ABC | 6.5 ± 0.3 BC | 10.2 ± 0.1 A | 10.3 ± 0.3 A |
| 22:5n-6 | 1.2 ± 0.0 B | 0.3 ± 0.0 C | 1.3 ± 0.1 B | 1.0 ± 0.1 AB | 0.8 ± 0.1 A | 0.3 ± 0.0 C | 0.8 ± 0.1 A |
| 22:4n-3 | 1.5 ± 0.1 C | 0.0 ± 0.0 AB | 0.1 ± 0.0 AC | 0.2 ± 0.1 AC | 0.0 ± 0.0 B | 0.0 ± 0.0 AB | 0.1 ± 0.0 ABC |
| 22:5n-3 | 3.0 ± 0.1 CD | 2.5 ± 0.1 AC | 2.5 ± 0.1 AC | 2.3 ± 0.1 A | 1.7 ± 0.1 B | 3.0 ± 0.0 D | 2.4 ± 0.1 A |
| 22:6n-3 | 12.1 ± 0.1 AC | 6.3 ± 0.2 D | 9.6 ± 0.6 ADE | 11.3 ± 1.1 ACD | 7.7 ± 0.9 AD | 14.4 ± 1.5 BCE | 20.1 ± 3.0 B |
| 24PUFA | 4.3 ± 0.3 B | 1.0 ± 0.1 AB | 1.1 ± 0.1 BC | 1.0 ± 0.2 AC | 0.7 ± 0.0 A | 0.8 ± 0.0 A | 0.6 ± 0.1 AC |
| Fatty Acid | Charr | Whitefish | Muksun | Inconnu | Broad Whitefish | Round Whitefish | Whitefish nd |
|---|---|---|---|---|---|---|---|
| 14:0 | 1.2 ± 0.1 AB | 0.9 ± 0.0 A | 1.1 ± 0.1 AB | 1.5 ± 0.2 B | 1.2 ± 0.1 AB | 0.9 ± 0.1 A | 1.4 ± 0.1 B |
| 15:0 | 0.2 ± 0.0 A | 0.2 ± 0.0 A | 0.4 ± 0.0 CD | 0.3 ± 0.0 BC | 0.5 ± 0.0 D | 0.2 ± 0.0 A | 0.3 ± 0.0 AB |
| 16:0 | 24.2 ± 0.4 A | 29.4 ± 0.5 BC | 26.3 ± 0.6 AB | 25.9 ± 0.5 AB | 27.3 ± 0.8 AB | 29.1 ± 1.3 BC | 31.7 ± 1.1 C |
| 16:1n-9 | 0.3 ± 0.0 AB | 0.1 ± 0.0 A | 0.3 ± 0.0 BC | 0.2 ± 0.1 AB | 0.5 ± 0.1 C | 0.2 ± 0.0 AB | 0.3 ± 0.1 BC |
| 16:1n-7 | 1.3 ± 0.1 A | 2.2 ± 0.1 BC | 1.9 ± 0.2 AB | 2.3 ± 0.1 B | 2.9 ± 0.2 B | 4.1 ± 0.2 D | 3.0 ± 0.2 C |
| 15-17BFA | 0.6 ± 0.0 C | 0.1 ± 0.0 A | 0.4 ± 0.0 BC | 0.3 ± 0.0 AB | 0.6 ± 0.1 C | 0.2 ± 0.0 AB | 0.3 ± 0.1 AB |
| 16PUFA | 0.0 ± 0.0 A | 0.2 ± 0.0 B | 0.1 ± 0.1 AB | 0.1 ± 0.0 AB | 0.1 ± 0.0 AB | 0.3 ± 0.0 C | 0.1 ± 0.0 AB |
| 17:0 | 0.2 ± 0.0 A | 0.2 ± 0.0 AB | 0.3 ± 0.0 B | 0.2 ± 0.0 AB | 0.3 ± 0.0 B | 0.1 ± 0.0 A | 0.2 ± 0.0 A |
| 18:0 | 2.8 ± 0.1 AB | 2.1 ± 0.1 A | 3.0 ± 0.1 B | 2.9 ± 0.3 AB | 2.2 ± 0.2 A | 3.4 ± 0.2 B | 2.8 ± 0.3 AB |
| 18:1n-9 | 6.2 ± 0.3 AB | 6.5 ± 0.2 AB | 6.6 ± 0.4 AB | 7.8 ± 0.4 B | 6.1 ± 0.5 A | 5.6 ± 0.3 A | 6.9 ± 0.3 AB |
| 18:1n-7 | 1.6 ± 0.1 A | 1.7 ± 0.1 AB | 2.2 ± 0.2 BC | 2.4 ± 0.1 C | 2.5 ± 0.1 C | 3.3 ± 0.2 D | 2.4 ± 0.3 C |
| 18:2n-6 | 0.7 ± 0.0 A | 0.8 ± 0.0 A | 1.0 ± 0.0 A | 1.1 ± 0.1 A | 2.5 ± 0.4 B | 1.6 ± 0.0 A | 1.4 ± 0.3 A |
| 18:3n-3 | 0.6 ± 0.0 AB | 0.4 ± 0.0 A | 1.0 ± 0.1 ABC | 1.0 ± 0.2 ABC | 2.1 ± 0.4 C | 1.3 ± 0.1 BC | 0.7 ± 0.1 ABC |
| 18:4n-3 | 0.1 ± 0.0 AB | 0.0 ± 0.0 A | 0.2 ± 0.0 B | 0.2 ± 0.0 AB | 0.1 ± 0.0 AB | 0.3 ± 0.0 B | 0.2 ± 0.1 AB |
| ∑20:1 | 0.2 ± 0.0 BC | 0.1 ± 0.0 A | 0.3 ± 0.0 C | 0.1 ± 0.0 ABC | 0.1 ± 0.0 AB | 0.2 ± 0.0 ABC | 0.2 ± 0.1 AC |
| 20:2n-6 | 0.2 ± 0.0 BC | 0.0 ± 0.0 A | 0.1 ± 0.0 AB | 0.1 ± 0.0 A | 0.2 ± 0.0 C | 0.1 ± 0.0 AC | 0.1 ± 0.0 AB |
| 20:4n-6 | 3.9 ± 0.1 BC | 3.0 ± 0.1 AB | 4.1 ± 0.2 C | 3.8 ± 0.3 BC | 5.6 ± 0.3 D | 2.4 ± 0.1 A | 3.2 ± 0.2 ABC |
| 20:3n-3 | 0.5 ± 0.0 C | 0.1 ± 0.0 A | 0.2 ± 0.0 B | 0.1 ± 0.0 AB | 0.2 ± 0.0 B | 0.1 ± 0.0 AB | 0.1 ± 0.0 AB |
| 20:4n-3 | 1.1 ± 0.0 B | 0.3 ± 0.0 A | 0.5 ± 0.0 AB | 0.5 ± 0.1 AB | 0.4 ± 0.1 A | 0.5 ± 0.0 AB | 0.4 ± 0.0 A |
| 20:5n-3 | 7.9 ± 0.2 A | 16.6 ± 0.6 D | 12.5 ± 0.3 BC | 11.2 ± 1.1 BC | 10.9 ± 0.4 B | 13.6 ± 1.0 C | 10.0 ± 0.5 AB |
| 22:5n-6 | 2.4 ± 0.1 C | 0.6 ± 0.0 A | 2.0 ± 0.3 C | 1.7 ± 0.2 BC | 2.2 ± 0.2 C | 0.4 ± 0.0 A | 0.9 ± 0.1 AB |
| 22:4n-3 | 0.2 ± 0.0 B | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.0 ± 0.0 A |
| 22:5n-3 | 2.5 ± 0.0 A | 2.7 ± 0.2 AB | 2.5 ± 0.1 A | 2.4 ± 0.3 A | 2.6 ± 0.1 AB | 3.2 ± 0.3 B | 2.1 ± 0.1 A |
| 22:6n-3 | 39.9 ± 0.5 D | 31.1 ± 0.7 BC | 31.8 ± 0.5 C | 32.4 ± 0.9 C | 26.4 ± 0.6 A | 27.9 ± 0.7 AB | 30.1 ± 1.1 BC |
| 24PUFA | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.4 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Sum FA | 3.0 ± 0.2 AB | 3.3 ± 0.5 AB | 3.2 ± 0.9 AB | 2.6 ± 0.2 AB | 3.4 ± 0.2 B | 3.2 ± 0.7 AB | 2.1 ± 0.2 A |
| Fatty Acid | Charr | Whitefish | Muksun | Inconnu | Broad Whitefish | Round Whitefish | Whitefish nd |
|---|---|---|---|---|---|---|---|
| 14:0 | 4.4 ± 0.1 CD | 3.3 ± 0.1 B | 4.8 ± 0.3 D | 3.9 ± 0.0 BC | 2.7 ± 0.2 A | 3.1 ± 0.2 B | 3.5 ± 0.4 ABC |
| 15:0 | 0.3 ± 0.0 B | 0.2 ± 0.0 AB | 0.5 ± 0.0 C | 0.4 ± 0.0 BC | 0.5 ± 0.1 C | 0.2 ± 0.0 A | 0.3 ± 0.0 AB |
| 16:0 | 16.7 ± 0.4 C | 14.6 ± 0.2 AB | 14.6 ± 0.8 AB | 15.9 ± 0.7 B | 17.4 ± 0.3 C | 12.9 ± 0.4 A | 15.7 ± 0.5 B |
| 16:1n-9 | 0.8 ± 0.3 B | 0.3 ± 0.0 A | 0.4 ± 0.0 AB | 0.4 ± 0.1 AB | 0.8 ± 0.1 B | 0.3 ± 0.0 A | 0.5 ± 0.1 AB |
| 16:1n-7 | 7.9 ± 0.4 A | 19.4 ± 0.8 C | 12.6 ± 1.3 B | 15.9 ± 1.4 BC | 13.2 ± 0.7 B | 24.5 ± 1.2 D | 15.7 ± 0.9 BC |
| 15-17BFA | 2.0 ± 0.2 BCD | 1.0 ± 0.0 AB | 1.9 ± 0.1 CD | 1.1 ± 0.1 ABC | 2.8 ± 0.4 D | 0.7 ± 0.1 A | 1.4 ± 0.1 AB |
| 16PUFA | 0.4 ± 0.1 A | 4.8 ± 0.2 C | 3.0 ± 0.6 B | 1.6 ± 0.4 AB | 1.3 ± 0.2 AB | 5.4 ± 0.7 C | 2.2 ± 0.5 B |
| 17:0 | 0.2 ± 0.0 AB | 0.3 ± 0.0 BC | 0.3 ± 0.0 C | 0.2 ± 0.0 AB | 0.5 ± 0.0 D | 0.1 ± 0.0 A | 0.3 ± 0.0 BC |
| 18:0 | 3.4 ± 0.1 C | 1.9 ± 0.0 A | 2.4 ± 0.1 A | 2.5 ± 0.3 AB | 3.2 ± 0.1 BC | 2.5 ± 0.1 A | 2.6 ± 0.3 A |
| 18:1n-9 | 19.2 ± 0.6 BC | 21.5 ± 0.4 C | 16.8 ± 1.0 AB | 18.9 ± 1.4 B | 17.8 ± 0.8 AB | 14.6 ± 0.8 A | 18.3 ± 0.6 B |
| 18:1n-7 | 2.9 ± 0.4 A | 4.2 ± 0.1 AB | 4.6 ± 0.4 BC | 4.9 ± 0.2 BC | 5.9 ± 0.3 C | 5.9 ± 0.4 C | 4.8 ± 0.5 BC |
| 18:2n-6 | 3.2 ± 0.1 AB | 2.2 ± 0.1 A | 3.0 ± 0.1 AB | 3.0 ± 0.4 AB | 5.4 ± 0.6 D | 5.1 ± 0.1 CD | 3.9 ± 0.3 BC |
| 18:3n-3 | 2.6 ± 0.1 AB | 1.4 ± 0.1 A | 3.0 ± 0.2 BC | 2.4 ± 0.3 A | 4.0 ± 0.5 C | 3.6 ± 0.4 BC | 2.2 ± 0.2 AB |
| 18:4n-3 | 1.6 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.2 | 1.5 ± 0.2 | 1.9 ± 0.3 | 2.1 ± 0.4 |
| ∑20:1 | 1.3 ± 0.0 BC | 1.0 ± 0.1 AB | 1.9 ± 0.2 C | 2.0 ± 0.9 ABC | 1.7 ± 0.3 BC | 0.7 ± 0.1 A | 1.3 ± 0.1 ABC |
| 20:2n-6 | 0.9 ± 0.0 C | 0.2 ± 0.0 A | 0.6 ± 0.0 B | 0.4 ± 0.0 AB | 0.4 ± 0.1 AB | 0.3 ± 0.0 AB | 0.5 ± 0.1 B |
| 20:4n-6 | 1.7 ± 0.0 BC | 1.4 ± 0.0 B | 2.1 ± 0.1 D | 1.9 ± 0.2 CD | 2.3 ± 0.1 D | 0.6 ± 0.0 A | 1.7 ± 0.1 BCD |
| 20:3n-3 | 1.4 ± 0.0 C | 0.1 ± 0.0 A | 0.5 ± 0.1 BC | 0.4 ± 0.0 ABC | 0.3 ± 0.0 AB | 0.2 ± 0.0 AB | 0.4 ± 0.1 AB |
| 20:4n-3 | 2.7 ± 0.1 C | 0.7 ± 0.1 AB | 1.2 ± 0.1 BC | 1.2 ± 0.1 ABC | 0.6 ± 0.0 A | 0.9 ± 0.1 AB | 0.9 ± 0.1 AB |
| 20:5n-3 | 4.4 ± 0.1 A | 9.4 ± 0.2 D | 9.0 ± 0.3 D | 6.5 ± 1.0 BC | 5.3 ± 0.4 AB | 7.0 ± 0.5 BC | 8.6 ± 0.5 CD |
| 22:5n-6 | 0.9 ± 0.0 D | 0.2 ± 0.0 AB | 1.0 ± 0.1 D | 0.7 ± 0.1 CD | 0.5 ± 0.1 BC | 0.0 ± 0.0 A | 0.4 ± 0.1 AB |
| 22:4n-3 | 1.3 ± 0.0 B | 0.0 ± 0.0 A | 0.1 ± 0.0 A | 0.2 ± 0.1 A | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.1 ± 0.0 A |
| 22:5n-3 | 2.7 ± 0.1 B | 2.2 ± 0.1 B | 2.4 ± 0.2 B | 2.1 ± 0.2 B | 1.4 ± 0.1 A | 2.3 ± 0.1 B | 2.1 ± 0.2 B |
| 22:6n-3 | 9.9 ± 0.2 C | 4.1 ± 0.2 B | 6.4 ± 0.3 C | 7.8 ± 0.8 C | 4.1 ± 0.3 B | 2.4 ± 0.2 A | 6.6 ± 0.5 C |
| 24PUFA | 2.9 ± 0.1 B | 0.7 ± 0.1 A | 0.9 ± 0.1 AB | 0.6 ± 0.3 A | 0.7 ± 0.0 A | 0.6 ± 0.1 A | 0.7 ± 0.1 A |
| Sum FA | 30.3 ± 4.7 AB | 33.2 ± 6.9 AB | 41.6 ± 15.0 B | 18.7 ± 7.0 AB | 19.5 ± 7.0 AB | 4.9 ± 1.2 A | 2.0 ± 0.5 A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sushchik, N.N.; Makhutova, O.N.; Rudchenko, A.E.; Glushchenko, L.A.; Shulepina, S.P.; Kolmakova, A.A.; Gladyshev, M.I. Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes. Biomolecules 2020, 10, 419. https://doi.org/10.3390/biom10030419
Sushchik NN, Makhutova ON, Rudchenko AE, Glushchenko LA, Shulepina SP, Kolmakova AA, Gladyshev MI. Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes. Biomolecules. 2020; 10(3):419. https://doi.org/10.3390/biom10030419
Chicago/Turabian StyleSushchik, Nadezhda N., Olesia N. Makhutova, Anastasia E. Rudchenko, Larisa A. Glushchenko, Svetlana P. Shulepina, Anzhelika A. Kolmakova, and Michail I. Gladyshev. 2020. "Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes" Biomolecules 10, no. 3: 419. https://doi.org/10.3390/biom10030419
APA StyleSushchik, N. N., Makhutova, O. N., Rudchenko, A. E., Glushchenko, L. A., Shulepina, S. P., Kolmakova, A. A., & Gladyshev, M. I. (2020). Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes. Biomolecules, 10(3), 419. https://doi.org/10.3390/biom10030419







