Development of a Monoclonal Antibody Specific to the IgM Heavy Chain of Bighead Catfish (Clarias macrocephalus): A Biomolecular Tool for the Detection and Quantification of IgM Molecules and IgM+ Cells in Clarias Catfish
Abstract
:1. Introduction
2. Results
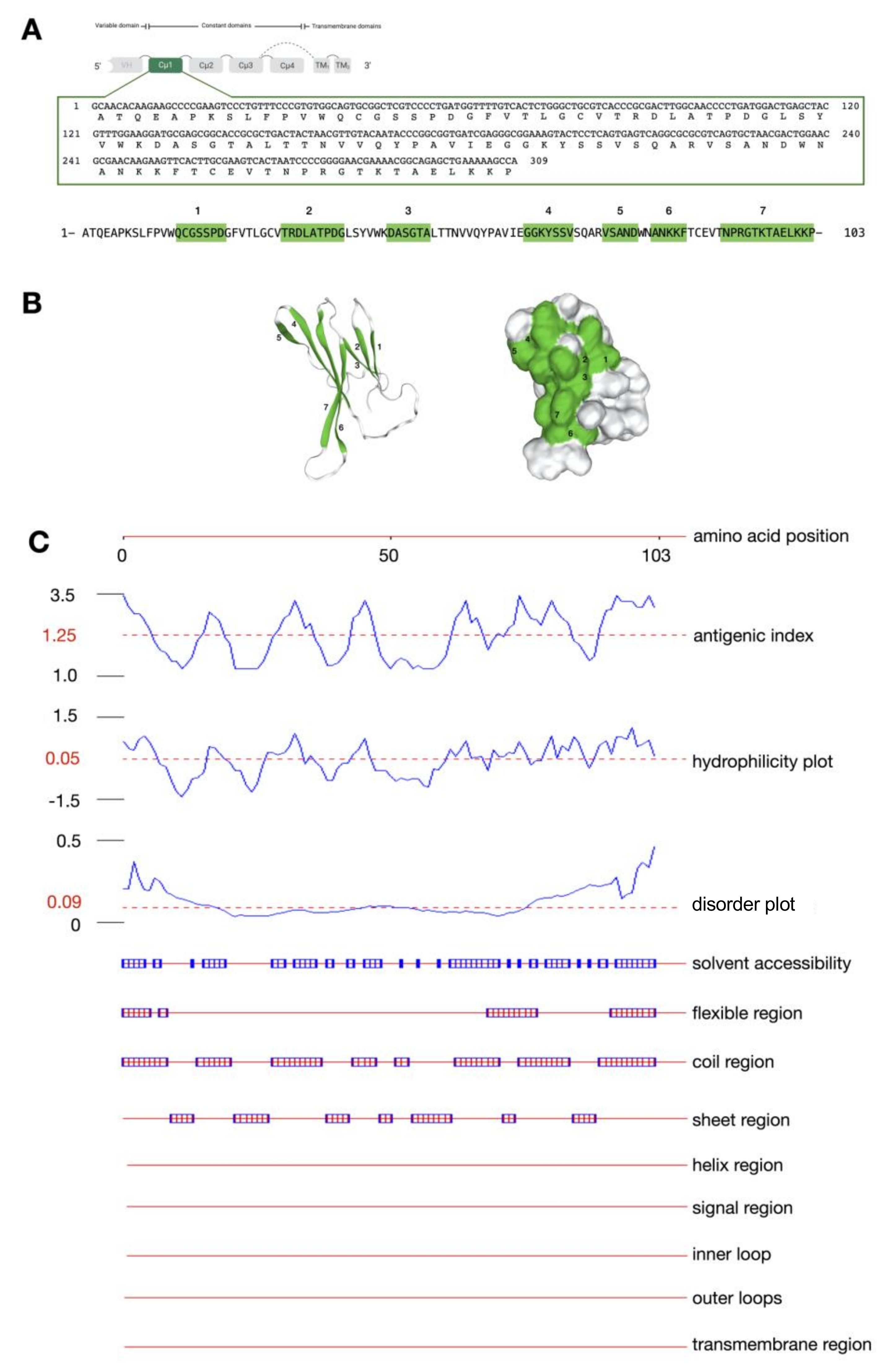
2.1. Molecular Cloning, Characterization, and Antigenicity Analysis of the IgMHCμ1 Sequence of Bighead Catfish
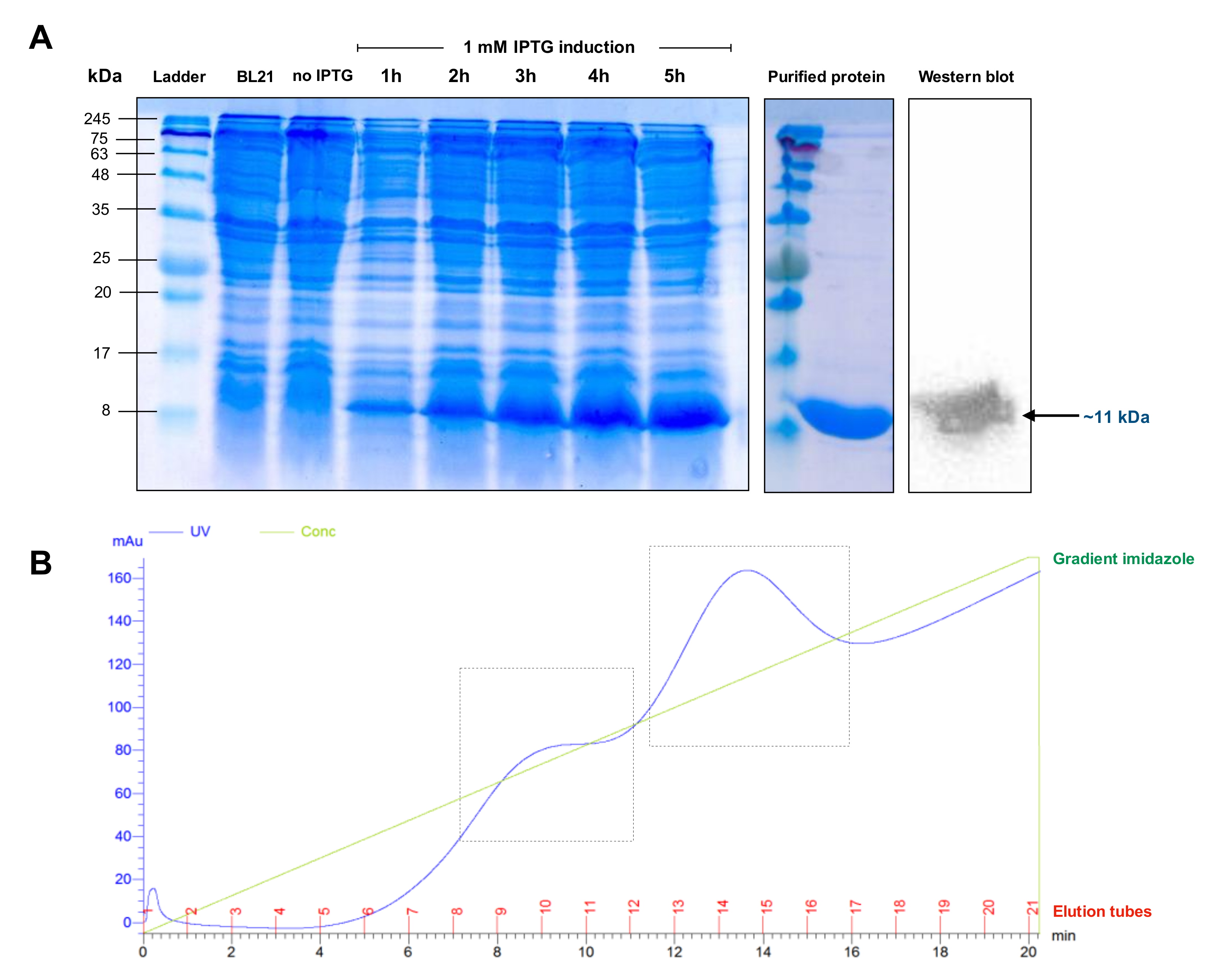
2.2. Expression of the IgMHCμ1 Recombinant Protein
2.3. Production of the IgMHCμ1 mAb against IgMH in Catfish
2.4. Characterization and Application of the IgMHCμ1 mAb against IgMH in Catfish
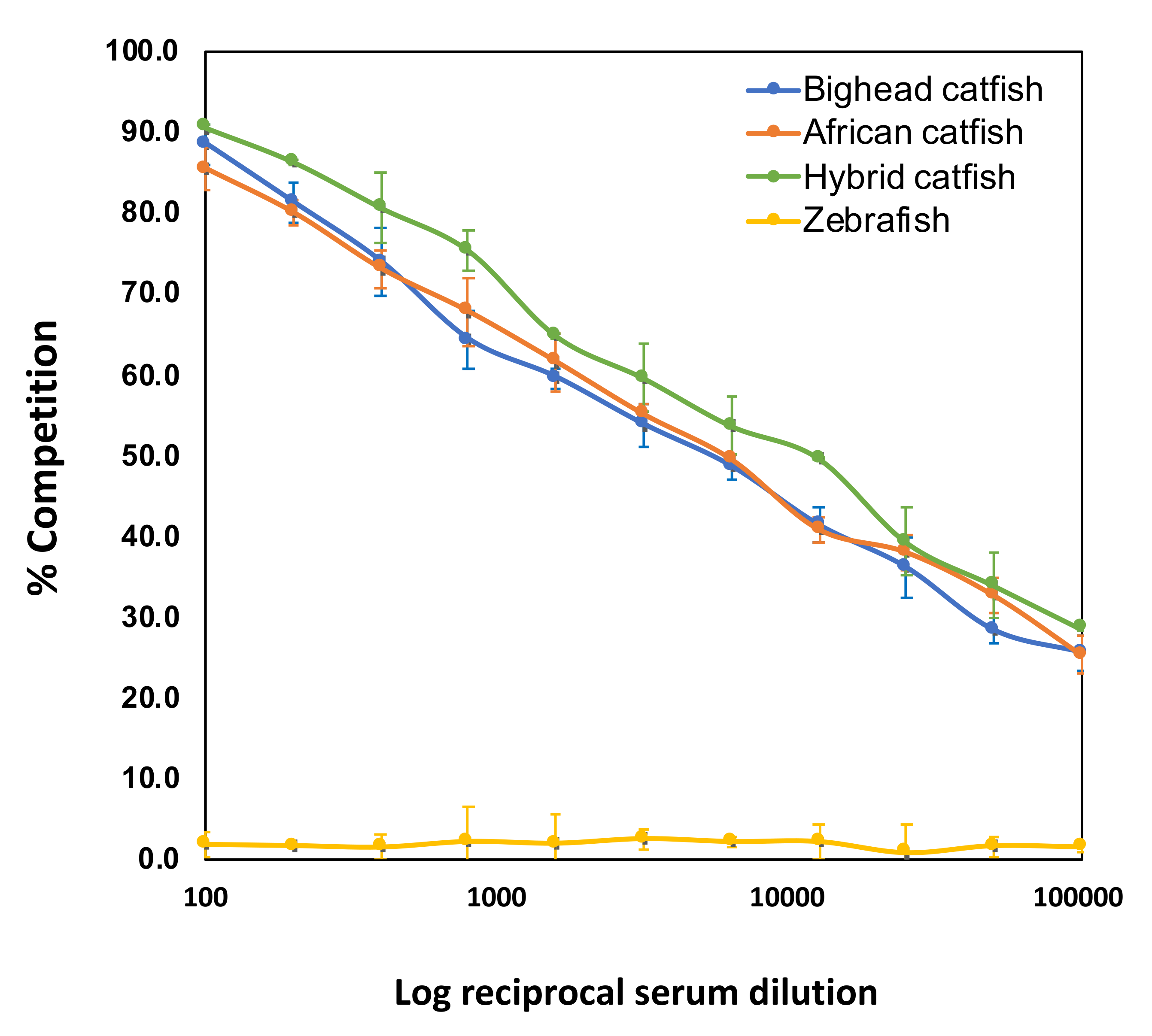
2.4.1. Establishment and c-ELISA Analysis
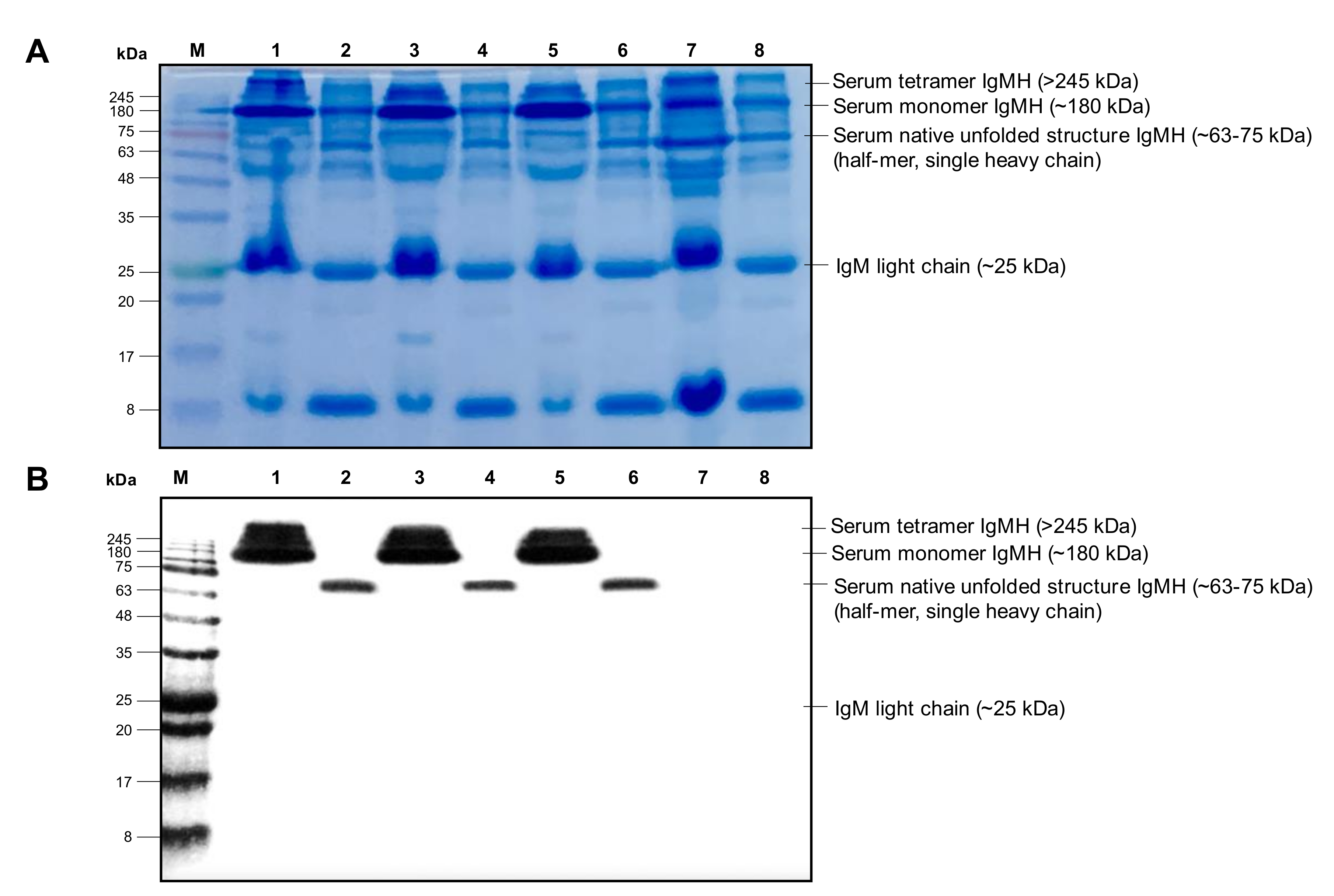
2.4.2. Western Blot Analysis
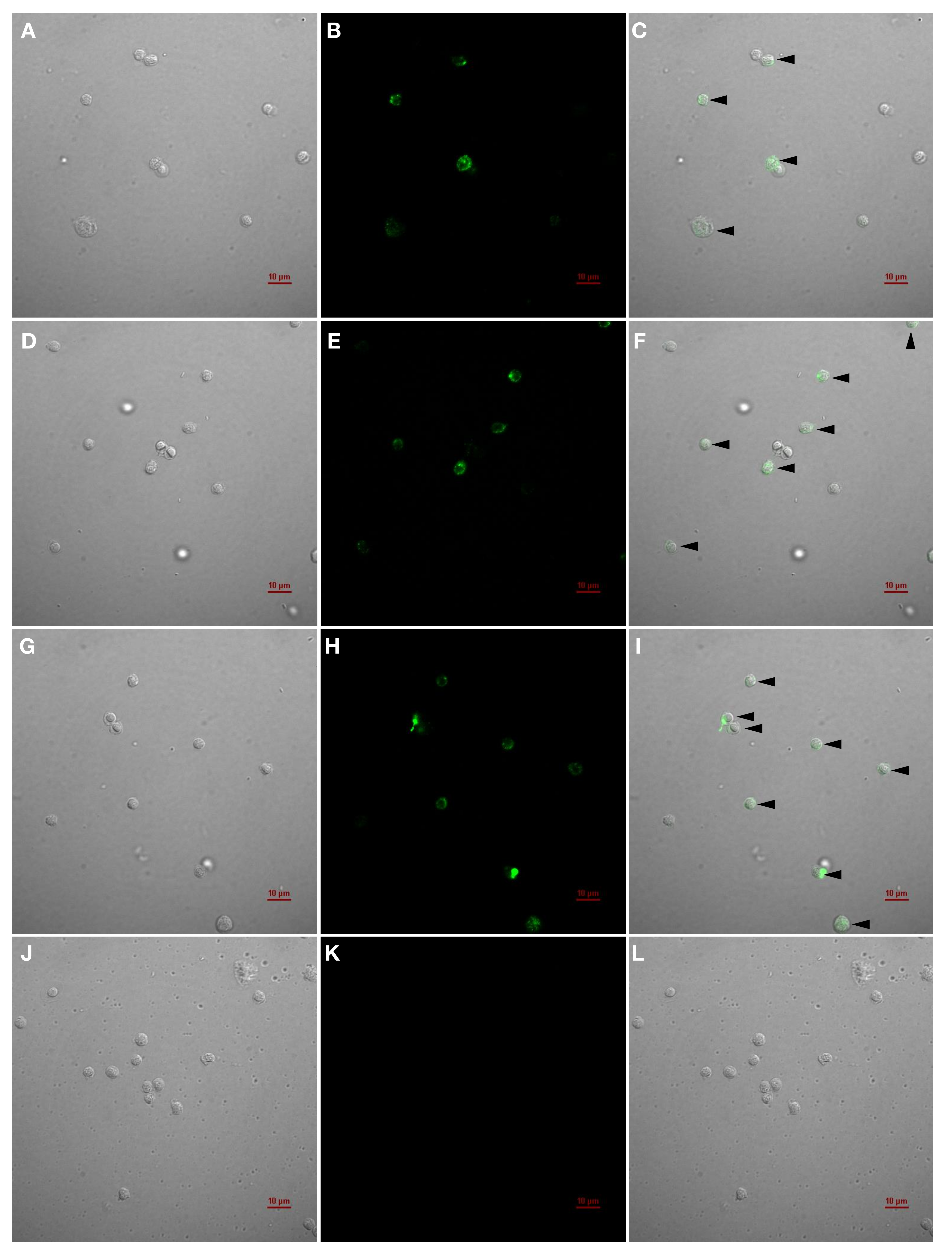
2.4.3. IIFAT for the Detection of IgM+ Cells
2.4.4. Flow Cytometry for the Quantification of IgM+ Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Peripheral Blood Leukocytes (PBLs)
4.3. Isolation of RNA and cDNA Synthesis
4.4. Specific Primer Design and Amplification of cDNAs Encoding Cμ1 of the IgM Heavy Chain Protein
4.5. Cloning of the IgM Heavy Chain cDNA Sequences
4.6. Antigenicity Characterization of the IgMHCμ1 Sequence of Bighead Catfish
4.7. Expression and Induced Overexpression of Recombinant Protein
4.8. Protein Purification
4.9. Production and Characterization of a Monoclonal Antibody (mAb) against the IgM Heavy Chain Protein
4.10. Characterization of Specific mAbs against the IgM Heavy Chain Protein of Catfish
4.10.1. Indirect ELISA Analysis
4.10.2. Western Blot Analysis
4.10.3. Indirect Immunofluorescent Assay Test (IIFAT) for the Detection and Quantification of IgM+ Cells
4.10.4. Flow Cytometry Analysis for the Quantification of IgM+ Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Fishstat Plus Rome; Universal Software for fishery statistical time series; FAO Fisheries and Aquaculture Department: Rome, Italy, 2017. [Google Scholar]
- Na-Nakorn, U.; Kamonrat, W.; Ngamsiri, T. Genetic diversity of walking catfish, Clarias macrocephalus, in Thailand and evidence of genetic introgression from introduced farmed C. gariepinus. Aquaculture 2004, 240, 145–163. [Google Scholar] [CrossRef]
- Chatchaiphan, S.; Srisapoome, P.; Kim, J.H.; Devlin, R.H.; Na-Nakorn, U. De novo transcriptome characterization and growth-related gene expression profiling of diploid and triploid bighead catfish (Clarias macrocephalus Gunther, 1864). Mar. Biotechnol. (NY) 2017, 19, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Srisapoome, P.; Chatchaiphan, S.; Bunnoy, A.; Koonawootrittriron, S.; Na-Nakorn, U. Heritability of immunity traits and disease resistance of bighead catfish, Clarias macrocephalus Günther, 1864. Fish Shellfish Immunol. 2019, 92, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Na-Nakorn, U.; Chantsawang, S.; Tarnchalanukit, W. Response to mass selection for disease resistance in walking catfish, Clarias macrocephalus. J. Appl. Aquaculture 1995, 4, 65–74. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Probiotic effects of a novel strain, Acinetobacter KU011TH, on the growth performance, immune responses, and resistance against Aeromonas hydrophila of bighead catfish (Clarias macrocephalus Günther, 1864). Microorganisms 2019, 7, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suanyuk, N.; Rogge, M.; Thune, R.; Watthanaphiromsakul, M.; Champhat, N.; Wiangkum, W. Mortality and pathology of hybrid catfish, Clarias macrocephalus (Gunther) x Clarias gariepinus (Burchell), associated with Edwardsiella ictaluri infection in southern Thailand. J. Fish. Dis. 2014, 37, 385–395. [Google Scholar] [CrossRef]
- Beck, B.H.; Farmer, B.D.; Straus, D.L.; Li, C.; Peatman, E. Putative roles for a rhamnose binding lectin in Flavobacterium columnare pathogenesis in channel catfish Ictalurus punctatus. Fish Shellfish Immunol. 2012, 33, 1008–1015. [Google Scholar] [CrossRef]
- Eirna-liza, N.; Saad, C.R.; Hassim, H.A.; Karim, M. The effects of dietary inclusion of garlic on growth performance and disease resistance of African catfish (Clarias gariepinus) fingerlings against Aeromonas hydrophila infection. J. Environ. Biol. 2016, 37, 817–824. [Google Scholar]
- Hikima, J.; Jung, T.S.; Aoki, T. Immunoglobulin genes and their transcriptional control in teleosts. Dev. Comp. Immunol. 2011, 35, 924–936. [Google Scholar] [CrossRef]
- Warr, G.W. The immunoglobulin genes of fish. Dev. Comp. Immunol. 1995, 19, 1–12. [Google Scholar] [CrossRef]
- Burton, D.R.; Woof, J.M. Human antibody effector function. Adv. Immunol. 1992, 51, 1–84. [Google Scholar] [PubMed]
- Whyte, S.K. The innate immune response of finfish–A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.R.; Warr, G.W. Fish immunoglobulins and the genes that encode them. Annu. Rev. Fish. Dis. 1992, 2, 201–221. [Google Scholar] [CrossRef]
- Harris, L.J.; Larson, S.B.; McPherson, A. Comparison of intact antibody structures and the implications for effector function. Adv. Immunol. 1999, 72, 191–208. [Google Scholar] [PubMed]
- Purcell, M.K.; Bromage, E.S.; Silva, J.; Hansen, J.D.; Badil, S.M.; Woodson, J.C.; Hershberger, P.K. Production and characterization of monoclonal antibodies to IgM of Pacific herring (Clupea pallasii). Fish Shellfish Immunol. 2012, 33, 552–558. [Google Scholar] [CrossRef]
- Sood, N.; Chaudhary, D.K.; Rathore, G.; Pradhan, P.K.; Punia, P.; Jena, J.K. Monoclonal antibodies recognizing serum immunoglobulins and surface immunoglobulin-positive cells of Catla catla (Hamilton, 1822). Fish Shellfish Immunol. 2013, 34, 1738–1739. [Google Scholar] [CrossRef]
- Jansson, E.; Grönvik, K.-O.; Johannisson, A.; Näslund, K.; Westergren, E.; Pilström, L. Monoclonal antibodies to lymphocytes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2003, 14, 239–257. [Google Scholar] [CrossRef]
- Yang, S.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Development of monoclonal antibodies against IgM of sea bass (Lateolabrax japonicus) and analysis of phagocytosis by mIgM+ lymphocytes. Fish Shellfish Immunol. 2018, 78, 372–382. [Google Scholar] [CrossRef]
- Faisal, M.; Standish, I.F.; Vogelbein, M.A.; Millard, E.V.; Kaattari, S.L. Production of a monoclonal antibody against of muskellunge (Esox masquinongy) IgM heavy chain and its use in development of an indirect ELISA for titrating circulating antibodies against VHSV-IVB. Fish Shellfish Immunol. 2019, 88, 464–471. [Google Scholar] [CrossRef]
- Li, Q.; Zhan, W.; Xing, J.; Sheng, X. Production, characterisation and applicability of monoclonal antibodies to immunoglobulin of Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2007, 23, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Thuvander, A.; Fossum, C.; Lorenzen, N. Monoclonal antibodies to salmonid immunoglobulin: Characterization and applicability in immunoassays. Dev. Comp. Immunol. 1990, 14, 415–423. [Google Scholar] [CrossRef]
- Magnad ÓTtir, B.Ó.; Kristj ÁNsd ÓTtir, H.; Gudmundsd ÓTtir, S. Characterisation of monoclonal antibodies to separate epitopes on salmon IgM heavy chain. Fish Shellfish Immunol. 1996, 6, 185–198. [Google Scholar] [CrossRef]
- Scapigliati, G.; Meloni, S.; Buonocore, F.; Bossu, P.; Prugnoli, D.; Secombes, C.J. Immunopurification of B lymphocytes from sea bass Dicentrarchus labrax (L.). Mar. Biotechnol. (NY) 2003, 5, 214–221. [Google Scholar] [CrossRef]
- Hsu, E.; Criscitiello, M.F. Diverse immunoglobulin light chain organizations in fish retain potential to revise B cell receptor specificities. J. Immunol. 2006, 177, 2452–2462. [Google Scholar] [CrossRef] [Green Version]
- Andersson, E.; Peixoto, B.; Tormanen, V.; Matsunaga, T. Evolution of the immunoglobulin M constant region genes of salmonid fish, rainbow trout (Oncorhynchus mykiss) and Arctic charr (Salvelinus alpinus): Implications concerning divergence time of species. Immunogenetics 1995, 41, 312–315. [Google Scholar] [CrossRef]
- Bengten, E.; Leanderson, T.; Pilstrom, L. Immunoglobulin heavy chain cDNA from the teleost Atlantic cod (Gadus morhua L.): Nucleotide sequences of secretory and membrane form show an unusual splicing pattern. Eur. J. Immunol. 1991, 21, 3027–3033. [Google Scholar] [CrossRef]
- Coscia, M.R.; Varriale, S.; De Santi, C.; Giacomelli, S.; Oreste, U. Evolution of the Antarctic teleost immunoglobulin heavy chain gene. Mol. Phylogenet. Evol. 2010, 55, 226–233. [Google Scholar] [CrossRef]
- Ghaffari, S.H.; Lobb, C.J. Cloning and sequence analysis of channel catfish heavy chain cDNA indicate phylogenetic diversity within the IgM immunoglobulin family. J. Immunol. 1989, 142, 1356–1365. [Google Scholar]
- Savan, R.; Aman, A.; Nakao, M.; Watanuki, H.; Sakai, M. Discovery of a novel immunoglobulin heavy chain gene chimera from common carp (Cyprinus carpio L.). Immunogenetics 2005, 57, 458–463. [Google Scholar] [CrossRef]
- Srisapoome, P.; Ohira, T.; Hirono, I.; Aoki, T. Genes of the constant regions of functional immunoglobulin heavy chain of Japanese flounder, Paralichthys olivaceus. Immunogenetics 2004, 56, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, X.-J.; Chen, D.-D.; Oriol Sunyer, J.; Zhang, Y.-A. Molecular characterization and expression analysis of three subclasses of IgT in rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2017, 70, 94–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litman, G.W.; Anderson, M.K.; Rast, J.P. Evolution of antigen binding receptors. Annu. Rev. Immunol. 1999, 17, 109–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, J.M.; Wiles, K.; Wood, K.J. The Acquired Immune System Response to Biomaterials. In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Oxford, UK, 2015; pp. 151–187. [Google Scholar]
- Hopp, T.P.; Woods, K.R. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 1981, 78, 3824–3828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Gu, F.; Huang, Z. Improved Chou-Fasman method for protein secondary structure prediction. BMC bioinformatics 2006, 7 Suppl. 4, S14. [Google Scholar] [CrossRef] [Green Version]
- Huzurbazar, S.; Kolesov, G.; Massey, S.E.; Harris, K.C.; Churbanov, A.; Liberles, D.A. Lineage-specific differences in the amino acid substitution process. J. Mol. Biol. 2010, 396, 1410–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yuan, X.; Mu, P.; Li, Q.; Ao, J.; Chen, X. Development of monoclonal antibody against IgM of large yellow croaker (Larimichthys crocea) and characterization of IgM+ B cells. Fish Shellfish Immunol. 2019, 91, 216–222. [Google Scholar] [CrossRef]
- Köllner, B.; Blohm, U.; Kotterba, G.; Fischer, U. A monoclonal antibody recognising a surface marker on rainbow trout (Oncorhynchus mykiss) monocytes. Fish Shellfish Immunol. 2001, 11, 127–142. [Google Scholar] [CrossRef]
- Li, Q.; Qi, R.-r.; Wang, Y.-n.; Qiao, G.; Ye, S.-g.; Li, H. Ontogenesis of coelomocytes in sea cucumber (Apostichopus japonicus) studied with probes of monoclonal antibody. Fish. Shellfish. Immunol. 2014, 41, 260–263. [Google Scholar] [CrossRef]
- Nakayasu, C.; Omori, M.; Hasegawa, S.; Kurata, O.; Okamoto, N. Production of a monoclonal antibody for carp (Cyprinus carpio L.) phagocytic cells and separation of the cells. Fish Shellfish Immunol. 1998, 8, 91–100. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Bjerknes, R.; Wergeland, H.I. Studies of Atlantic salmon (Salmo salar L.) blood, spleen and head kidney leucocytes using specific monoclonal antibodies, immunohistochemistry and flow cytometry. Fish. Shellfish. Immunol. 2000, 10, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Rathore, G.; Kumar, G.; Sood, N.; Kapoor, D.; Lakra, W.S. Development of monoclonal antibodies to rohu [Labeo rohita] immunoglobulins for use in immunoassays. Fish Shellfish Immunol. 2008, 25, 761–774. [Google Scholar] [CrossRef]
- Sood, N.; Chaudhary, D.K.; Rathore, G.; Singh, A.; Lakra, W.S. Monoclonal antibodies to snakehead, Channa striata immunoglobulins: Detection and quantification of immunoglobulin-positive cells in blood and lymphoid organs. Fish Shellfish Immunol. 2011, 30, 569–575. [Google Scholar] [CrossRef]
- Xing, J.; Tang, X.; Ni, Y.; Zhan, W. Application of monoclonal antibody against granulocytes of scallop Chlamys farreri on granulocytes occurrence at different developmental stages and antigenic cross-reactivity of granulocytes in five other bivalve species. Fish Shellfish Immunol. 2014, 36, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Akdis, M.; Andrä, I.; Annunziato, F.; Bacher, P.; Barnaba, V.; Battistini, L.; Bauer, W.M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017, 47, 1584–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilova, N.; Bussmann, J.; Jekosch, K.; Steiner, L.A. The immunoglobulin heavy-chain locus in zebrafish: Identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005, 6, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cerutti, A. New insights into the enigma of immunoglobulin D. Immunol. Rev. 2010, 237, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Edholm, E.S.; Bengten, E.; Stafford, J.L.; Sahoo, M.; Taylor, E.B.; Miller, N.W.; Wilson, M. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J. Immunol. 2010, 185, 4082–4094. [Google Scholar] [CrossRef] [Green Version]
- Pernis, B. IgD. In Encyclopedia of Immunology (Second Edition); Delves, P.J., Ed.; Elsevier: Oxford, UK, 1998; pp. 1199–1202. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E.; Jett, B.D. B Cell Development, Activation and Effector Functions. In Primer to the Immune Response (Second Edition); Mak, T.W., Saunders, M.E., Jett, B.D., Eds.; Academic Cell: Boston, MA, USA, 2014; pp. 111–142. [Google Scholar] [CrossRef]
- Flaherty, D.K. Antibodies. In Immunology for Pharmacy; Mosby: Saint Louis, MO, USA, 2012; pp. 70–78. [Google Scholar] [CrossRef]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 1968, 97, 77–89. [Google Scholar]
- Tang, X.; Liu, F.; Sheng, X.; Xing, J.; Zhan, W. Production, characterization and application of monoclonal antibody against immunoglobulin D heavy chain of flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2017, 64, 401–406. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briand, L.; Marcion, G.; Kriznik, A.; Heydel, J.M.; Artur, Y.; Garrido, C.; Seigneuric, R.; Neiers, F. A self-inducible heterologous protein expression system in Escherichia coli. Sci. Rep. 2016, 6, 33037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowther, J.R. The ELISA guidebook. Methods. Mol. Biol. 2000, 149, 1–413. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.S.; Kim, Y.R.; Im, S.P.; Kim, S.W.; Lazarte, J.M.S.; Kim, J.; Thompson, K.D.; Suh, J.P.; Jung, T.S. Development of a monoclonal antibody against the CD3ε of olive flounder (Paralichthys olivaceus) and its application in evaluating immune response related to CD3ε. Fish Shellfish Immunol. 2017, 65, 179–185. [Google Scholar] [CrossRef]


| Fish | mAbs | IgM+ Cells (%) | References |
|---|---|---|---|
| Bighead catfish (Clarias macrocephalus) | IgMHCμ1 mAb IgMHCμ1 mAb IgMHCμ1 mAb | 38.0–39.9 | Present study Present study Present study |
| African catfish (Clarias gariepinus) | 45.6–53.2 | ||
| Hybrid catfish (C. macrocephalus x C. gariepinus) | 58.7–60.0 | ||
| Japanese halibut (Paralichthys olivaceus) | 2D8 | 40.48 | [22] |
| Rainbow trout (Oncorhynchus mykiss) | 3D7, 4A8, 3E10,4C10 | 35–51 | [23] |
| Atlantic salmon (Salmo salar) | Type II mAb | 33 | [24] |
| European bass (Dicentrarchus labrax) | DLIg3 | 21 | [25] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Development of a Monoclonal Antibody Specific to the IgM Heavy Chain of Bighead Catfish (Clarias macrocephalus): A Biomolecular Tool for the Detection and Quantification of IgM Molecules and IgM+ Cells in Clarias Catfish. Biomolecules 2020, 10, 567. https://doi.org/10.3390/biom10040567
Bunnoy A, Na-Nakorn U, Srisapoome P. Development of a Monoclonal Antibody Specific to the IgM Heavy Chain of Bighead Catfish (Clarias macrocephalus): A Biomolecular Tool for the Detection and Quantification of IgM Molecules and IgM+ Cells in Clarias Catfish. Biomolecules. 2020; 10(4):567. https://doi.org/10.3390/biom10040567
Chicago/Turabian StyleBunnoy, Anurak, Uthairat Na-Nakorn, and Prapansak Srisapoome. 2020. "Development of a Monoclonal Antibody Specific to the IgM Heavy Chain of Bighead Catfish (Clarias macrocephalus): A Biomolecular Tool for the Detection and Quantification of IgM Molecules and IgM+ Cells in Clarias Catfish" Biomolecules 10, no. 4: 567. https://doi.org/10.3390/biom10040567
APA StyleBunnoy, A., Na-Nakorn, U., & Srisapoome, P. (2020). Development of a Monoclonal Antibody Specific to the IgM Heavy Chain of Bighead Catfish (Clarias macrocephalus): A Biomolecular Tool for the Detection and Quantification of IgM Molecules and IgM+ Cells in Clarias Catfish. Biomolecules, 10(4), 567. https://doi.org/10.3390/biom10040567







