Soluble Epoxide Hydrolase Inhibition to Face Neuroinflammation in Parkinson’s Disease: A New Therapeutic Strategy
Abstract
:1. Parkinson’s Disease Outline
2. Therapeutic Strategies for Parkinson’s Disease
3. Pathological Hallmarks of PD and the Role of Inflammation
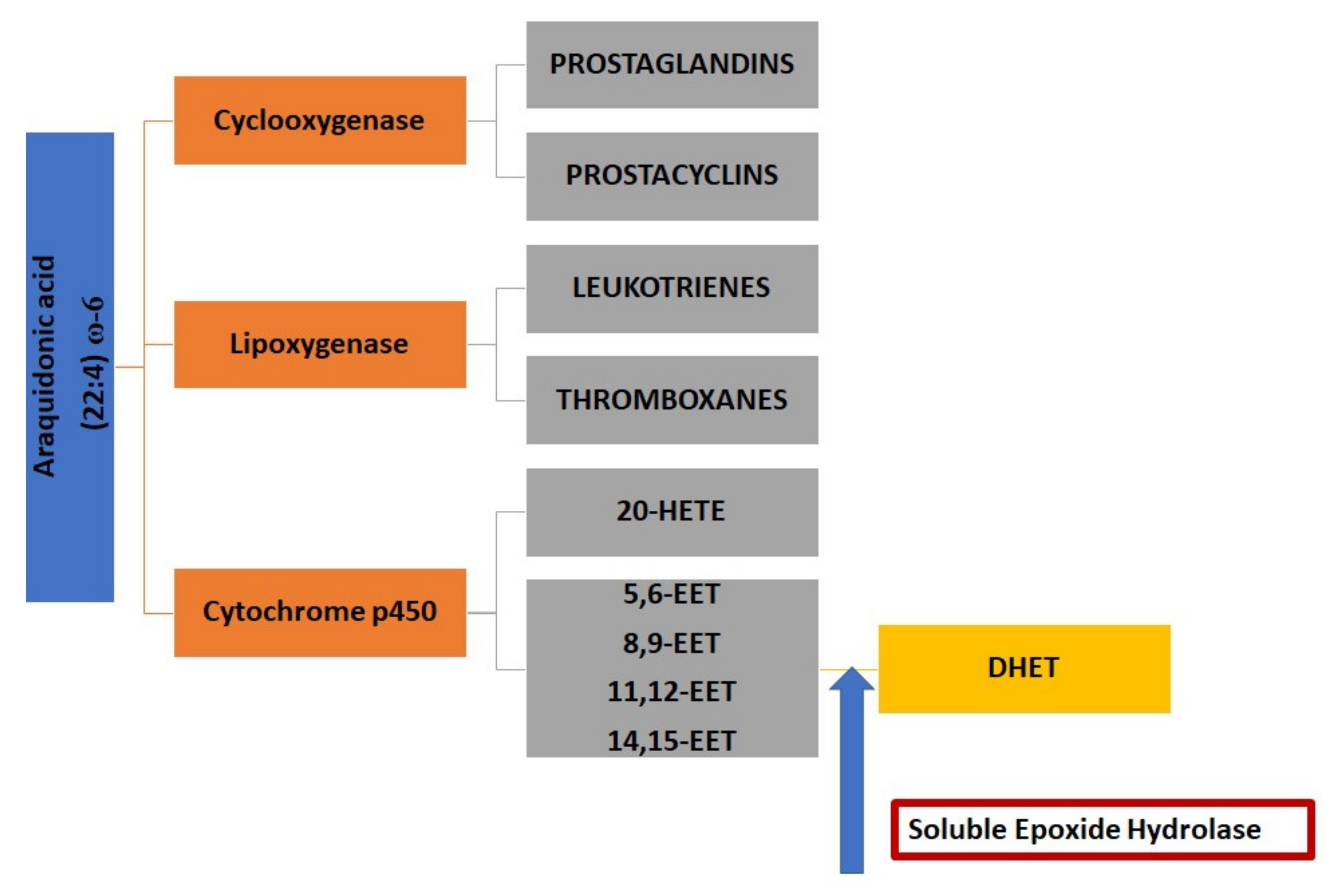
4. The Role of Soluble Epoxide Hydrolase and Epoxy Fatty Acids in Neuroinflammation
5. Soluble Epoxide Hydrolase in Central Nervous System Disorders
6. sEH-Phosphatase Activity and Neurodegenerative Diseases
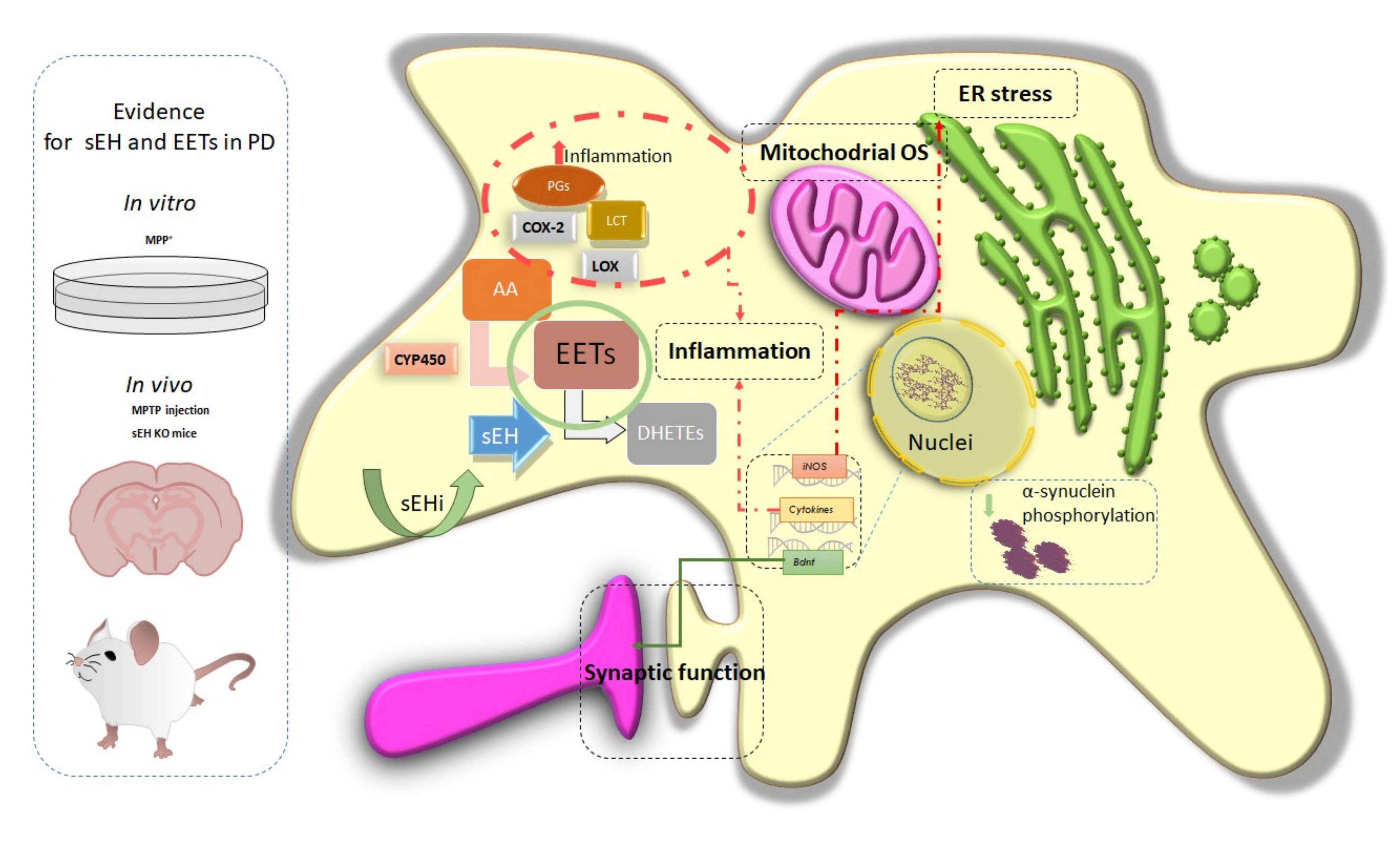
7. Soluble Epoxide Hydrolase in Parkinson’s Disease
8. Therapeutic Use of sEH Modulation in Parkinson’s Disease
Funding
Conflicts of Interest
References
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Mhyre, T.R.; Nw, R.; Boyd, J.T.; Hall, G.; Room, C. Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease. In Introduction and Technical Survey: Protein Aggregation and Fibrillogenesis; Springer: Basel, Switzerland, 2012; Volume 65, ISBN 978-94-007-5415-7. [Google Scholar]
- Jamwal, S.; Kumar, P. Insight into the Emerging Role of Striatal Neurotransmitters in the Pathophysiology of Parkinson’s Disease and Huntington’s Disease: A Review. Curr. Neuropharmacol. 2018, 17, 165–175. [Google Scholar] [CrossRef]
- Paredes-Rodriguez, E.; Vegas-Suarez, S.; Morera-Herreras, T.; De Deurwaerdere, P.; Miguelez, C. The Noradrenergic System in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; O’Malley, K. Axon degeneration in Parkinson’s disease. Exp. Neurol. 2013, 246, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Wanneveich, M.; Moisan, F.; Jacqmin-Gadda, H.; Elbaz, A.; Joly, P. Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010-2030) in France. Mov. Disord. 2018, 33, 1449–1455. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Ball, N.; Teo, W.P.; Chandra, S.; Chapman, J. Parkinson’s disease and the environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Gasser, T.; Hardy, J.; Mizuno, Y. Milestones in PD genetics. Mov. Disord. 2011, 26, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, A.J.; Murugesan, R.; Devi, S.V.; Meera, M.; Madhumala, G.; Padmaja, M.V.; Ramesh, A.; Banerjee, A.; Sushmitha, S.; Khokhlov, A.N. Current trends in etiology, prognosis and therapeutic aspects of Parkinson’s disease: A review. Acta bio-medica Atenei Parm. 2017, 88, 249. [Google Scholar]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schapira, A.H.V.; McDermott, M.P.; Barone, P.; Comella, C.L.; Albrecht, S.; Hsu, H.H.; Massey, D.H.; Mizuno, Y.; Poewe, W.; Rascol, O. Pramipexole in patients with early Parkinson’s disease (PROUD): A randomised delayed-start trial. Lancet Neurol. 2013, 12, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Bendi, V.S.; Shou, J.; Joy, S.; Torres-Russotto, D. Motor fluctuations and levodopa-induced dyskinesias in dopa-responsive dystonia. Parkinsonism Relat. Disord. 2018, 50, 126–127. [Google Scholar] [CrossRef]
- Faggiani, E.; Benazzouz, A. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: From history to the interaction with the monoaminergic systems. Prog. Neurobiol. 2017, 151, 139–156. [Google Scholar] [CrossRef]
- Tanner, C.M.; Comella, C.L. When brawn benefits brain: Physical activity and Parkinson’s disease risk. Brain 2015, 138, 238–239. [Google Scholar] [CrossRef] [Green Version]
- Weisskopf, M.G.; Weuve, J.; Nie, H.; Saint-Hilaire, M.-H.; Sudarsky, L.; Simon, D.K.; Hersh, B.; Schwartz, J.; Wright, R.O.; Hu, H. Association of cumulative lead exposure with Parkinson’s disease. Environ. Health Perspect. 2010, 118, 1609–1613. [Google Scholar] [CrossRef]
- Pezzoli, G.; Cereda, E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 2013, 80, 2035–2041. [Google Scholar] [CrossRef]
- Ritz, B.; Lee, P.-C.; Lassen, C.F.; Arah, O.A. Parkinson disease and smoking revisited: Ease of quitting is an early sign of the disease. Neurology 2014, 83, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, K.R.; Schapira, A.H. V Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.-T. New pharmacological avenues for the treatment of L-DOPA-induced dyskinesias in Parkinson’s disease: Targeting glutamate and adenosine receptors. Expert Opin. Investig. Drugs 2012, 21, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Fujita, M.; Asai, N.; Saki, M.; Mori, A. Safety and effectiveness of istradefylline in patients with Parkinson’s disease: Interim analysis of a post-marketing surveillance study in Japan. Expert Opin. Pharmacother. 2018, 19, 1635–1642. [Google Scholar] [CrossRef] [Green Version]
- LeWitt, P.A.; Guttman, M.; Tetrud, J.W.; Tuite, P.J.; Mori, A.; Chaikin, P.; Sussman, N.M.; 6002-US-005 Study Group. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: A double-blind, randomized, multicenter clinical trial (6002-US-005). Ann. Neurol. 2008, 63, 295–302. [Google Scholar] [CrossRef]
- Pinna, A. Adenosine A 2A receptor antagonists in Parkinson’s disease: Progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs 2014, 28, 455–474. [Google Scholar] [CrossRef]
- Hauser, R.A.; Olanow, C.W.; Kieburtz, K.D.; Pourcher, E.; Docu-Axelerad, A.; Lew, M.; Kozyolkin, O.; Neale, A.; Resburg, C.; Meya, U. Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: A phase 2b, double-blind, randomised trial. Lancet Neurol. 2014, 13, 767–776. [Google Scholar] [CrossRef]
- Litim, N.; Morissette, M.; Di Paolo, T. Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease: An update from the last 5 years of research. Neuropharmacology 2017, 115, 166–179. [Google Scholar] [CrossRef]
- Rascol, O.; Fox, S.; Gasparini, F.; Kenney, C.; Di Paolo, T.; Gomez-Mancilla, B. Use of metabotropic glutamate 5-receptor antagonists for treatment of levodopa-induced dyskinesias. Parkinsonism Relat. Disord. 2014, 20, 947–956. [Google Scholar] [CrossRef]
- Petrov, D.; Pedros, I.; de Lemos, M.L.; Pallàs, M.; Canudas, A.M.; Lazarowski, A.; Beas-Zarate, C.; Auladell, C.; Folch, J.; Camins, A. Mavoglurant as a treatment for Parkinson’s disease. Expert Opin. Investig. Drugs 2014, 23, 1165–1179. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Ascherio, A.; Beal, M.F.; Cudkowicz, M.E.; Curhan, G.C.; Hare, J.M.; Rudolph, A. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: A randomized clinical trial. JAMA Neurol 2014, 71, 141–150. [Google Scholar]
- Biglan, K.M.; Oakes, D.; Lang, A.E.; Hauser, R.A.; Hodgeman, K.; Greco, B.; Lowell, J.; Rockhill, R.; Shoulson, I.; Venuto, C. A novel design of a Phase III trial of isradipine in early Parkinson disease (STEADY-PD III). Ann. Clin. Transl. Neurol. 2017, 4, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. Jama 2015, 313, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Espay, A.J. Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations. Mov. Disord. 2018, 33, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Dave, K.D.; Kordower, J.H. Therapeutic approaches to target alpha-synuclein pathology. Exp. Neurol. 2017, 298, 225–235. [Google Scholar] [CrossRef]
- Di Maio, R.; Hoffman, E.K.; Rocha, E.M.; Keeney, M.T.; Sanders, L.H.; De Miranda, B.R.; Zharikov, A.; Van Laar, A.; Stepan, A.F.; Lanz, T.A. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 2018, 10, eaar5429. [Google Scholar] [CrossRef] [Green Version]
- Athauda, D.; Foltynie, T. Insulin resistance and Parkinson’s disease: A new target for disease modification? Prog. Neurobiol. 2016, 145, 98–120. [Google Scholar] [CrossRef]
- Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Shults, C.M. A randomized clinical trial of high-dosage coenzyme Q10 in early parkinson disease no evidence of benefit. JAMA Neurol. 2014, 75, 543–552. [Google Scholar] [CrossRef]
- Wu, H.-F.; Kao, L.-T.; Shih, J.-H.; Kao, H.-H.; Chou, Y.-C.; Li, I.-H.; Kao, S. Pioglitazone use and Parkinson’s disease: A retrospective cohort study in Taiwan. BMJ Open 2018, 8, e023302. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, W.; Li, G.; Chen, J.; Guan, X.; Chen, X.; Guan, Z. Neuroprotective Effect and Mechanism of Thiazolidinedione on Dopaminergic Neurons in Vivo and in Vitro in Parkinson’s Disease. PPAR Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, K.; Voutilainen, M.H.; Domanskyi, A.; Airavaara, M. AAV vector-mediated gene delivery to substantia nigra dopamine neurons: Implications for gene therapy and disease models. Genes 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren Olanow, C.; Bartus, R.T.; Baumann, T.L.; Factor, S.; Boulis, N.; Stacy, M.; Turner, D.A.; Marks, W.; Larson, P.; Starr, P.A. Gene delivery of neurturin to putamen and substantia nigra in P arkinson disease: A double-blind, randomized, controlled trial. Ann. Neurol. 2015, 78, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Und Halbach, O.V.B. Synucleins and their relationship to Parkinson’s disease. Cell Tissue Res. 2004, 318, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Greenamyre, J.T.; Hastings, T.G. Parkinson’s--divergent causes, convergent mechanisms. Science (80-. ) 2004, 304, 1120–1122. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, S210–S212. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.A.; Kraneveld, A.D. Exploring Braak’s hypothesis of Parkinson’s disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Akiyama, H.; McGeer, E.G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 1988, 24, 574–576. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Szymkowski, D.E.; Smith, C.G.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. 2006, 26, 9365–9375. [Google Scholar] [CrossRef]
- Girard-Joyal, O.; Ismail, N. Effect of LPS treatment on tyrosine hydroxylase expression and Parkinson-like behaviors. Horm. Behav. 2017, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.S.; Café-Mendes, C.C.; Britto, L.R.G. NADPH oxidase and the degeneration of dopaminergic neurons in parkinsonian mice. Oxid. Med. Cell. Longev. 2013, 2013, 157857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef]
- Jump, D.B. Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr. Opin. Lipidol. 2002, 13, 155–164. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Laye, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Green, P.; Mann, J.J.; Rapoport, S.I.; Sublette, M.E. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 2015, 1597, 220–246. [Google Scholar] [CrossRef] [Green Version]
- Imig, J.D.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009, 8, 794–805. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; von Schacky, C.; Luft, F.C.; Muller, D.N. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar] [CrossRef] [Green Version]
- Imig, J.D. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol. Ther. 2018, 192, 1–19. [Google Scholar] [CrossRef]
- Imig, J.D. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012, 92, 101–130. [Google Scholar] [CrossRef] [Green Version]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Urquhart, P.; Nicolaou, A.; Woodward, D.F. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 2015, 1851, 366–376. [Google Scholar] [CrossRef]
- Westphal, C.; Konkel, A.; Schunck, W.-H. Cytochrome p450 enzymes in the bioactivation of polyunsaturated fatty acids and their role in cardiovascular disease. In Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450; Springer: Basel, Switzerland, 2015; pp. 151–187. [Google Scholar]
- Marowsky, A.; Burgener, J.; Falck, J.R.; Fritschy, J.-M.; Arand, M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 2009, 163, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.M.; McReynolds, C.B.; Schmidt, W.K.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 2017, 180, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, C.; Gong, W.; Li, Y.; Edin, M.L.; Zeldin, D.C.; Wang, D.W. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J. Pharmacol. Exp. Ther. 2011, 339, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Jia, J.; Nelson, J.; Goyagi, T.; Klaus, J.; Alkayed, N.J. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010, 91, 68–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sura, P.; Sura, R.; EnayetAllah, A.E.; Grant, D.F. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 2008, 56, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, C.; White, I.; Johansson, C.; Stark, A.; Meijer, J. Localization of the human soluble epoxide hydrolase gene (EPHX2) to chromosomal region 8p21-p12. Hum. Genet. 1995, 95, 356–358. [Google Scholar] [CrossRef]
- Gomez, G.A.; Morisseau, C.; Hammock, B.D.; Christianson, D.W. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry 2004, 43, 4716–4723. [Google Scholar] [CrossRef]
- Gill, S.S.; Hammock, B.D.; Yamamoto, I.; Casida, J.E. Preliminary chromatographic studies on the metabolites and photodecomposition products of the juvenoid 1-(4′-ethilphenoxy)-6, 7-epoxy-3, 7-dimethyl-2-octene. In Insect Juvenile Hormones; Academic Press: New York, NY, USA, 1972; pp. 177–189. [Google Scholar]
- Hammock, B.D.; Storms, D.H.; Grant, D.F. Epoxide hydrolases. Compr. Toxicol. 1997, 3, 283–305. [Google Scholar]
- Draper, A.J.; Hammock, B.D. Soluble epoxide hydrolase in rat inflammatory cells is indistinguishable from soluble epoxide hydrolase in rat liver. Toxicol. Sci. an Off. J. Soc. Toxicol. 1999, 50, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Draper, A.J.; Hammock, B.D. Inhibition of soluble and microsomal epoxide hydrolase by zinc and other metals. Toxicol. Sci. an Off. J. Soc. Toxicol. 1999, 52, 26–32. [Google Scholar] [CrossRef]
- Morisseau, C.; Schebb, N.H.; Dong, H.; Ulu, A.; Aronov, P.A.; Hammock, B.D. Role of soluble epoxide hydrolase phosphatase activity in the metabolism of lysophosphatidic acids. Biochem. Biophys. Res. Commun. 2012, 419, 796–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacos, N.; Capdevila, J.; Falck, J.R.; Manna, S.; Martin-Wixtrom, C.; Gill, S.S.; Hammock, B.D.; Estabrook, R.W. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch. Biochem. Biophys. 1983, 223, 639–648. [Google Scholar] [CrossRef]
- Greene, J.F.; Newman, J.W.; Williamson, K.C.; Hammock, B.D. Toxicity of epoxy fatty acids and related compounds to cells expressing human soluble epoxide hydrolase. Chem. Res. Toxicol. 2000, 13, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science (80-.) 1999, 285, 1276–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Schmelzer, K.; Lee, T.-S.; Fang, X.; Zhu, Y.; Spector, A.A.; Gill, S.; Morisseau, C.; Hammock, B.D. The antiinflammatory effect of laminar flow: The role of PPARγ, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2005, 102, 16747–16752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarriello, S.; Tuazon, J.P.; Corey, S.; Schimmel, S.; Rajani, M.; Gorsky, A.; Incontri, D.; Hammock, B.D.; Borlongan, C.V. Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders. Prog. Neurobiol. 2019, 172, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, K.R.; Kubala, L.; Newman, J.W.; Kim, I.-H.; Eiserich, J.P.; Hammock, B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. USA 2005, 102, 9772–9777. [Google Scholar] [CrossRef] [Green Version]
- Prakash, R.; Carmichael, S.T. Blood–brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 2015, 28, 556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Koerner, I.P.; Noppens, R.; Grafe, M.; Tsai, H.-J.; Morisseau, C.; Luria, A.; Hammock, B.D.; Falck, J.R.; Alkayed, N.J. Soluble epoxide hydrolase: A novel therapeutic target in stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Davis, C.M.; Edin, M.L.; Lee, C.R.; Zeldin, D.C.; Alkayed, N.J. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLoS ONE 2013, 8, e61244. [Google Scholar] [CrossRef]
- Wang, J.; Fujiyoshi, T.; Kosaka, Y.; Raybuck, J.D.; Lattal, K.M.; Ikeda, M.; Herson, P.S.; Koerner, I.P. Inhibition of soluble epoxide hydrolase after cardiac arrest/cardiopulmonary resuscitation induces a neuroprotective phenotype in activated microglia and improves neuronal survival. J. Cereb. Blood Flow Metab. 2013, 33, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliff, J.J.; Alkayed, N.J. Soluble epoxide hydrolase inhibition: Targeting multiple mechanisms of ischemic brain injury with a single agent. Future Neurol. 2009, 4, 179–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorrance, A.M.; Rupp, N.; Pollock, D.M.; Newman, J.W.; Hammock, B.D.; Imig, J.D. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2005, 46, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, G.; Corda, E.; Martino, P.A.; Dall’Ara, P.; Bareggi, S.R.; Bondiolotti, G.; Iulini, B.; Mazza, M.; Casalone, C.; Hwang, S.H. Therapeutic activity of inhibition of the soluble epoxide hydrolase in a mouse model of scrapie. Life Sci. 2013, 92, 1145–1150. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.W.; Young, J.M.; Borkar, R.N.; Woltjer, R.L.; Quinn, J.F.; Silbert, L.C.; Grafe, M.R.; Alkayed, N.J. Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat. 2014, 113, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Inceoglu, B.; Zolkowska, D.; Yoo, H.J.; Wagner, K.M.; Yang, J.; Hackett, E.; Hwang, S.H.; Lee, K.S.S.; Rogawski, M.A.; Morisseau, C. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS ONE 2013, 8, e80922. [Google Scholar] [CrossRef] [Green Version]
- Hung, Y.-W.; Hung, S.-W.; Wu, Y.-C.; Wong, L.-K.; Lai, M.-T.; Shih, Y.-H.; Lee, T.-S.; Lin, Y.-Y. Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain. Behav. Immun. 2015, 43, 118–129. [Google Scholar] [CrossRef]
- Hung, T.-H.; Shyue, S.-K.; Wu, C.-H.; Chen, C.-C.; Lin, C.-C.; Chang, C.-F.; Chen, S.-F. Deletion or inhibition of soluble epoxide hydrolase protects against brain damage and reduces microglia-mediated neuroinflammation in traumatic brain injury. Oncotarget 2017, 8, 103236. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, M.; Ishima, T.; Morisseau, C.; Yang, J.; Wagner, K.M.; Zhang, J.; Yang, C.; Yao, W.; Dong, C. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc. Natl. Acad. Sci. USA 2016, 113, E1944–E1952. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K. Soluble epoxide hydrolase: A new therapeutic target for depression. Expert Opin. Ther. Targets 2016, 20, 1149–1151. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.L. Depression and Parkinson’s disease: A review. Am. J. Psychiatry 1992, 149, 443–454. [Google Scholar] [PubMed]
- Swardfager, W.; Hennebelle, M.; Yu, D.; Hammock, B.D.; Levitt, A.J.; Hashimoto, K.; Taha, A.Y. Metabolic/inflammatory/vascular comorbidity in psychiatric disorders; soluble epoxide hydrolase (sEH) as a possible new target. Neurosci. Biobehav. Rev. 2018, 87, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, W.; Cao, X.; Zeng, Y.; Qin, X.; Zhu, M.; Ren, J.; Wu, Z.; Huang, Q.; Zhang, Y.; Wang, M. Astrocytic epoxyeicosatrienoic acid signaling in the medial prefrontal cortex modulates depressive-like behaviors. J. Neurosci. 2019, 39, 4606–4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Q.; Ma, M.; Yang, J.; Nonaka, R.; Yamaguchi, A.; Ishikawa, K.I.; Kobayashi, K.; Murayama, S.; Hwang, S.H.; Saiki, S.; et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E5815–E5823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, P.; Narayanan, J.; Harder, D.R. Differential effect of amyloid beta on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience 2011, 194, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Griñán-Ferré, C.; Codony, S.; Pujol, E.; Yang, J.; Leiva, R.; Escolano, C.; Puigoriol-Illamola, D.; Companys-Alemany, J.; Corpas, R.; Sanfeliu, C. Pharmacological inhibition of soluble epoxide hydrolase as a new therapy for Alzheimer’s Disease. bioRxiv 2019, 605055. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.T.; Lee, K.I.; Chen, C.H.; Lee, T.S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflammation 2019, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Liu, J.; Dong, R.; Zhu, J.; Tao, C.; Zheng, R.; Zhu, S. 14, 15-epoxyeicosatrienoic acid promotes production of brain derived neurotrophic factor from astrocytes and exerts neuroprotective effects during ischaemic injury. Neuropathol. Appl. Neurobiol. 2016, 42, 607–620. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Hsu, P.-C.; Hung, C.-C.; Hu, Y.-Y.; Huang, Y.-J.; Gan, Y.-L.; Lin, C.-H.; Shie, F.-S.; Chang, W.-K.; Kao, L.-S. Soluble Epoxide Hydrolase Inhibition Attenuates Excitotoxicity Involving 14, 15-Epoxyeicosatrienoic Acid–Mediated Astrocytic Survival and Plasticity to Preserve Glutamate Homeostasis. Mol. Neurobiol. 2019, 56, 8451–8474. [Google Scholar] [CrossRef]
- Bianco, R.A.; Agassandian, K.; Cassell, M.D.; Spector, A.A.; Sigmund, C.D. Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res. 2009, 1291, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imig, J.D. Epoxyeicosatrienoic acids and 20-hydroxyeicosatetraenoic acid on endothelial and vascular function. In Advances in Pharmacology; Elsevier: San Diego, CA, USA, 2016; Volume 77, pp. 105–141. ISBN 1054-3589. [Google Scholar]
- Liu, Y.; Dang, H.; Li, D.; Pang, W.; Hammock, B.D.; Zhu, Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet–induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS ONE 2012, 7, e39165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahabi, P.; Siest, G.; Meyer, U.A.; Visvikis-Siest, S. Human cytochrome P450 epoxygenases: Variability in expression and role in inflammation-related disorders. Pharmacol. Ther. 2014, 144, 134–161. [Google Scholar] [CrossRef] [PubMed]
- EnayetAllah, A.E.; Luria, A.; Luo, B.; Tsai, H.-J.; Sura, P.; Hammock, B.D.; Grant, D.F. Opposite regulation of cholesterol levels by the phosphatase and hydrolase domains of soluble epoxide hydrolase. J. Biol. Chem. 2008, 283, 36592–36598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfrieger, F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. C. 2003, 60, 1158–1171. [Google Scholar] [CrossRef]
- Bjoörkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Domingues, M.F.; Callai-Silva, N.; Piovesan, A.R.; Carlini, C.R. Soluble Epoxide Hydrolase and Brain Cholesterol Metabolism. Front. Mol. Neurosci. 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Chen, C.-S.; Patterson, M.C.; O’Brien, J.F.; Pagano, R.E.; Wheatley, C.L. Broad screening test for sphingolipid-storage diseases. Lancet 1999, 354, 901–905. [Google Scholar] [CrossRef]
- Sillence, D.J.; Puri, V.; Marks, D.L.; Butters, T.D.; Dwek, R.A.; Pagano, R.E.; Platt, F.M. Glucosylceramide modulates membrane traffic along the endocytic pathway. J. Lipid Res. 2002, 43, 1837–1845. [Google Scholar] [CrossRef] [Green Version]
- Salvioli, R.; Scarpa, S.; Ciaffoni, F.; Tatti, M.; Ramoni, C.; Vanier, M.T.; Vaccaro, A.M. Glucosylceramidase mass and subcellular localization are modulated by cholesterol in Niemann-Pick disease type C. J. Biol. Chem. 2004, 279, 17674–17680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.; George-Hyslop, P.H.S.; Pericak-Vance, M.A.; Joo, S.H.; Rosi, B.L.; Gusella, J.F.; Crapper-MacLachlan, D.R.; Alberts, M.J. Association of apolipoprotein E allele ϵ4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43, 1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farin, F.M.; Janssen, P.; Quigley, S.; Abbott, D.; Hassett, C.; Smith-Weller, T.; Franklin, G.M.; Swanson, P.D.; Longstreth Jr, W.T.; Omiecinski, C.J. Genetic polymorphisms of microsomal and soluble epoxide hydrolase and the risk of Parkinson’s disease. Pharmacogenet. Genomics 2001, 11, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Sedelis, M.; Schwarting, R.K.W.; Huston, J.P. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2001, 125, 109–125. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141. [Google Scholar] [CrossRef]
- Qin, X.; Wu, Q.; Lin, L.; Sun, A.; Liu, S.; Li, X.; Cao, X.; Gao, T.; Luo, P.; Zhu, X. Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism. Mol. Neurobiol. 2015, 52, 187–195. [Google Scholar] [CrossRef]
- Huang, Y.; Shu, H.; Li, L.; Zhen, T.; Zhao, J.; Zhou, X.; Luo, W. L-DOPA-Induced Motor Impairment and Overexpression of Corticostriatal Synaptic Components Are Improved by the mGluR5 Antagonist MPEP in 6-OHDA-Lesioned Rats. ASN Neuro 2018, 10, 1759091418811021. [Google Scholar] [CrossRef]
- Fecchio, C.; Palazzi, L.; Polverino de Laureto, P. α-Synuclein and polyunsaturated fatty acids: Molecular basis of the interaction and implication in neurodegeneration. Molecules 2018, 23, 1531. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, Y.; Okada, Y.; Akamatsu, W.; Koike, M.; Kuzumaki, N.; Hayakawa, H.; Nihira, T.; Kobayashi, T.; Ohyama, M.; Sato, S. Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol. Brain 2012, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.T.; Estrada, C.; Maatouk, L.; Vyas, S. Inflammation in Parkinson’s disease: Role of glucocorticoids. Front. Neuroanat. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, V.H. Innate inflammation in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the role of neuroinflammation in the progression of Parkinson’s disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, R.; Verbitsky, M.; Kisselev, S.; Browne, A.; Mejia-Sanatana, H.; Louis, E.D.; Cote, L.J.; Andrews, H.; Waters, C. Genome-wide association study identifies candidate genes for Parkinson’s disease in an Ashkenazi Jewish population. BMC Med. Genet. 2011, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pihlstrøm, L.; Axelsson, G.; Bjørnarå, K.A.; Dizdar, N.; Fardell, C.; Forsgren, L.; Holmberg, B.; Larsen, J.P.; Linder, J.; Nissbrandt, H. Supportive evidence for 11 loci from genome-wide association studies in Parkinson’s disease. Neurobiol. Aging 2013, 34, 1708-e7. [Google Scholar] [CrossRef] [Green Version]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 and neuroinflammation: Partners in crime in Parkinson’s disease? J. Neuroinflammation 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Lampe, J.B.; Gossrau, G.; Herting, B.; Kempe, A.; Sommer, U.; Füssel, M.; Weber, M.; Koch, R.; Reichmann, H. HLA typing and Parkinson’s disease. Eur. Neurol. 2003, 50, 64–68. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Hammock, B.D.; Velmurugan, D.; Bharath, M.M.S. Possible role of Epoxyeicosatrienoic acid in prevention of oxidative stress mediated neuroinflammation in Parkinson disorders. Med. Hypotheses 2016, 93, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson’s Disease. Exp. Neurobiol. 2019, 28, 554. [Google Scholar] [CrossRef] [Green Version]
- Musanti, R.; Parati, E.; Lamperti, E.; Ghiselli, G. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem. Med. Metab. Biol. 1993, 49, 133–142. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakamura, Y.; Kiyozuka, T.; Aoyagi, J.; Hirayama, T.; Nagata, R.; Ito, H.; Iwamoto, K.; Murata, K.; Yoshii, Y. Serological profiles of urate, paraoxonase-1, ferritin and lipid in Parkinson’s disease: Changes linked to disease progression. Neurodegener. Dis. 2011, 8, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, H.; Tian, Y.; Xu, F.; Chen, X.; Wang, K. Reduced serum levels of triglyceride, very low density lipoprotein cholesterol and apolipoprotein B in Parkinson’s disease patients. PLoS ONE 2013, 8, e75743. [Google Scholar] [CrossRef] [Green Version]
- Borlongan, C. V Fatty acid chemical mediator provides insights into the pathology and treatment of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 6322–6324. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Anderson, G.D.; McGiff, J.C. The red blood cell participates in regulation of the circulation by producing and releasing epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2012, 98, 91–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.H.; Pavlov, T.S.; Christain, S.V.; Neckář, J.; Staruschenko, A.; Gauthier, K.M.; Capdevila, J.H.; Falck, J.R.; Campbell, W.B.; Imig, J.D. Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin. Sci. 2014, 127, 463–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Krishnamurthy, P.T.; Thomas, P.; Hammock, B.D.; Hwang, S.H. Soluble epoxide hydrolase inhibitor, t-TUCB, protects against myocardial ischaemic injury in rats. J. Pharm. Pharmacol. 2014, 66, 1251–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Li, N.; Liu, J.; Harris, T.R.; Hammock, B.D.; Chiamvimonvat, N. Soluble epoxide hydrolase inhibitors and heart failure. Cardiovasc. Ther. 2011, 29, 99–111. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Lin, T.; Wang, T.; Tang, Y.; Dong, Y.; Wang, J. Epoxyeicosatrienoic acids–novel mechanism and pharmacological therapy of chronic renocardiac syndrome. Med. Hypotheses 2011, 76, 550–552. [Google Scholar] [CrossRef]
- Lee, K.S.S.; Liu, J.-Y.; Wagner, K.M.; Pakhomova, S.; Dong, H.; Morisseau, C.; Fu, S.H.; Yang, J.; Wang, P.; Ulu, A. Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy. J. Med. Chem. 2014, 57, 7016–7030. [Google Scholar] [CrossRef]
- Wagner, K.; Yang, J.; Inceoglu, B.; Hammock, B.D. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J. Pain 2014, 15, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Kodani, S.; Hammock, B.D. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014, 53, 108–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Vicario, C.; Alcaraz-Quiles, J.; García-Alonso, V.; Rius, B.; Hwang, S.H.; Titos, E.; Lopategi, A.; Hammock, B.D.; Arroyo, V.; Clària, J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: Role for omega-3 epoxides. Proc. Natl. Acad. Sci. USA 2015, 112, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.C. Soluble epoxide hydrolase inhibitors: A patent review. Expert Opin. Ther. Pat. 2010, 20, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Role of soluble epoxide hydrolase in metabolism of PUFAs in psychiatric and neurological disorders. Front. Pharmacol. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, S.; Morisseau, C.; Inceoglu, B.; Kamita, S.G.; De Nicola, G.R.; Nyegue, M.; Hammock, B.D. Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana Baillon: Synthesis, quantification, and measurement of biological activities in vitro and in vivo. PLoS ONE 2015, 10, e0117438. [Google Scholar] [CrossRef]
- Terashvili, M.; Sarkar, P.; Van Nostrand, M.; Falck, J.R.; Harder, D.R. The protective effect of astrocyte-derived 14, 15-EET on H2O2-induced cell injury in Astrocyte-dopaminergic neuronal cell line co-culture. Neuroscience 2012, 223, 68. [Google Scholar] [CrossRef] [Green Version]
- Abdu, E.; Bruun, D.A.; Yang, D.; Yang, J.; Inceoglu, B.; Hammock, B.D.; Alkayed, N.J.; Lein, P.J. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J. Neurochem. 2011, 117, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Munzenmaier, D.H.; Harder, D.R. Cerebral microvascular endothelial cell tube formation: Role of astrocytic epoxyeicosatrienoic acid release. Am. J. Physiol. Circ. Physiol. 2000, 278, H1163–H1167. [Google Scholar] [CrossRef]
- Sellers, K.W.; Sun, C.; Diez-Freire, C.; Waki, H.; Morisseau, C.; Falck, J.R.; Hammock, B.D.; Paton, J.F.; Raizada, M.K. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005, 19, 626–628. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Meng, Q.; Song, C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 226, 386–396. [Google Scholar] [CrossRef]
- Kamel, F.; Goldman, S.M.; Umbach, D.M.; Chen, H.; Richardson, G.; Barber, M.R.; Meng, C.; Marras, C.; Korell, M.; Kasten, M. Dietary fat intake, pesticide use, and Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodani, S.D.; Morisseau, C. Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie 2019, 159, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lakkappa, N.; Krishnamurthy, P.T.; Yamjala, K.; Hwang, S.H.; Hammock, B.D.; Babu, B. Evaluation of antiparkinson activity of PTUPB by measuring dopamine and its metabolites in Drosophila melanogaster: LC–MS/MS method development. J. Pharm. Biomed. Anal. 2018, 149, 457–464. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Pandareesh, M.D.; Hammock, B.D.; Hwang, S.H. Soluble epoxide hydrolase inhibitor, APAU, protects dopaminergic neurons against rotenone induced neurotoxicity: Implications for Parkinson’s disease. Neurotoxicology 2019, 70, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Atone, J.; Wagner, K.; Hashimoto, K.; Hammock, B.D. Prostaglandins and other lipid mediators cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106385. [Google Scholar] [CrossRef]

| Outline | Biological Substrate | Reference | |
|---|---|---|---|
| The protective effect of astrocyte-derived 14,15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-DA neuronal cell line co-culture. | Implication of sEH in PD pathology | Astrocyte-DA neuronal cell line co-culture | [160] |
| Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism | Implication of sEH in PD pathology | Mice | [131] |
| Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. | Implication of sEH in PD pathology | Human, mice | [108] |
| Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. | Role of EETs in neuroinflammation | Review | [167] |
| Evaluation of antiparkinson activity of PTUPB by measuring dopamine and its metabolites in Drosophila melanogaster: LC–MS/MS method development. | Therapeutic profile for sEHi in PD | Drosophila melanogaster | [168] |
| Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders. | Therapeutic profile for sEHi in CNS disorders | Review | [89] |
| Soluble epoxide hydrolase inhibitor, APAU, protects DA neurons against rotenone induced neurotoxicity: Implications for Parkinson’s disease. | Therapeutic profile for sEHi in PD | DA cell culture | [169] |
| Role of soluble epoxide hydrolase in metabolism of PUFAs in psychiatric and neurological disorders. | Therapeutic profile for sEH | Review | [158] |
| Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases. | Role of EETs in PD | Review | [170] |
| Compound | Chemical Structure | Reference |
|---|---|---|
| Honokiol (5,3′-diallyl-2,4′-dihydroxybiphenyl) |  | [153,160] |
| 12-(3-(adamantan-1-yl)ureido)dodecanoic acid (AUDA) |  | [92,96] |
| 1,3-bis (4-methoxybenzyl)urea (MMU) |  | [159] |
| N-(1-acetylpiperidin-4-yl)-N-(adamant-1-yl)urea (APAU) |  | [170] |
| (4-(5-phenyl-3-{3-[3-(4-trifluoromethyl-phenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide) (PTUPB) |  | [143] |
| 1-((1-Propionylpiperidin-4-yl)-3-(4-(trifluoromethoxy) phenyl)) urea (TPPU) |  | [109] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallàs, M.; Vázquez, S.; Sanfeliu, C.; Galdeano, C.; Griñán-Ferré, C. Soluble Epoxide Hydrolase Inhibition to Face Neuroinflammation in Parkinson’s Disease: A New Therapeutic Strategy. Biomolecules 2020, 10, 703. https://doi.org/10.3390/biom10050703
Pallàs M, Vázquez S, Sanfeliu C, Galdeano C, Griñán-Ferré C. Soluble Epoxide Hydrolase Inhibition to Face Neuroinflammation in Parkinson’s Disease: A New Therapeutic Strategy. Biomolecules. 2020; 10(5):703. https://doi.org/10.3390/biom10050703
Chicago/Turabian StylePallàs, Mercè, Santiago Vázquez, Coral Sanfeliu, Carles Galdeano, and Christian Griñán-Ferré. 2020. "Soluble Epoxide Hydrolase Inhibition to Face Neuroinflammation in Parkinson’s Disease: A New Therapeutic Strategy" Biomolecules 10, no. 5: 703. https://doi.org/10.3390/biom10050703
APA StylePallàs, M., Vázquez, S., Sanfeliu, C., Galdeano, C., & Griñán-Ferré, C. (2020). Soluble Epoxide Hydrolase Inhibition to Face Neuroinflammation in Parkinson’s Disease: A New Therapeutic Strategy. Biomolecules, 10(5), 703. https://doi.org/10.3390/biom10050703









