Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
2.2. X-ray Crystallography
2.2.1. Crystallization
2.2.2. Data Collection
2.2.3. Data Processing
2.3. CA Inhibition Studies
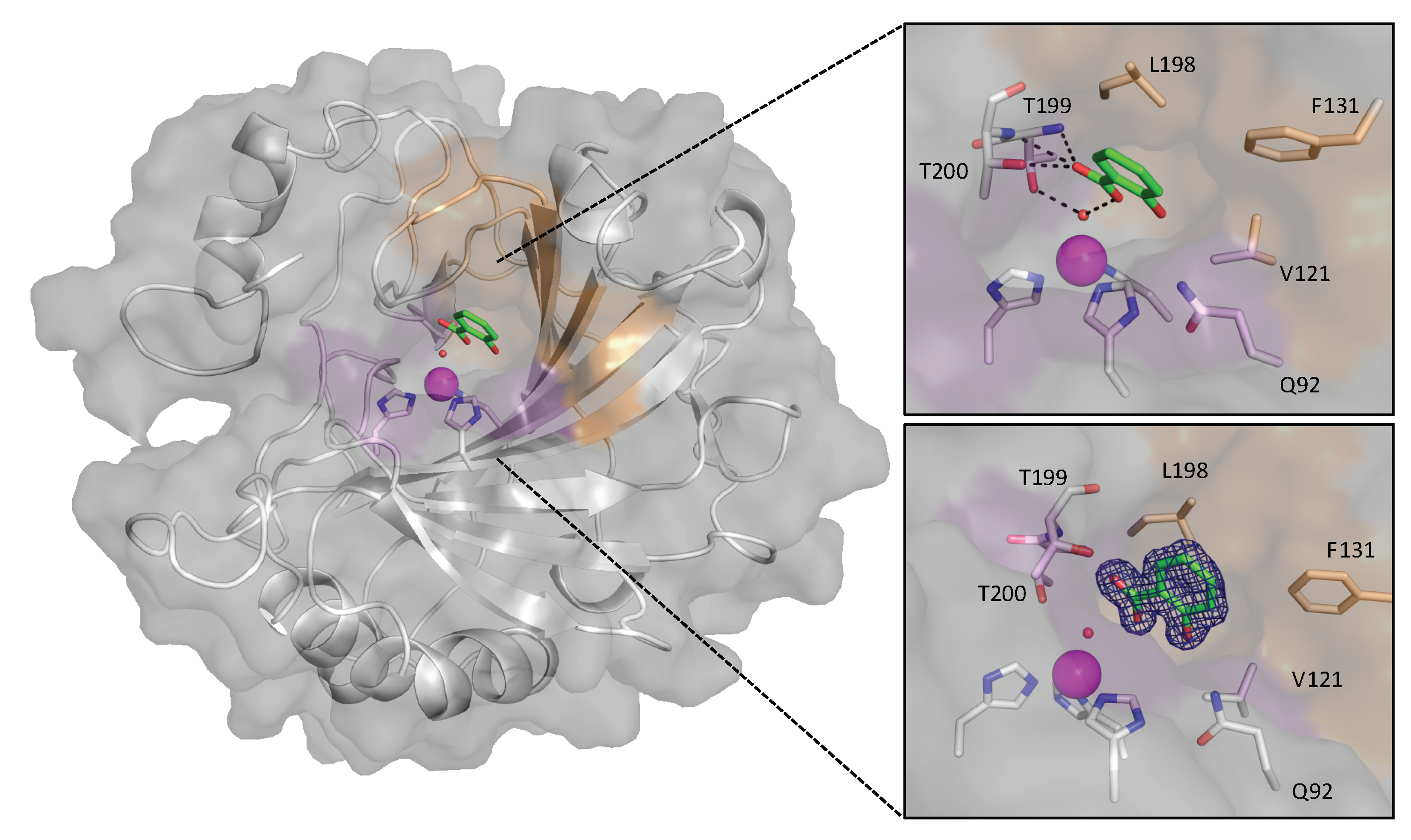
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase I. stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar]
- Lomelino, C.L.; Andring, J.T.; McKenna, R. Structural insights into the catalytic mechanism of carbonic anhydrase. In Carbonic Anhydrases: Biochemistry, Mechanism of Action and Therapeutic Applications; Nova Science: Hauppauge, NY, USA, 2018. [Google Scholar]
- Eriksson, A.E.; Jones, T.A.; Liljas, A. Refined structure of human carbonic anhydrase II at 2.0 A resolution. Proteins 1988, 4, 274–282. [Google Scholar] [CrossRef]
- Frost, S.C. Physiological functions of the alpha class of carbonic anhydrases. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Frost, S.C., McKenna, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 9–30. [Google Scholar] [CrossRef]
- Steiner, H.; Jonsson, B.-H.; Lindskog, S. The catalytic mechanism of carbonic anhydrase. Eur. J. Biochem. 1975, 59, 253–259. [Google Scholar] [CrossRef]
- Brown, D.; Kumpulainen, T.; Roth, J.; Orci, L. Immunohistochemical localization of carbonic anhydrase in postnatal and adult rat kidney. Am. J. Physiol. 1983, 245, F110–F118. [Google Scholar] [CrossRef]
- Moini, M.; Demars, S.M.; Huang, H. Analysis of carbonic anhydrase in human red blood cells using capillary electrophoresis/electrospray ionization-mass spectrometry. Anal. Chem. 2002, 74, 3772–3776. [Google Scholar] [CrossRef]
- Jakubowski, M.; Szahidewicz-Krupska, E.; Doroszko, A. The human carbonic anhydrase II in platelets: An underestimated field of its activity. BioMed Res. Int. 2018, 2018, 4548353. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, J. Carbonic Anhydrases: Biochemistry, Mechanism of Action and Therapeutic Applications; Nova Science Publisher: Hauppauge, NY, USA, 2018. [Google Scholar]
- Bozdag, M.; Carta, F.; Ceruso, M.; Ferraroni, M.; McDonald, P.C.; Dedhar, S.; Supuran, C.T. Discovery of 4-Hydroxy-3-(3-(Phenylureido)Benzenesulfonamides as SLC-0111 analogues for the treatment of hypoxic tumors overexpressing carbonic anhydrase IX. J. Med. Chem. 2018, 61, 6328–6338. [Google Scholar] [CrossRef]
- Andreucci, E.; Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Laurenzana, A.; Carta, F.; Supuran, C.T.; Calorini, L. The carbonic anhydrase IX inhibitor SLC-0111 sensitises cancer cells to conventional chemotherapy. J. Enzyme Inhib. Med. Chem. 2019, 34, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Safety Study of SLC-0111 in Subjects with Advanced Solid Tumours-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02215850 (accessed on 1 November 2019).
- Scozzafava, A.; Supuran, C.T. Glaucoma and the applications of carbonic anhydrase inhibitors. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; pp. 349–359. [Google Scholar] [CrossRef]
- Nocentini, A.; Ceruso, M.; Bua, S.; Lomelino, C.L.; Andring, J.T.; McKenna, R.; Lanzi, C.; Sgambellone, S.; Pecori, R.; Matucci, R.; et al. Discovery of β-Adrenergic receptors blocker-carbonic anhydrase inhibi-tor hybrids for multitargeted antiglaucoma therapy. J. Med. Chem. 2018, 61, 5380–5394. [Google Scholar] [CrossRef] [PubMed]
- Lomelino, C.L.; Supuran, C.T.; McKenna, R. Non-classical inhibition of carbonic anhydrase. Int. J. Mol. Sci. 2016, 17, 1150. [Google Scholar] [CrossRef] [PubMed]
- Lomelino, C.L.; McKenna, R. Carbonic anhydrase II in complex with carboxylic acid-based inhibitors. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2019, 75, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Cau, Y.; Vullo, D.; Mori, M.; Dreassi, E.; Supuran, C.T.; Botta, M. Potent and selective carboxylic acid inhibitors of tumor-associated carbonic anhydrases IX and XII. Molecules 2017, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of mammalian isoforms I–XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg. Med. Chem. 2008, 16, 7424–7428. [Google Scholar] [CrossRef] [PubMed]
- Atallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for prevention of preeclampsia. Drugs 2017, 77, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Cao, Y.; Chan, A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Rev. Cancer 2016, 16, 173–186. [Google Scholar] [CrossRef]
- Ma, J.; Cai, Z.; Wei, H.; Liu, X.; Zhao, Q.; Zhang, T. The anti-tumor effect of aspirin: What we know and what we expect. Biomed. Pharmacother. 2017, 95, 656–661. [Google Scholar] [CrossRef]
- Kyle, M.E.; Wang, J.C.; Shin, J.J. Ubiquitous aspirin: A systematic review of its impact on sensorineural hearing loss. Otolaryngol.-Head Neck Surg. 2015, 152, 23–41. [Google Scholar] [CrossRef]
- Rowland, M.; Riegelman, S.; Harris, P.A.; Sholkoff, S.D. Absorption kinetics of aspirin in man follow oral administration of an aqueous solution. J. Pharm. Sci. 1972, 61, 379–385. [Google Scholar] [CrossRef]
- Jakubowski, M.; Dębski, J.; Szahidewicz-Krupska, E.; Turek-Jakubowska, A.; Gawryś, J.; Gawryś, K.; Skomro, R.; Derkacz, A.; Doroszko, A. Platelet carbonic anhydrase II, a forgot-ten enzyme, may be responsible for aspirin resistance. Oxidative Med. Cell. Longev. 2017, 2017, 3132063. [Google Scholar] [CrossRef]
- Avvaru, B.S.; Kim, C.U.; Sippel, K.H.; Gruner, S.M.; Agbandje-McKenna, M.; Silverman, D.N.; McKenna, R. A short, strong hydrogen bond in the active site of human carbonic an-hydrase II. Biochemistry 2010, 49, 249–251. [Google Scholar] [CrossRef]
- Tanhauser, S.M.; Jewell, D.A.; Tu, C.K.; Silverman, D.N.; Laipis, P.J. A T7 Expression vector optimized for site-directed mutagenesis using oligodeoxyribonucleotide cassettes. Gene 1992, 117, 113–117. [Google Scholar] [CrossRef]
- Pinard, M.A.; Boone, C.D.; Rife, B.D.; Supuran, C.T.; McKenna, R. Structural study of interaction between brinzolamide and dorzolamide inhibition of human carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 7210–7215. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Torres, N.A.; Mahon, B.P.; Boone, C.D.; Pinard, M.A.; Tu, C.; Ng, R.; Agbandje-McKenna, M.; Silverman, D.; Scott, K.; McKenna, R. Structural and biophysical characterization of the α-carbonic anhydrase from the gammaproteobacterium thiomicrospira crunogena XCL-2: Insights into engineering thermostable enzymes for CO2 sequestration. Acta Crystallogr. D Biol. Crystallogr. 2015, 71 Pt 8, 1745–1756. [Google Scholar] [CrossRef]
- Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL; Schrödinger, LLC: New York, NY, USA, 2002. [Google Scholar]
- Tashian, R.E.; Douglas, D.P.; Yu, Y.-S.L. Esterase and hydrase activity of carbonic an-hydrase-I from primate erythrocytes. Biochem. Biophys. Res. Commun. 1964, 14, 256–261. [Google Scholar] [CrossRef]
- Domsic, J.F.; Avvaru, B.S.; Kim, C.U.; Gruner, S.M.; Agbandje-McKenna, M.; Silverman, D.N.; McKenna, R. Entrapment of carbon dioxide in the active site of carbonic anhydrase II. J. Biol. Chem. 2008, 283, 30766–30771. [Google Scholar] [CrossRef] [PubMed]
- Uda, N.R.; Seibert, V.; Stenner-Liewen, F.; Müller, P.; Herzig, P.; Gondi, G.; Zeidler, R.; van Dijk, M.; Zippelius, A.; Renner, C. Esterase activity of carbonic anhydrases serves as surrogate for selecting antibodies blocking hydratase activity. J. Enzyme Inhib. Med. Chem. 2015, 30, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Bua, S.; Lomelino, C.; Murray, A.B.; Osman, S.M.; ALOthman, Z.A.; Bozdag, M.; Abdel-Aziz, H.A.; Eldehna, W.M.; McKenna, R.; Nocentini, A.; et al. “A sweet combination”: Developing saccharin and acesulfame k structures for selectively targeting the tumor-associated carbonic anhydrases IX and XII. J. Med. Chem. 2020, 63, 321–333. [Google Scholar] [CrossRef]
- Non-linear Regression, GraphPad Prism Version 8.0.0 for Mac, GraphPad Software, San Diego, CA, USA. Available online: www.graphpad.com (accessed on 15 December 2019).
- Hakansson, K.; Briand, C.; Zaitsev, V.; Xue, Y.; Liljas, A. Wild-type and E106Q mutant carbonic anhydrase complexed with acetate. Acta Crystallogr. Sect. D 1994, 50, 101–104. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andring, J.; Combs, J.; McKenna, R. Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II. Biomolecules 2020, 10, 527. https://doi.org/10.3390/biom10040527
Andring J, Combs J, McKenna R. Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II. Biomolecules. 2020; 10(4):527. https://doi.org/10.3390/biom10040527
Chicago/Turabian StyleAndring, Jacob, Jacob Combs, and Robert McKenna. 2020. "Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II" Biomolecules 10, no. 4: 527. https://doi.org/10.3390/biom10040527
APA StyleAndring, J., Combs, J., & McKenna, R. (2020). Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II. Biomolecules, 10(4), 527. https://doi.org/10.3390/biom10040527




