Trends in the Recent Patent Literature on Cholinesterase Reactivators (2016–2019)
Abstract
:1. Introduction
2. Cholinesterase Enzymes–AChE and BChE
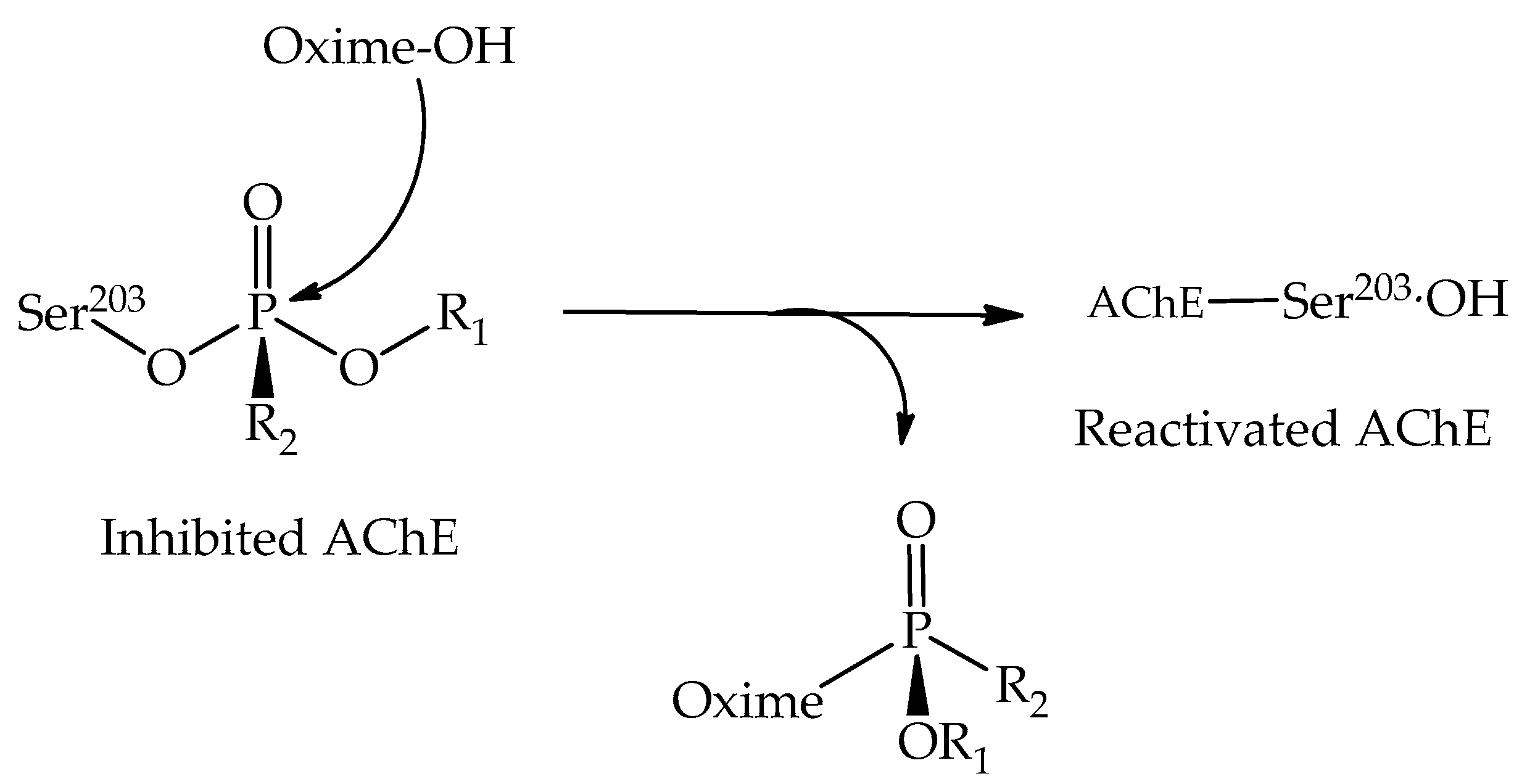
3. AChE Inhibition Processes
4. AChE Reactivation Processes
5. Update of the Recent Patents in Literature from 2016 to 2019
6. Expert Commentary
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ganesan, K.; Raza, S.; Vijayaraghavan, R. Chemical warfare agents. J. Pharm. Bioallied Sci. 2010, 2, 166–178. [Google Scholar] [CrossRef] [PubMed]
- El-Ebiary, A.A.; Elsharkawy, R.E.; Soliman, N.A.; Soliman, M.A.; Hashem, A.A. N -acetylcysteine in Acute Organophosphorus Pesticide Poisoning: A Randomized, Clinical Trial. Basic Clin. Pharmacol. Toxicol. 2016, 119, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, R.M.; Read, R.W. Biological markers of exposure to organophosphorus nerve agents. Arch. Toxicol. 2013, 87, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Weng, Y.B.; Zhen, G.S.; Li, F.J.; Jin, A.C.; Liu, J.; Pany, S. Clinical emergency treatment of 68 critical patients with severe organophosphorus poisoning and prognosis analysis after rescue. Medicine 2017, 96, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Kassa, J.; Korabecny, J.; Nepovimova, E.; Jun, D. The influence of modulators of acetylcholinesterase on the resistance of mice against soman and on the effectiveness of antidotal treatment of soman poisoning in mice. J. Appl. Biomed. 2018, 16, 10–14. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Palikov, V.A.; Palikova, Y.A.; Dyachenko, I.A.; Shamborant, O.G.; Smirnov, I.V.; Masson, P.; Gabibov, A.G. Application of Tetrameric Recombinant Human Butyrylcholinesterase as a Biopharmaceutical for Amelioration of Symptoms of Acute Organophosphate Poisoning. Bull. Exp. Biol. Med. 2017, 163, 430–435. [Google Scholar] [CrossRef]
- Ranjith, K.G.K.; Nagabhushana, S.; Ranganatha, M.; Virupakshappa, K. Clinical Pattern and Outcome of Organophosphorus Compound Poisoning. J. Evol. Med. Dent. Sci. 2016, 5, 3030–3033. [Google Scholar]
- Masson, P.; Nachon, F. Cholinesterase reactivators and bioscavengers for pre- and post-exposure treatments of organophosphorus poisoning. J. Neurochem. 2017, 142, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Srivastava, R.K.; Athar, M. Biological and environmental hazards associated with exposure to chemical warfare agents: Arsenicals. Ann. N. Y. Acad. Sci. 2016, 1378, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Main, M.J.; Cooper, N.J.; Briggs, M.E.; Cooper, A.I.; Adams, D.J. Swellable functional hypercrosslinked polymer networks for the uptake of chemical warfare agents. Polym. Chem. 2017, 8, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.R.; Borges, I.; Figueroa-Villar, J.D.; De Castro, A.T. Defesa química: Histórico, classificação dos agentes de guerra e ação dos neurotóxicos. Quim. Nova 2012, 35, 2083–2091. [Google Scholar] [CrossRef] [Green Version]
- Worek, F.; Thiermann, H.; Szinicz, L.; Eyer, P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem. Pharmacol. 2004, 68, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Thiermann, H.; Worek, F.; Wille, T. Precision cut lung slices as test system for candidate therapeutics in organophosphate poisoning. Toxicology 2017, 389, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Alencar Filho, E.B.; Santos, A.A.; Oliveira, B.G. A quantum chemical study of molecular properties and QSPR modeling of oximes, amidoximes and hydroxamic acids with nucleophilic activity against toxic organophosphorus agents. J. Mol. Struct. 2017, 1133, 338–347. [Google Scholar] [CrossRef]
- Malfatti, M.A.; Enright, H.A.; Be, N.A.; Kuhn, E.A.; Hok, S.; McNerney, M.W.; Lao, V.; Nguyen, T.H.; Lightstone, F.C.; Carpenter, T.S.; et al. The biodistribution and pharmacokinetics of the oxime acetylcholinesterase reactivator RS194B in guinea pigs. Chem. Biol. Interact. 2017, 277, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Kuča, K.; Kassa, J. A comparison of the ability of a new bispyridinium oxime—1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J. Enzym. Inhib. Med. Chem. 2003, 18, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus compounds as chemical warfare agents: A review. Sect. Title Toxicol. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Patrick, G.L.; Spencer, J. An Introduction to Medicinal Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 2009; ISBN 9780199234479. [Google Scholar]
- Lemke, T.L.; David, A. Williams Foye’s Principles of Medicinal Chemistry, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; ISBN 978-0781768795. [Google Scholar]
- Bergmann, F.; Wilson, I.B.; Nachmansohn, D. Acetylcholinesterase. IX. Structural features determining the inhibition by amino acids and related compounds. J. Biol. Chem. 1950, 186, 693–703. [Google Scholar]
- Wilson, I.B.; Bergmann, F. Acetylcholinesterase. VIII. Dissociation constants of the active groups. J. Biol. Chem. 1950, 186, 683–692. [Google Scholar]
- Wilson, I.B.; Bergmann, F.; Nachmansohn, D. Acetylcholinesterase. X. Mechanism of the catalysis of acylation reactions. J. Biol. Chem. 1950, 186, 781–790. [Google Scholar] [PubMed]
- Siegel, G.J. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- De Giacoppo, J.O.S.; De Lima, W.E.A.; Kuca, K.; Da Cunha, E.F.F.; França, T.C.C.; De Ramalho, T.C. Guerra química: Perspectivas no estudo de reativadores da enzima acetilcolinesterase inibida por organofosforados. Rev. Virtual Quim. 2014, 6, 653–670. [Google Scholar]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovarik, Z.; Bosak, A.; Latas, T. Exploring the Active Sites of Cholinesterases by Inhibition with Bambuterol and Haloxon. Croat. Chem. Acta 2003, 76, 63–67. [Google Scholar]
- Saxena, A.; Redman, A.M.G.; Jiang, X.; Lockridge, O.; Doctor, B.P. Differences in Active Site Gorge Dimensions of Cholinesterases Revealed by Binding of Inhibitors to Human Butyrylcholinesterase. Biochemistry 1997, 36, 14642–14651. [Google Scholar] [CrossRef]
- Rosenberry, T.L. Catalysis by acetylcholinesterase: Evidence that the rate-limiting step for acylation with certain substrates precedes general acid-base catalysis. Proc. Natl. Acad. Sci. USA 1975, 72, 3834–3838. [Google Scholar] [CrossRef] [Green Version]
- Lockridge, O. Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. Pharmacol. Ther. 1990, 47, 35–60. [Google Scholar] [CrossRef] [Green Version]
- Pezzementi, L.; Nachon, F.; Chatonnet, A. Evolution of acetylcholinesterase and butyrylcholinesterase in the vertebrates: An atypical butyrylcholinesterase from the medaka oryzias latipes. PLoS ONE 2011, 6, 17396. [Google Scholar] [CrossRef]
- Ventura, A.L.M.; Abreu, P.A.; Freitas, R.C.C.; Sathler, P.C.; Loureiro, N.; Castro, H.C. Colinergic system: Revisiting receptors, regulation and the relationship with Alzheimer disease, schizophrenia, epilepsy and smoking. Rev. Psiquiatr. Clin. 2010, 37, 74–80. [Google Scholar]
- de Castro, A.A.; Prandi, I.G.; Kuca, K.; Ramalho, T.C. Enzimas degradantes de organofosforados: Base molecular e perspectivas para biorremediação enzimática de agroquímicos. Ciência e Agrotecnologia 2017, 41, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.F.; de Castro, A.A.; Soares, F.V.; Soares Leal, D.H.; da Cunha, E.F.F.; Mancini, D.T.; Ramalho, T.C. Development of technologies applied to the biodegradation of warfare nerve agents: Theoretical evidence for asymmetric homogeneous catalysis. Chem. Biol. Interact. 2019, 308, 323–331. [Google Scholar] [CrossRef]
- de Castro, A.A.; Soares, F.V.; Pereira, A.F.; Silva, T.C.; Silva, D.R.; Mancini, D.T.; Caetano, M.S.; da Cunha, E.F.F.; Ramalho, T.C. Asymmetric biodegradation of the nerve agents Sarin and VX by human dUTPase: Chemometrics, molecular docking and hybrid QM/MM calculations. J. Biomol. Struct. Dyn. 2019, 37, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.V.; de Castro, A.A.; Pereira, A.F.; Leal, D.H.S.; Mancini, D.T.; Krejcar, O.; Ramalho, T.C.; da Cunha, E.F.F.; Kuca, K. Theoretical Studies Applied to the Evaluation of the DFPase Bioremediation Potential against Chemical Warfare Agents Intoxication. Int. J. Mol. Sci. 2018, 19, 1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Castro, A.A.; Assis, L.C.; Silva, D.R.; Corrêa, S.; Assis, T.M.; Gajo, G.C.; Soares, F.V.; Ramalho, T.C. Computational enzymology for degradation of chemical warfare agents: Promising technologies for remediation processes. AIMS Microbiol. 2017, 3, 108–135. [Google Scholar] [CrossRef] [PubMed]
- De Giacoppo, J.O.S.; França, T.C.C.; da Cunha, E.F.F.; Abagyan, R.; Mancini, D.T.; Ramalho, T.C. Molecular modeling and in vitro reactivation study between the oxime BI-6 and acetylcholinesterase inhibited by different nerve agents. J. Biomol. Struct. Dyn. 2015, 33, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.L.C.; Ramalho, T.C.; Fiqueroa-Villar, J.D. A Theoretical and Experimental 13C and 15N NMR Investigation of Guanylhydrazones in Solution. Mag. Reson. Chem. 2003, 41, 983–988. [Google Scholar] [CrossRef]
- Ramalho, T.C.; de Castro, A.A.; Silva, D.R.; Silva, M.C.; Franca, T.C.C.; Bennion, B.J.; Kuca, K. Computational Enzymology and Organophosphorus Degrading Enzymes: Promising Approaches Toward Remediation Technologies of Warfare Agents and Pesticides. Curr. Med. Chem. 2016, 23, 1041–1061. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, B.; Singh, N.; Acharya, J.R.; Musilek, K.; Kuca, K.; Ghosh, K. Development and Structural Modifications of Cholinesterase Reactivators against Chemical Warfare Agents in Last Decade: A Review. Mini-Rev. Med. Chem. 2014, 15, 58–72. [Google Scholar] [CrossRef]
- Santos, L.A.; Prandi, I.G.; De Ramalho, T.C. Could Quantum Mechanical Properties Be Reflected on Classical Molecular Dynamics? The Case of Halogenated Organic Compounds of Biological Interest. Front. Chem. 2019, 7, 848. [Google Scholar] [CrossRef] [Green Version]
- Benschop, H.P.; De Jong, L.P.A. Nerve Agent Stereoisomers: Analysis, Isolation, and Toxicology. Acc. Chem. Res. 1988, 21, 368–374. [Google Scholar] [CrossRef]
- Melzer, M.; Chen, J.C.H.; Heidenreich, A.; Gäb, J.; Koller, M.; Kehe, K.; Blum, M.M. Reversed enantioselectivity of diisopropyl fluorophosphatase against organophosphorus nerve agents by rational design. J. Am. Chem. Soc. 2009, 131, 17226–17232. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, P.; Lee, Y.-J.; Kim, B.; Seo, S.S. In silico approaches to evaluate the molecular properties of organophosphate compounds to inhibit acetylcholinesterase activity in housefly. J. Biomol. Struct. Dyn. 2019, 37, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Bhattacharjee, S.; Cannon, J.; Tang, S.; Yang, K.; Bowden, S.; Varnau, V.; O’Konek, J.J.; Choi, S.K. Reactivity and mechanism of α-nucleophile scaffolds as catalytic organophosphate scavengers. Org. Biomol. Chem. 2019, 17, 3951–3963. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.M.; Topczewski, J.J. Compounds and Methods to Treat Organophosphorus Poisoning. U.S. Patent 2016/151342 A1, 2 June 2016. [Google Scholar]
- Da Petronilho, E.C.; Figueroa-Villar, J.D. Agents for defense against chemical warfare: Reactivators of the inhibited acetylcholinesterase with organophosphorus neurotoxic compounds. Rev. Virtual Quim. 2014, 6, 671–686. [Google Scholar] [CrossRef]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef] [PubMed]
- Ordentlich, A.; Barak, D.; Sod-Moriah, G.; Kaplan, D.; Mizrahi, D.; Segall, Y.; Kronman, C.; Karton, Y.; Lazar, A.; Marcus, D.; et al. Stereoselectivity toward VX is determined by interactions with residues of the acyl pocket as well as of the peripheral anionic site of AChE. Biochemistry 2004, 43, 11255–11265. [Google Scholar] [CrossRef]
- Alvim, R.S.; Vaiss, V.S.; Leitão, A.A.; Borges, I. Theoretical chemistry at the service of the chemical defense: Degradation of nerve agents in magnesium oxide and hydroxide surface. Rev. Virtual Quim. 2014, 6, 687–723. [Google Scholar] [CrossRef]
- Cavalcanti, L.P.A.N.; De Aguiar, A.P.; Lima, J.A.; Lima, A.L.S. Organophosphorous poisoning: Treatment and analytical methodologies applied in evaluation of reactivation and inhibition of acetylcholinesterase. Rev. Virtual Quim. 2016, 8, 739–766. [Google Scholar] [CrossRef]
- Zilker, T. Medical management of incidents with chemical warfare agents. Toxicology 2005, 214, 221–231. [Google Scholar] [CrossRef]
- Dichtwald, S.; Weinbroum, A.A. Bioterrorism and the anaesthesiologist’s perspective. Best Pract. Res. Clin. Anaesthesiol. 2008, 22, 477–502. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, I.B. Acetylcholinesterase. XI. Reversibility of tetraethyl pyrophosphate. J. Biol. Chem. 1951, 190, 111–117. [Google Scholar] [PubMed]
- Wilson, I.B.; Ginsburg, S. A powerful reactivator of alkylphosphate-inhibited acetylcholinesterase. Biochim. Biophys. Acta 1955, 18, 168–170. [Google Scholar] [CrossRef]
- Petroianu, G.A. The history of pyridinium oximes as nerve gas antidotes: The British contribution. Pharmazie 2013, 68, 916–918. [Google Scholar]
- Worek, F.; Thiermann, H. The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol. Ther. 2013, 139, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Franjesevic, A.J.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase. Chemistry 2019, 25, 5337–5371. [Google Scholar] [CrossRef]
- Wang, J.; Gu, J.; Leszczynski, J.; Feliks, M.; Sokalski, W.A. Oxime-Induced Reactivation of Sarin-Inhibited AChE: A Theoretical Mechanisms Study. J. Phys. Chem. B 2007, 111, 2404–2408. [Google Scholar] [CrossRef]
- Artursson, E.; Akfur, C.; Hörnberg, A.; Worek, F.; Ekström, F. Reactivation of tabun-hAChE investigated by structurally analogous oximes and mutagenesis. Toxicology 2009, 265, 108–114. [Google Scholar] [CrossRef]
- Kuca, K.; Jun, D.; Musilek, K. Structural Requirements of Acetylcholinesterase Reactivators. Mini-Rev. Med. Chem. 2006, 6, 269–277. [Google Scholar] [CrossRef]
- Lundy, P.M.; Raveh, L.; Amitai, G. Development of the Bisquaternary Oxime HI-6 Toward Clinical Use in the Treatment of Organophosphate Nerve Agent Poisoning. Toxicol. Rev. 2006, 25, 231–243. [Google Scholar] [CrossRef]
- Kitagawa, D.; Cavalcante, S.; de Paula, R.; Rodrigues, R.; Bernardo, L.; da Silva, M.; da Silva, T.; dos Santos, W.; Granjeiro, J.; de Almeida, J.; et al. In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon. Biomolecules 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Kuca, K.; Nepovimova, E.; Wu, Q.; de Souza, F.R.; de Castro Ramalho, T.; Franca, T.C.C.; Musilek, K. Experimental hydrophilic reactivator: Bisoxime with three positive charges. Chem. Pap. 2019, 73, 777–782. [Google Scholar] [CrossRef]
- Polisel, D.A.; de Castro, A.A.; Mancini, D.T.; da Cunha, E.F.F.; França, T.C.C.; Ramalho, T.C.; Kuca, K. Slight difference in the isomeric oximes K206 and K203 makes huge difference for the reactivation of organophosphorus-inhibited AChE: Theoretical and experimental aspects. Chem. Biol. Interact. 2019, 309, 108671. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Holas, O.; Kuca, K.; Jun, D.; Dohnal, V.; Opletalova, V.; Dolezal, M. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J. Enzyme Inhib. Med. Chem. 2008, 23, 70–76. [Google Scholar] [CrossRef]
- Kuca, K.; Musilek, K.; Jun, D.; Zdarova-Karasova, J.; Nepovimova, E.; Soukup, O.; Hrabinova, M.; Mikler, J.; Franca, T.C.C.; Da Cunha, E.F.F.; et al. A newly developed oxime K203 is the most effective reactivator of tabun-inhibited acetylcholinesterase. BMC Pharmacol. Toxicol. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuča, K.; Petroianu, G.A. Oximes as pretreatment before acute exposure to paraoxon. J. Appl. Toxicol. 2019, 39, 1506–1515. [Google Scholar] [CrossRef]
- Jaćević, V.; Nepovimova, E.; Kuča, K. Toxic Injury to Muscle Tissue of Rats Following Acute Oximes Exposure. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jaćević, V.; Nepovimova, E.; Kuča, K. Acute Toxic Injuries of Rat’s Visceral Tissues Induced by Different Oximes. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Katz, F.S.; Pecic, S.; Tran, T.H.; Trakht, I.; Schneider, L.; Zhu, Z.; Ton-That, L.; Luzac, M.; Zlatanic, V.; Damera, S.; et al. Discovery of New Classes of Compounds that Reactivate Acetylcholinesterase Inhibited by Organophosphates. ChemBioChem 2015, 16, 2205–2215. [Google Scholar] [CrossRef]
- de Koning, M.C.; Horn, G.; Worek, F.; van Grol, M. Discovery of a potent non-oxime reactivator of nerve agent inhibited human acetylcholinesterase. Eur. J. Med. Chem. 2018, 157, 151–160. [Google Scholar] [CrossRef]
- Cadieux, C.L.; Wang, H.; Zhang, Y.; Koenig, J.A.; Shih, T.M.; McDonough, J.; Koh, J.; Cerasoli, D. Probing the activity of a non-oxime reactivator for acetylcholinesterase inhibited by organophosphorus nerve agents. Chem. Biol. Interact. 2016, 259, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niessen, K.V.; Seeger, T.; Rappenglück, S.; Wein, T.; Höfner, G.; Wanner, K.T.; Thiermann, H.; Worek, F. In vitro pharmacological characterization of the bispyridinium non-oxime compound MB327 and its 2- and 3-regioisomers. Toxicol. Lett. 2018, 293, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Franjesevic, A.J.; Corrigan, T.S.; Coldren, W.H.; Dicken, R.; Sillart, S.; DeYong, A.; Yoshino, N.; Smith, J.; Fabry, S.; et al. Demonstration of In Vitro Resurrection of Aged Acetylcholinesterase after Exposure to Organophosphorus Chemical Nerve Agents. J. Med. Chem. 2018, 61, 7034–7042. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Malinak, D.; Nepovimova, E.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. SAR study to find optimal cholinesterase reactivator against organophosphorous nerve agents and pesticides. Arch. Toxicol. 2016, 90, 2831–2859. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Thiermann, H.; Wille, T. Oximes in organophosphate poisoning: 60 years of hope and despair. Chem. Biol. Interact. 2016, 259, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kovalevsky, A.; Blumenthal, D.K.; Cheng, X.; Taylor, P.; Radić, Z. Limitations in current acetylcholinesterase structure–based design of oxime antidotes for organophosphate poisoning. Ann. N. Y. Acad. Sci. 2016, 1378, 41–49. [Google Scholar] [CrossRef]
- Carletti, E.; Colletier, J.-P.; Dupeux, F.; Trovaslet, M.; Masson, P.; Nachon, F. Structural Evidence That Human Acetylcholinesterase Inhibited by Tabun Ages through O-Dealkylation. J. Med. Chem. 2010, 53, 4002–4008. [Google Scholar] [CrossRef]
- Kalisiak, J.; Ralph, E.C.; Zhang, J.; Cashman, J.R. Amidine−Oximes: Reactivators for Organophosphate Exposure. J. Med. Chem. 2011, 54, 3319–3330. [Google Scholar] [CrossRef]
- Quinn, M.D.; Topczewski, J.; Yasapala, N.; Lodge, A. Why is Aged Acetylcholinesterase So Difficult to Reactivate? Molecules 2017, 22, 1464. [Google Scholar] [CrossRef] [Green Version]
- Chambers, J.E.; Chambers, H.W.; Meek, E.C. Novel Oximes for Reactivating Butyrylcholinesterase. U.S. Patent 2017/0258774 A1, 14 September 2017. [Google Scholar]
- Chambers, J.E.; Meek, E.C.; Bennett, J.P.; Bennett, W.S.; Chambers, H.W.; Leach, C.A.; Pringle, R.B.; Wills, R.W. Novel substituted phenoxyalkyl pyridinium oximes enhance survival and attenuate seizure-like behavior of rats receiving lethal levels of nerve agent surrogates. Toxicology 2016, 339, 51–57. [Google Scholar] [CrossRef]
- Kovarik, Z.; Ciban, N.; Radić, Z.; Simeon-Rudolf, V.; Taylor, P. Active site mutant acetylcholinesterase interactions with 2-PAM, HI-6, and DDVP. Biochem. Biophys. Res. Commun. 2006, 342, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Musilova, L.; Kuca, K.; Jung, Y.-S.; Jun, D. In vitro oxime-assisted reactivation of paraoxon-inhibited human acetylcholinesterase and butyrylcholinesterase. Clin. Toxicol. 2009, 47, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.; Musilova, L.; Pohanka, M.; Jung, Y.-S.; Bostik, P.; Kuca, K. Reactivation of Human Acetylcholinesterase and Butyrylcholinesterase Inhibited by Leptophos-Oxon with Different Oxime Reactivators in Vitro. Int. J. Mol. Sci. 2010, 11, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.; Musilova, L.; Musilek, K.; Kuca, K. In Vitro Ability of Currently Available Oximes to Reactivate Organophosphate Pesticide-Inhibited Human Acetylcholinesterase and Butyrylcholinesterase. Int. J. Mol. Sci. 2011, 12, 2077–2087. [Google Scholar] [CrossRef] [Green Version]
- Valdez, C.A.; Be, N.A.; Alfatti, M.A.; Enright, H.A.; Bennion, B.J.; Carpenter, T.S.; Hok, S.; lao, H.L.; Nguyen, T.H. Compounds for Central Reactivation of Organophosphorus-Based Compound-Inhibited Acetylcolinesterase and/or Inactivation of Organophosphorus-Based Acetylcholinesterase Inhibitors and Related Compositions Methods and Systems for Making and Using Them. U.S. Patent 2019/0152920 A1, 23 May 2019. [Google Scholar]
- Batool, A.; Kamal, M.A.; Rizvi, S.M.D.; Rashid, S. Topical Discoveries on Multi-Target Approach to Manage Alzheimer’s Disease. Curr. Drug Metab. 2018, 19, 704–713. [Google Scholar] [CrossRef]
- Du, W.-J.; Guo, J.-J.; Gao, M.-T.; Hu, S.-Q.; Dong, X.-Y.; Han, Y.-F.; Liu, F.-F.; Jiang, S.; Sun, Y. Brazilin inhibits amyloid β-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity. Sci. Rep. 2015, 5, 7992. [Google Scholar] [CrossRef] [Green Version]
- Khavrutskii, I.; Wallqvist, A. Compositions and Methods for Reactivating Cholinesterases. WO Patent 2017/218886 A1, 21 December 2017. [Google Scholar]
- Hardman, J.G.; Limbird, L.E.; Gilman, A.G. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 10th ed.; McGraw-Hill: New York, NY, USA, 2001; ISBN 0-07-135469-7. [Google Scholar]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Nepovimova, E.; Malinak, D.; Kucera, T.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. Progress in acetylcholinesterase reactivators and in the treatment of organophosphorus intoxication: A patent review (2006–2016). Expert Opin. Ther. Pat. 2017, 27, 971–985. [Google Scholar] [CrossRef]



















| Time, Hours | |||
|---|---|---|---|
| 1.00 | 2.45 (± 0.7) | 1.16 (± 0.06) | 1.28 (± 0.8) |
| 4.00 | 5.67 (± 0.6) | 1.16 (± 0.06) | 4.50 (± 0.6) |
| 12.00 | 6.46 (± 1.1) | 1.16 (± 0.06) | 5.30 (± 1.1) |
| 24.00 | 8.70 (± 1.3) | 0.00 (± 0.06) | 8.70 (± 1.3) |
| 48.00 | 7.03 (± 0.8) | 0.00 (± 0.06) | 7.03 (± 0.8) |
| BChE Reactivation Tested Oxime Molecules | ||
|---|---|---|
| Tested Phenoxyalkyl Pyridinium Oxime Molecule | Alkyl Linker Chain Length (n) | Phenoxy Substitute Moiety (R) |
| Oxime 12 (OX12) | 5 | 4-CH3 –O– |
| Oxime 14 (OX14) | 4 | 4-Cl– |
| Oxime 28 (OX28) | 4 | 4-CH3CH2C(:O)– |
| Oxime 31 (OX31) | 3 | 3-CH=CHCH=CH-4 |
| Oxime 32 (OX32) | 4 | 3-CH=CHCH=CH-4 |
| Oxime 59 (OX59) | 4 | 4-Ph–CH2–O– |
| Oxime 98 (OX98) | 4 | 4-(CH3)3CCH2C(CH3)2 – |
| Oxime 99 (OX99) | 5 | 4-(CH3)3CCH2C(CH3)2 – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Castro, A.A.; Assis, L.C.; Soares, F.V.; Kuca, K.; Polisel, D.A.; da Cunha, E.F.F.; Ramalho, T.C. Trends in the Recent Patent Literature on Cholinesterase Reactivators (2016–2019). Biomolecules 2020, 10, 436. https://doi.org/10.3390/biom10030436
de Castro AA, Assis LC, Soares FV, Kuca K, Polisel DA, da Cunha EFF, Ramalho TC. Trends in the Recent Patent Literature on Cholinesterase Reactivators (2016–2019). Biomolecules. 2020; 10(3):436. https://doi.org/10.3390/biom10030436
Chicago/Turabian Stylede Castro, Alexandre A., Letícia C. Assis, Flávia V. Soares, Kamil Kuca, Daniel A. Polisel, Elaine F. F. da Cunha, and Teodorico C. Ramalho. 2020. "Trends in the Recent Patent Literature on Cholinesterase Reactivators (2016–2019)" Biomolecules 10, no. 3: 436. https://doi.org/10.3390/biom10030436
APA Stylede Castro, A. A., Assis, L. C., Soares, F. V., Kuca, K., Polisel, D. A., da Cunha, E. F. F., & Ramalho, T. C. (2020). Trends in the Recent Patent Literature on Cholinesterase Reactivators (2016–2019). Biomolecules, 10(3), 436. https://doi.org/10.3390/biom10030436







