Fluorogenic and Bioorthogonal Modification of RNA Using Photoclick Chemistry
Abstract
1. Introduction
2. Materials and Methods
General
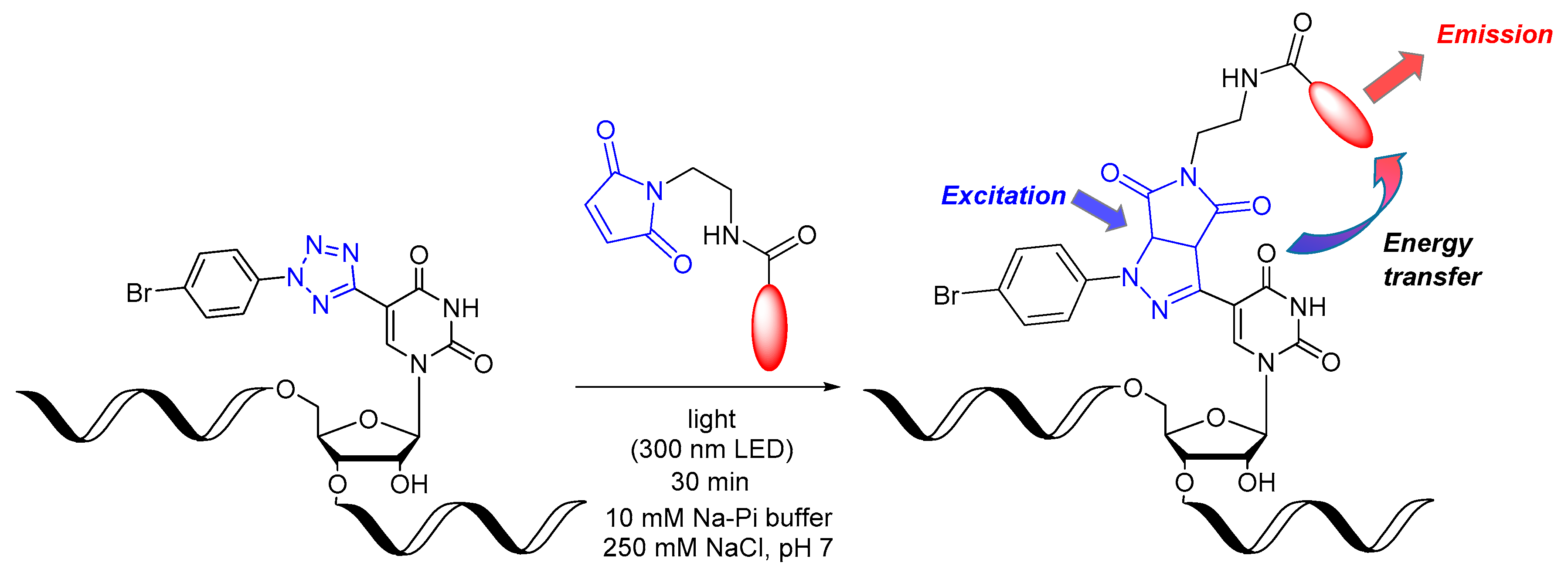
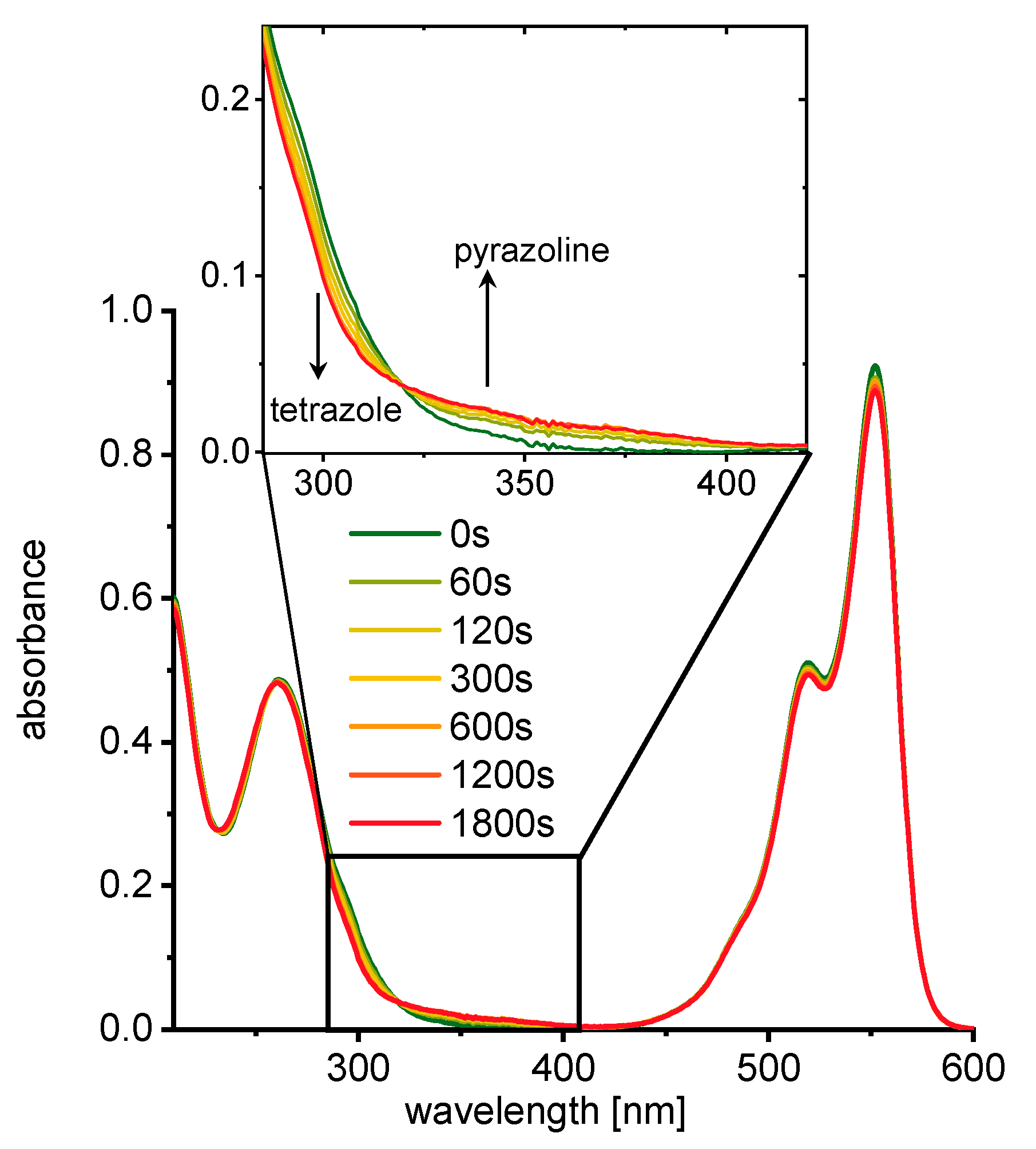
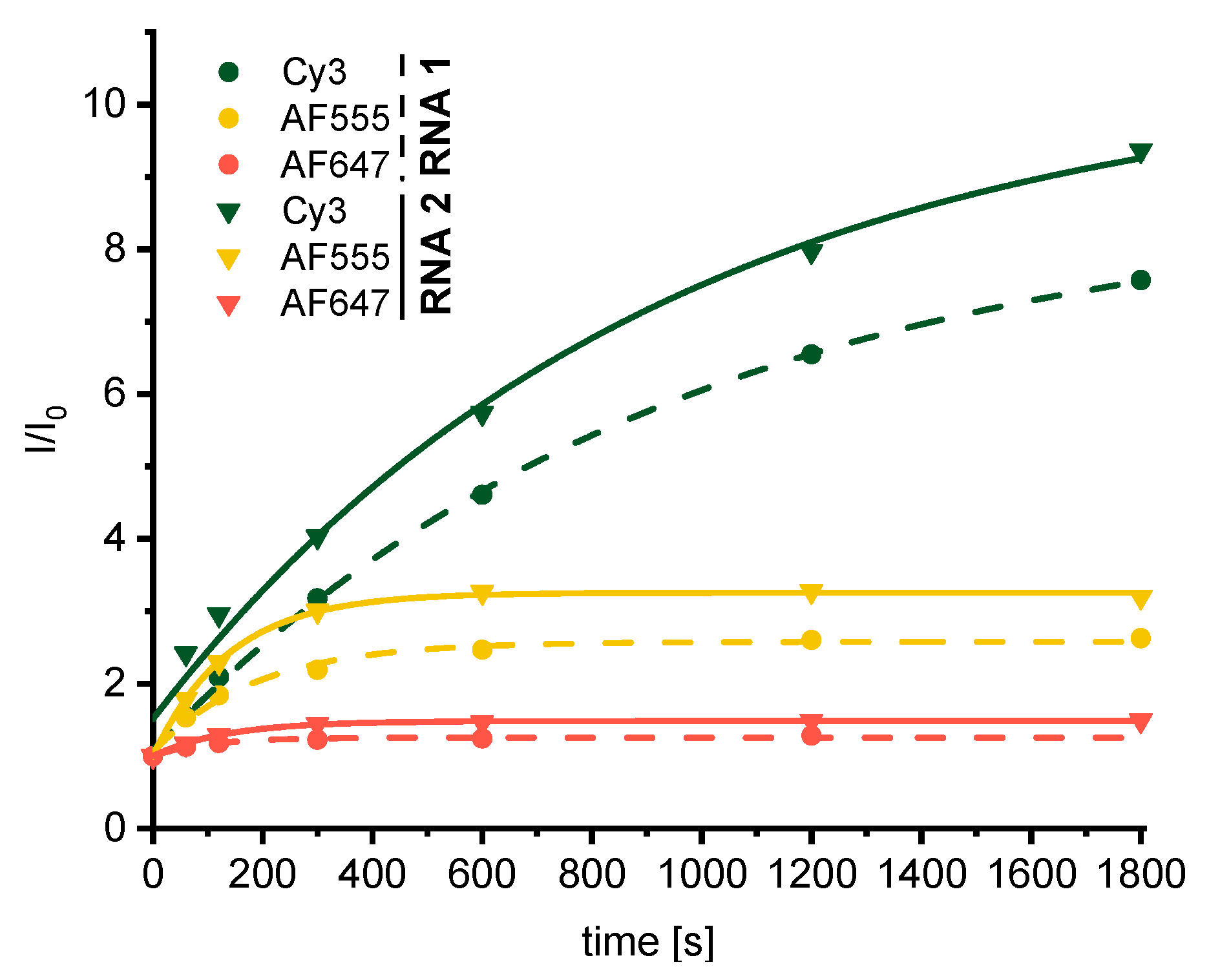
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Row, R.D.; Prescher, J.A. Constructing New Bioorthogonal Reagents and Reactions. Accounts Chem. Res. 2018, 51, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.; Bertozzi, C.R. Design strategies for bioorthogonal smart probes. Org. Biomol. Chem. 2014, 12, 9307–9320. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K. The Future of Bioorthogonal Chemistry. ACS Central Sci. 2018, 4, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as Copper(I)-Stabilizing Ligands in Catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef]
- Hong, V.; Steinmetz, N.F.; Manchester, M.; Finn, M.G. Labeling Live Cells by Copper-Catalyzed Alkyne−Azide Click Chemistry. Bioconjugate Chem. 2010, 21, 1912–1916. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Chen, P.R. Transition metal-mediated bioorthogonal protein chemistry in living cells. Chem. Soc. Rev. 2014, 43, 6511–6526. [Google Scholar] [CrossRef]
- Kennedy, D.; McKay, C.S.; Legault, M.C.B.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular Consequences of Copper Complexes Used To Catalyze Bioorthogonal Click Reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Weissleder, R.; Hilderbrand, S.A. Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjugate Chem. 2008, 19, 2297–2299. [Google Scholar] [CrossRef]
- Lang, K.; Mayer, S. Tetrazines in Inverse-Electron-Demand Diels–Alder Cycloadditions and Their Use in Biology. Synthesis 2016, 49, 830–848. [Google Scholar] [CrossRef]
- Kamber, D.N.; Liang, Y.; Blizzard, R.J.; Liu, F.; Mehl, R.A.; Houk, K.N.; Prescher, J.A. 1,2,4-Triazines Are Versatile Bioorthogonal Reagents. J. Am. Chem. Soc. 2015, 137, 8388–8391. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Chin, J.W. Bioorthogonal Reactions for Labeling Proteins. ACS Chem. Biol. 2014, 9, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef]
- Lim, R.K.V.; Lin, Q. Photoinducible Bioorthogonal Chemistry: A Spatiotemporally Controllable Tool to Visualize and Perturb Proteins in Live Cells. Accounts Chem. Res. 2011, 44, 828–839. [Google Scholar] [CrossRef]
- Nadler, A.; Schultz, C. The Power of Fluorogenic Probes. Angew. Chem. Int. Ed. 2013, 52, 2408–2410. [Google Scholar] [CrossRef]
- Wu, H.; Devaraj, N.K. Advances in Tetrazine Bioorthogonal Chemistry Driven by the Synthesis of Novel Tetrazines and Dienophiles. Accounts Chem. Res. 2018, 51, 1249–1259. [Google Scholar] [CrossRef]
- Wieczorek, A.; Werther, P.; Euchner, J.; Wombacher, R. Green- to far-red-emitting fluorogenic tetrazine probes - synthetic access and no-wash protein imaging inside living cells. Chem. Sci. 2017, 8, 1506–1510. [Google Scholar] [CrossRef]
- Lehmann, B.; Wagenknecht, H.-A. Fluorogenic “photoclick”-type labelling of DNA using a Cy3 dye. Org. Biomol. Chem. 2018, 16, 7579–7582. [Google Scholar] [CrossRef]
- Hudson, R.H.; Wojciechowski, F. The detrimental effect of orotic acid substitution in the peptide nucleic acid strand on the stability of PNA2:NA triple helices. Can. J. Chem. 2005, 83, 1731–1740. [Google Scholar] [CrossRef]
- Clovis, J.S.; Eckell, A.; Huisgen, R.; Sustmann, R. 1.3-Dipolare Cycloadditionen, XXV. Der Nachweis des freien Diphenylnitrilimins als Zwischenstufe bei Cycloadditionen. Eur. J. Inorg. Chem. 1967, 100, 60–70. [Google Scholar] [CrossRef]
- Blasco, E.; Sugawara, Y.; Lederhose, P.; Blinco, J.P.; Kelterer, A.-M.; Barner-Kowollik, C. Understanding Reactivity Patterns in Light-Induced Nitrile Imine Mediated Tetrazole-Ene Cycloadditions. ChemPhotoChem 2017, 1, 159–163. [Google Scholar] [CrossRef]
- Vorbrüggen, H.; Krolikiewicz, K. Neue Katalysatoren für die Nucleosidsynthese. Angew. Chem. 1975, 87, 417. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Kakehi, A.; Tanaka, Y.; Ito, S.; Kondo, K. A Facile Synthesis of 2,5-Disubstituted Tetrazoles by the Reaction of Phenylsulfonylhydrazones with Arenediazonium Salts. Bull. Chem. Soc. Jpn. 1976, 49, 1920–1923. [Google Scholar]
- Hakimelahi, G.H.; Proba, Z.A.; Ogilvie, K.K. New catalysts and procedures for the dimethoxytritylation and selective silylation of ribonucleosides. Can. J. Chem. 1982, 60, 1106–1113. [Google Scholar] [CrossRef]
- Tridgett, M.; Moore-Kelly, C.; Duprey, J.-L.H.A.; Iturbe, L.O.; Tsang, C.W.; Little, H.A.; Sandhu, S.K.; Hicks, M.R.; Dafforn, T.R.; Rodger, A. Linear dichroism of visible-region chromophores using M13 bacteriophage as an alignment scaffold. RSC Adv. 2018, 8, 29535–29543. [Google Scholar] [CrossRef]

| Dye Adduct of | RNA1 | RNA2 |
|---|---|---|
| Cy3 | 27% | 31% (70%) a |
| AF555 | 78% | 84% |
| AF647 | 48% | 48% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krell, K.; Wagenknecht, H.-A. Fluorogenic and Bioorthogonal Modification of RNA Using Photoclick Chemistry. Biomolecules 2020, 10, 480. https://doi.org/10.3390/biom10030480
Krell K, Wagenknecht H-A. Fluorogenic and Bioorthogonal Modification of RNA Using Photoclick Chemistry. Biomolecules. 2020; 10(3):480. https://doi.org/10.3390/biom10030480
Chicago/Turabian StyleKrell, Katja, and Hans-Achim Wagenknecht. 2020. "Fluorogenic and Bioorthogonal Modification of RNA Using Photoclick Chemistry" Biomolecules 10, no. 3: 480. https://doi.org/10.3390/biom10030480
APA StyleKrell, K., & Wagenknecht, H.-A. (2020). Fluorogenic and Bioorthogonal Modification of RNA Using Photoclick Chemistry. Biomolecules, 10(3), 480. https://doi.org/10.3390/biom10030480





