Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of cHP Isomers
2.2. Experimental Design and Treatments
2.3. Determination of Cellular Viability
2.4. Determination of AChE, TACE, and BACE1 Activities
2.5. Determination of TAC and TOS Levels
2.6. Determination of Apoptosis and Necrosis by Fluorescence Microscopy and Flow Cytometry
2.7. Determination of the Molecular Genetic Basis of Neuroprotection by cHP1-4
2.8. Determination of Cytotoxic and Genotoxic Potentials of cHP1-4
2.9. Statistical Analysis
3. Results
3.1. cHP Isomers Provided a Different Degree of Neuroprotection against Aβ1-42 Induced Cell Death in In Vitro AD Model
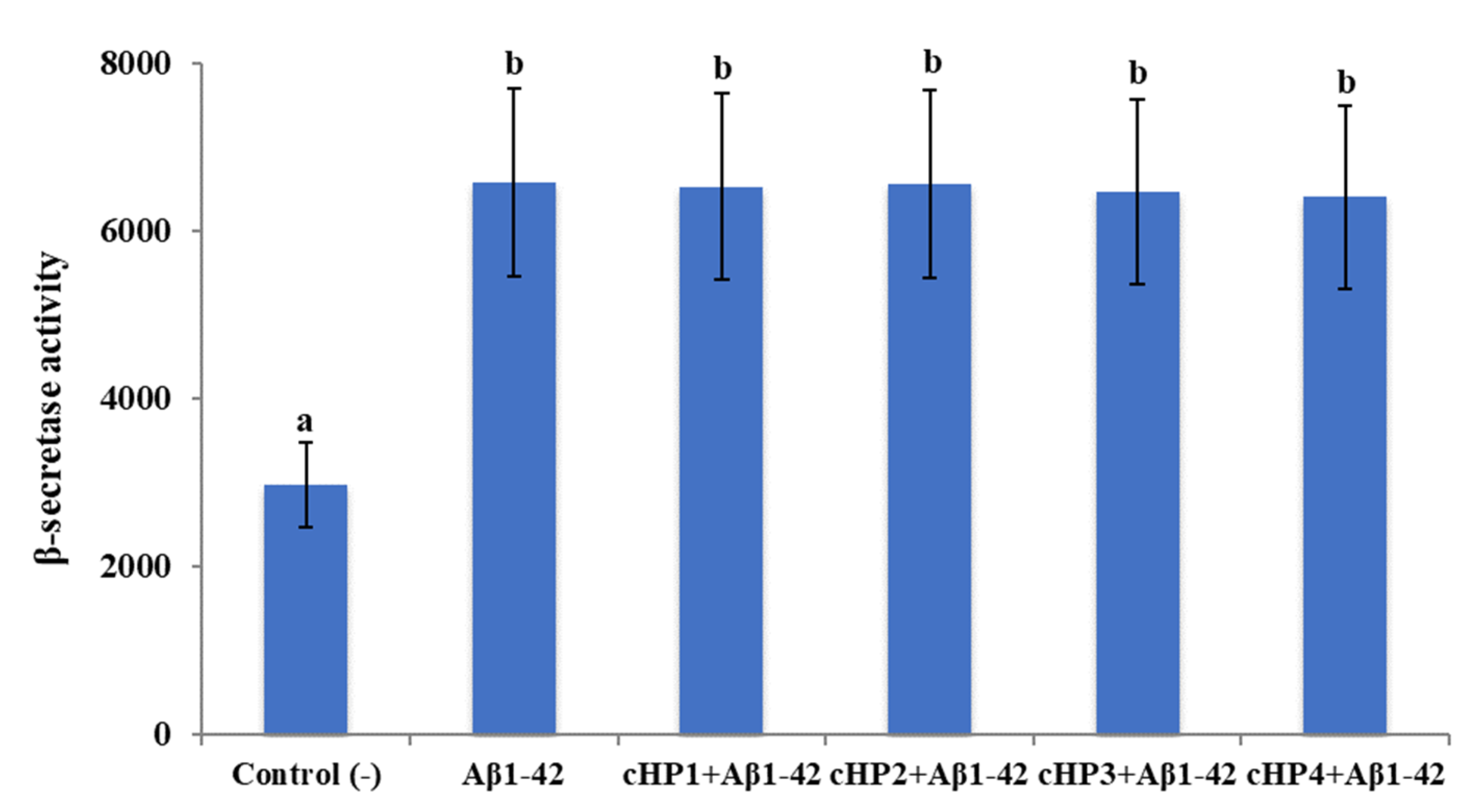
3.2. The Applications with cHP Isomers Altered the Activity of AChE but not the Activity of TACE and BACE1
3.3. cHP1-4 Supported Total Antioxidant Capacity without Altering Total Oxidative Status Levels in the Cellular AD Model
3.4. cHP4 Provided In Vitro Protection to Neuron-Like Cells against Apoptotic and Necrotic Effects by Aβ1-42 in Cellular AD Model
3.5. cHP4 Modulated the Alterations of Gene Expressions by Aβ1-42 Exposure
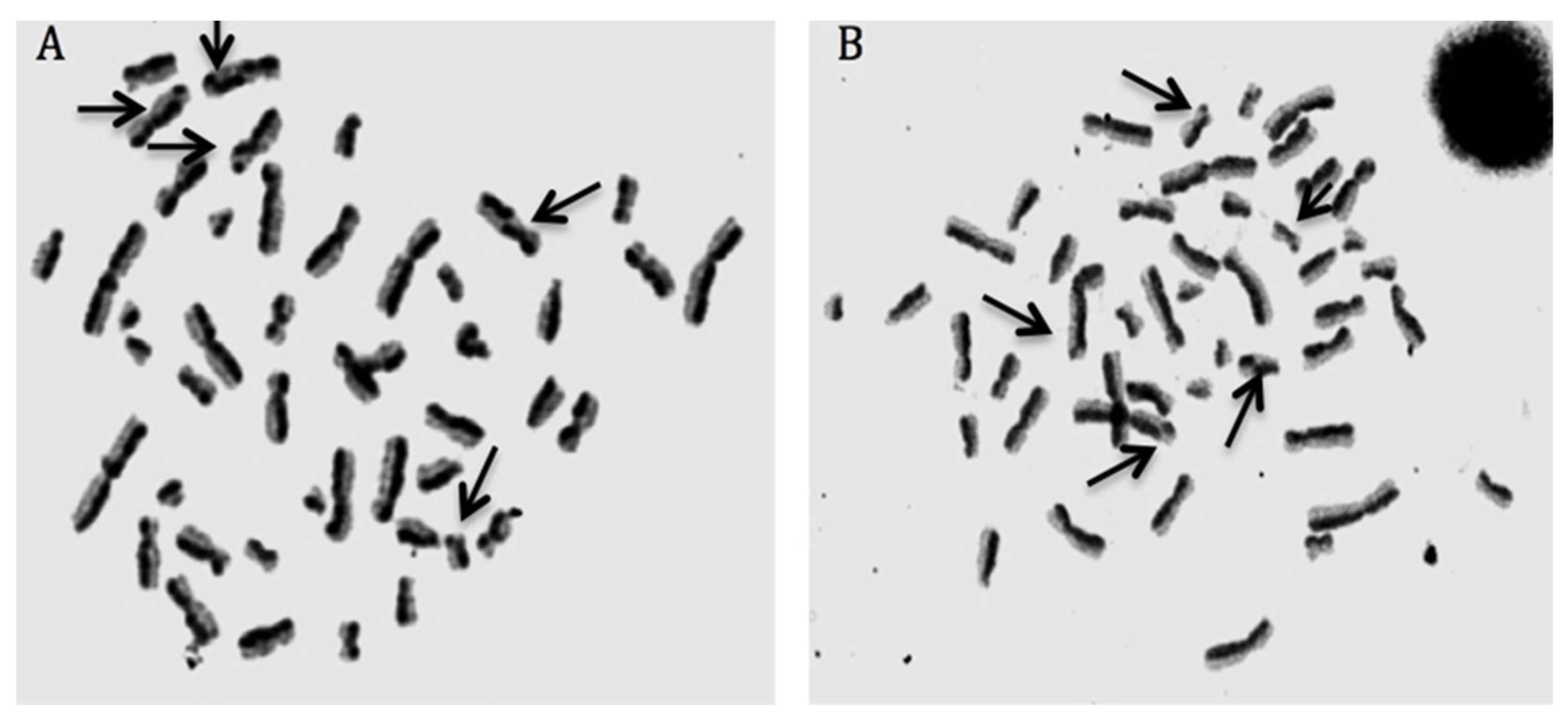
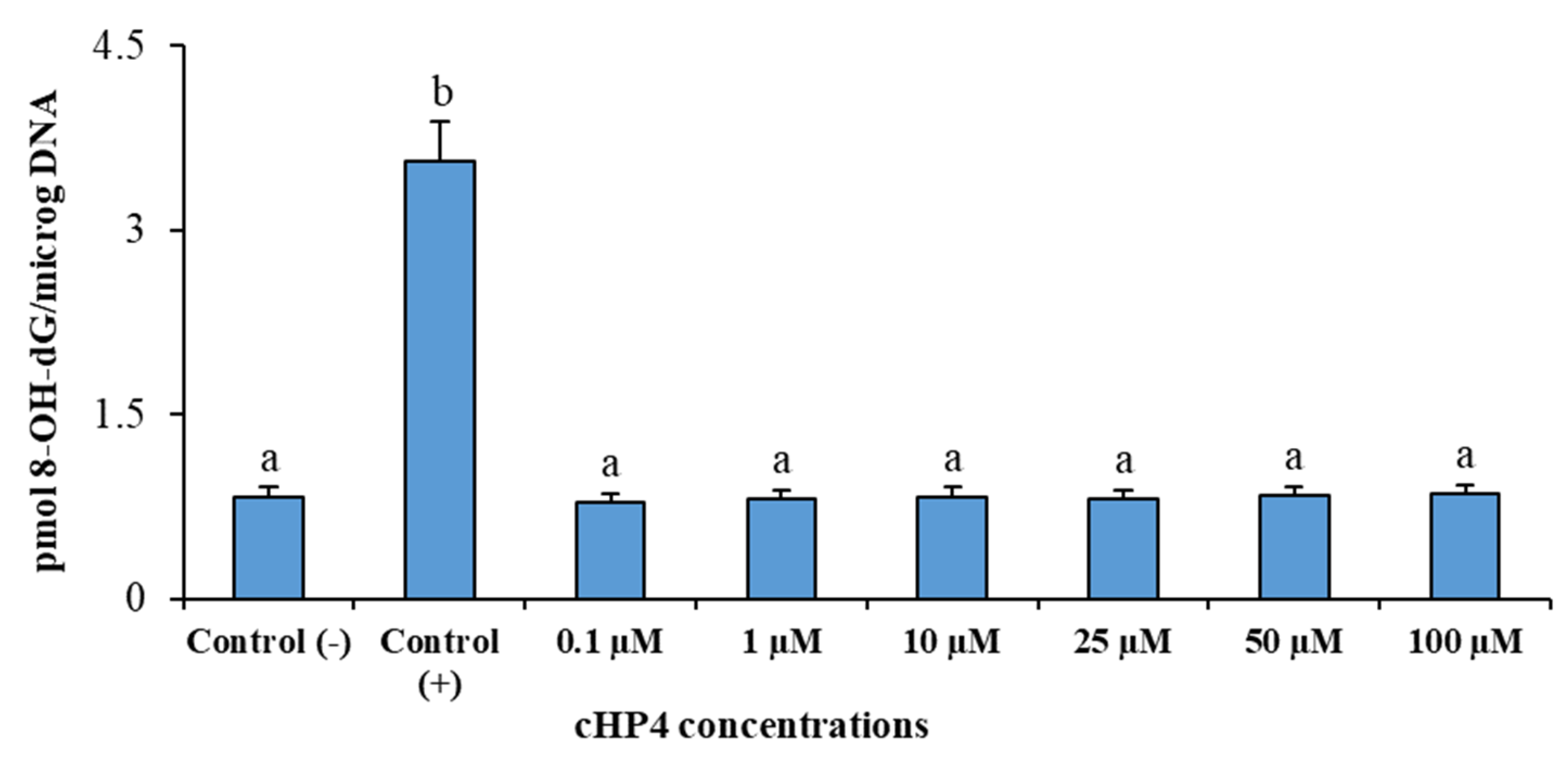
3.6. cHP4 Exhibited Noncytotoxic and Non-Genotoxic Features in Cultured Human Whole Blood Cells
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Minelli, A.; Conte, C.; Grottelli, S.; Bellezza, M.; Cacciatore, I.; Bolanos, J.P. Cyclo(his-pro) promotes cytoprotection by activating Nrf2-mediated up-regulation of antioxidant defence. J. Cell Mol. Med. 2009, 13, 1149–1161. [Google Scholar] [CrossRef] [Green Version]
- Minelli, A.; Grottelli, S.; Mierla, A. Cyclo(His-Pro) exerts anti-inflammatory effects by modulating NF-κB and Nrf2 signalling. Int. J. Biochem. Cell Biol. 2012, 44, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Grottelli, S.; Ferrari, I.; Pietrini, G. The role of cyclo(His-Pro) in neurodegeneration. Int. J. Mol. Sci. 2016, 17, 1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grottelli, S.; Mezzasoma, L.; Scarpelli, P.; Cacciatore, I.; Cellini, B.; Bellezza, I. Cyclo(His-Pro) inhibits NLRP3 inflammasome cascade in ALS microglial cells. Mol. Cell Neurosci. 2019, 94, 23–31. [Google Scholar] [CrossRef]
- Prasad, C.; Jayaraman, A.; Robertson, H.J.F.; Rao, J.K. Is all cyclo(His-Pro) derived from thyrotropin-releasing hormone? Neurochem. Res. 1987, 12, 767–774. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Grottelli, S.; Galli, F. Focus on cyclo(His-Pro): History and perspectives as antioxidant peptide. Amino Acids 2008, 35, 283–289. [Google Scholar] [CrossRef]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L. 2,5-Diketopiperazines as neuroprotective agents. Mini-Rev. Med. Chem. 2011, 12, 2–12. [Google Scholar] [CrossRef]
- Grottelli, S.; Costanzi, E.; Peirce, M.J.; Minelli, A.; Cellini, B.; Bellezza, I. Potential influence of cyclo(his-pro) on proteostasis: Impact on neurodegenerative diseases. Curr. Protein Pept. Sci. 2018, 19, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Grottelli, S.; Mierla, A.L.; Cacciatore, I.; Fornasari, E.; Roscini, L.; Cardinali, G.; Minelli, A. Neuroinflammation and endoplasmic reticulum stress are coregulated by cyclo(His-Pro) to prevent LPS neurotoxicity. Int. J. Biochem. Cell Biol. 2014, 51, 159–169. [Google Scholar] [CrossRef]
- Song, M.K.; Bischoff, D.S.; Song, A.M. Metabolic relationship between diabetes and Alzheimer’s Disease affected by Cyclo(His-Pro) plus zinc treatment. BBA Clin. 2017, 7, 41–54. [Google Scholar] [CrossRef]
- Kukla, M.J.; Breslin, H.J.; Bowden, C.R. Synthesis, characterization, and anorectic testing of the four stereoisomers of cyclo(histidylproline). J. Med. Chem. 1985, 28, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Jamsa, A.; Hasslund, K.; Cowburn, R.F. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem. Biophys. Res. Commun. 2004, 319, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Roberg, K.; Jerhammar, F. Autophagy of amyloid beta-protein in differentiated neuroblastoma cells exposed to oxidative stress. Neurosci. Lett. 2006, 394, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kgedal, K. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimer Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucinska, M.; Giron, M.D.; Piotrowska, H. Novel promising estrogenic receptor modulators: Cytotoxic and estrogenic activity of benzanilides and dithiobenzanilides. PLoS ONE 2016, 11, e0145615. [Google Scholar] [CrossRef]
- Patruno, A.; Fornasari, E.; Di Stefano, A.; Cerasa, L.S.; Marinelli, L.; Baldassarre, L.; Sozio, P.; Turkez, H.; Franceschelli, S.; Ferrone, A.; et al. Synthesis of a novel cyclic prodrug of S-allyl-glutathione able to attenuate LPS-induced ROS production through the inhibition of MAPK pathways in U937 cells. Mol. Pharm. 2015, 12, 66–74. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, N.; Li, C.; Chang, Q.; Liu, X.; Liao, Y.; Pan, R. Longistyline C acts antidepressant in vivo and neuroprotection in vitro against glutamate-induced cytotoxicity by regulating NMDAR/NR2B-ERK pathway in PC12 cells. PLoS ONE 2017, 12, e0183702. [Google Scholar] [CrossRef] [Green Version]
- Dirican, E.; Turkez, H. In vitro studies on protective effect of Glycyrrhiza glabra root extracts against cadmium-induced genetic and oxidative damage in human lymphocytes. Cytotechnology 2014, 66, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhao, Q.; Xiang, H.; Liu, M.; Zhang, Q.; Xue, W.; Song, B.; Yang, S. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, L.M.; Zhang, Y.; Qiao, J.T.; Zhang, C. Humanin protects neurons against apoptosis induced by Abeta31-35 through suppression of intrinsic pathway. Sheng Li Xue Bao 2010, 62, 93–100. [Google Scholar] [PubMed]
- Majstorović, I.; Vučević, D.; Pavlović, B.; Vasilijić, S.; Čolić, M. An anti-DEC-205 monoclonal antibody stimulates binding of thymocytes to rat thymic dendritic cells and promotes apoptosis of thymocytes. Cent. Eur. J. Immunol. 2014, 39, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, H.J.; O’Riordan, M.L. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat. Res. 1975, 31, 135–148. [Google Scholar] [CrossRef]
- Dirican, E.; Turkez, H.; Toğar, B. Modulatory effects of Thymbra spicata L. different extracts against the mercury induced genotoxicity in human lymphocytes in vitro. Cytotechnology 2012, 64, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M. The cytokinesis-block micronucleus technique: A detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. Fundam. Mol. Mech. Mutagen. 1993, 285, 35–44. [Google Scholar] [CrossRef]
- Turkez, H.; Tatar, A.; Hacimuftuoglu, A.; Ozdemir, E. Boric acid as a protector against paclitaxel genotoxicity. Acta Biochim. Pol. 2010, 57, 95–97. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Lau, W.K.W.; Yu, M.S.; Lai, C.S.W.; Yeung, S.C.; So, K.F.; Chang, R.C.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Uemura, K.; Kitagawa, N.; Kohno, R.; Kuzuya, A.; Kageyama, T.; Shibasaki, H.; Shimohama, S. Presenilin 1 mediates retinoic acid-induced differentiation of SH-SY5Y cells through facilitation of Wnt signaling. J. Neurosci. Res. 2003, 73, 166–175. [Google Scholar] [CrossRef]
- Constantinescu, R.; Constantinescu, A.T.; Reichmann, H.; Janetzky, B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. In Neuropsychiatric Disorders an Integrative Approach; Springer: Vienna, Austria, 2007; pp. 17–28. [Google Scholar]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2003, 5, 579–598. [Google Scholar] [CrossRef]
- Marinelli, L.; Fornasari, E.; Di Stefano, A.; Turkez, H.; Arslan, M.E.; Eusepi, P.; Ciulla, M.; Cacciatore, I. (R)-α-Lipoyl-Gly-L-Pro-L-Glu dimethyl ester as dual acting agent for the treatment of Alzheimer’s disease. Neuropeptides 2017, 66, 52–58. [Google Scholar] [CrossRef]
- Sutton, E.T.; Hellermann, G.R.; Thomas, T. β-Amyloid-induced endothelial necrosis and inhibition of nitric oxide production. Exp. Cell Res. 1997, 230, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.; Kim, Y.M.; Soon, C.H. Soluble neuregulin-1 from microglia enhances amyloid beta-induced neuronal death. CNS Neurol. Disord. Drug Targets 2016, 15, 918–926. [Google Scholar] [CrossRef] [PubMed]
- O’Barr, S.A.; Song, M.K.; Mendoza, K.H. Effects of zinc plus cyclo(his-pro) on pathology, learning and memory in a transgenic mouse model of Alzheimer’s disease. Alzheimer Dement. 2009, 5, 243. [Google Scholar] [CrossRef]
- Choi, J.R.; Shin, K.S.; Choi, C.Y.; Kang, S.J. PARP1 regulates the protein stability and proapoptotic function of HIPK2. Cell Death Dis. 2016, 7, e2438. [Google Scholar] [CrossRef] [Green Version]
- Martire, S.; Mosca, L.; d’Erme, M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer’s and Parkinson’s diseases. Mech. Ageing Dev. 2015, 146–148, 53–64. [Google Scholar] [CrossRef]
- Su, J.H.; Anderson, A.J.; Cribbs, D.H. Fas and Fas Ligand are associated with neuritic degeneration in the AD brain and participate in β-amyloid-induced neuronal death. Neurobiol. Dis. 2003, 12, 182–193. [Google Scholar] [CrossRef]
- Meng, P.; Yoshida, H.; Tanji, K. Carnosic acid attenuates apoptosis induced by amyloid-β 1-42 or 1-43 in SH-SY5Y human neuroblastoma cells. Neurosci. Res. 2015, 94, 1–9. [Google Scholar] [CrossRef]
- Wei, W.; Wang, X.; Kusiak, J.W. Signaling events in amyloid β-peptide-induced neuronal death and insulin-like growth factor I protection. J. Biol. Chem. 2002, 277, 17649–17656. [Google Scholar] [CrossRef] [Green Version]
- Ciesslik, M.; Czapski, G.A.; Strosznajder, J.B. The molecular mechanism of amyloid β42 peptide toxicity: The role of sphingosine kinase-1 and mitochondrial sirtuins. PLoS ONE 2015, 10, e0137193. [Google Scholar]
- Famer, D.; Crisby, M. Rosuvastatin reduces caspase-3 activity and up-regulates α-secretase in human neuroblastoma SH-SY5Y cells exposed to Aβ. Neurosci. Lett. 2004, 371, 209–214. [Google Scholar] [CrossRef]
- Li, R.; Lindholm, K.; Yang, L.B. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2004, 101, 3632–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiao, Y.J.; Su, M.H.; Lin, H.C.; Wu, C.R. Echinacoside ameliorates the memory impairment and cholinergic deficit induced by amyloid beta peptides via the inhibition of amyloid deposition and toxicology. Food Funct. 2017, 8, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Jasiecki, J.; Wasąg, B. Butyrylcholinesterase protein ends in the pathogenesis of alzheimer’s disease-could bche genotyping be helpful in alzheimer’s therapy? Biomolecules 2019, 9, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivieri, G.; Baysang, G.; Meier, F. N-acetyl-L-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: Effects on β-amyloid secretion and tau phosphorylation. J. Neurochem. 2001, 76, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Zhao, X. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010, 57, 547–555. [Google Scholar] [CrossRef]
- Bon, K.; Suh, H.J.; Ra, K.S.; Choi, J.W. Protective effect of cyclo(His-Pro) on streptozotocin-induced cytotoxicity and apoptosis in vitro. J. Microbiol. Biotechnol. 2011, 21, 218–227. [Google Scholar]
- Abiram, A.; Kolandaivel, P. Structural analysis and the effect of cyclo(His-Pro) dipeptide on neurotoxins-a dynamics and density functional theory study. J. Mol. Model. 2010, 16, 193–202. [Google Scholar] [CrossRef]
- Sato, H.; Tamba, M.; Ishii, T.; Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999, 274, 11455–11458. [Google Scholar] [CrossRef] [Green Version]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system x c-: Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef]
- Jiang, L.; Hickman, J.H.; Wang, S.J.; Gu, W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle 2015, 14, 2881–2885. [Google Scholar] [CrossRef]
- Liao, W.; Zheng, Y.; Fang, W.; Liao, S.; Xiong, Y.; Li, Y.; Xiao, S.; Zhang, X.; Liu, J. Dual specificity phosphatase 6 protects neural stem cells from β-amyloid-induced cytotoxicity through erk1/2 inactivation. Biomolecules 2018, 8, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyb, K.I.; Ansar, S.; Bean, J.; Michaelis, M.L. β-amyloid and endoplasmic reticulum stress reponses in primary neurons: Effects of drugs that interact with the cytoskeleton. J. Mol. Neurosci. 2006, 28, 111–123. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Ferreiro, E.; Oliveira, C.R. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 2191–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, C.G.; Wisén, S.; Gestwicki, J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1-42) aggregation in vitro. J. Biol. Chem. 2006, 281, 33182–33191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproul, A.A.; Jacob, S.; Pre, D. Characterization and molecular profiling of PSEN1 familial alzheimer’s disease iPSC-Derived neural progenitors. PLoS ONE 2014, 9, e84547. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; Omran, Z.; Zamzami, M.A. Signalling pathways in UHRF1-dependent regulation of tumor suppressor genes in cancer. J. Exp. Clin. Cancer Res. 2016, 35, 174. [Google Scholar] [CrossRef] [Green Version]
- Rhein, V.; Baysang, G.; Rao, S. Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol. Neurobiol. 2009, 29, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Wilhelmus, M.M.; De Waal, R.M.; Verbeek, M.M. Heat shock proteins and amateur chaperones in amyloid-beta accumulation and clearance in Alzheimer’s disease. Mol. Neurobiol. 2007, 35, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Ponzoni, M. Antiangiogenic strategies in neuroblastoma. Cancer Treat. Rev. 2005, 31, 27–34. [Google Scholar] [CrossRef]
- Priest, R.C.; Spaull, J.; Buckton, J. Immunomodulatory activity of a methionine aminopeptidase-2 inhibitor on B cell differentiation. Clin. Exp. Immunol. 2009, 155, 514–522. [Google Scholar] [CrossRef]
- Wu, M.F.; Yin, J.H.; Hwang, C.S. NAD attenuates oxidative DNA damages induced by amyloid beta-peptide in primary rat cortical neurons. Free Radic. Res. 2014, 48, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Nirzhor, S.S.R.; Khan, R.I.; Neelotpol, S. The biology of glial cells and their complex roles in alzheimer’s disease: New opportunities in therapy. Biomolecules 2018, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCBI (2019) ERCC1 ERCC Excision Repair 1, Endonuclease Non-Catalytic Subunit [Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/2067 (accessed on 14 January 2020).
- Braida, D.; Sala, M. Eptastigmine: Ten years of pharmacology, toxicology, pharmacokinetic, and clinical studies. CNS Drug Rev. 2001, 7, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Mihira, N.; Matsuba, Y.; Sasaguri, H.; Hashimoto, S.; Narasimhan, S.; Zhang, B.; Murayama, S.; Higuchi, M.; Lee, V.M.Y.; et al. Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J. Biol. Chem. 2019, 294, 12754–12765. [Google Scholar] [CrossRef]
- Bellezza, I.; Peirce, M.J.; Minelli, A. Cyclic Peptides in Neurological Disorders: The Case of Cyclo(His-Pro). In Quorum Sensing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–286. ISBN 9780128149065. [Google Scholar]






| Groups | % Cell Viability (MTT Assay) | % Cell Viability (LDH Assay) |
|---|---|---|
| Control (−) | 100 e | 100 g |
| 20 µM Aβ1-42 | 50.8 ± 3.7 a | 48.9 ± 2.5 a |
| Aβ + 0.1 µM cHP1 | 50.9 ± 3.2 a | 48.7 ± 2.7 a |
| Aβ + 1 µM cHP1 | 51.3 ± 4.0 a | 49.5 ± 3.0 a |
| Aβ + 10 µM cHP1 | 52.5 ± 3.7 a | 50.9 ± 2.5 a |
| Aβ + 25 µM cHP1 | 56.2 ± 4.4 ab | 53.6 ± 2.4 ab |
| Aβ + 50 µM cHP1 | 56.8 ± 3.5 ab | 57.1 ± 2.8 b |
| Aβ + 100 µM cHP1 | 57.9 ± 4.1 ab | 58.8 ± 3.0 b |
| Aβ + 0.1 µM cHP2 | 51.5 ± 3.3 a | 49.9 ± 2.8 a |
| Aβ + 1 µM cHP2 | 52.7 ± 4.2 a | 50.6 ± 3.1 a |
| Aβ + 10 µM cHP2 | 55.8 ± 5.0 ab | 52.9 ± 2.7 a |
| Aβ + 25 µM cHP2 | 57.1 ± 4.8 ab | 53.1 ± 2.8 ab |
| Aβ + 50 µM cHP2 | 56.9 ± 4.1 ab | 58.7 ± 2.4 b |
| Aβ + 100 µM cHP2 | 59.7 ± 3.8 b | 60.1 ± 3.3 b |
| Aβ + 0.1 µM cHP3 | 51.7 ± 4.1 a | 53.5 ± 3.0 ab |
| Aβ + 1 µM cHP3 | 55.5 ± 4.3 ab | 58.9 ± 2.5 b |
| Aβ + 10 µM cHP3 | 59.6 ± 4.7 b | 63.6 ± 2.8 bc |
| Aβ + 25 µM cHP3 | 66.5 ± 4.6 b | 66.8 ± 3.0 c |
| Aβ + 50 µM cHP3 | 69.0 ± 5.0 b | 72.4 ± 3.3 d |
| Aβ + 100 µM cHP3 | 71.3 ± 6.6 bc | 77.3 ± 3.5 de |
| Aβ + 0.1 µM cHP4 | 52.8 ± 3.6 a | 52.4 ± 2.7 ab |
| Aβ + 1 µM cHP4 | 55.9 ± 4.7 ab | 56.8 ± 2.9 b |
| Aβ + 10 µM cHP4 | 63.9 ± 5.2 b | 67.0 ± 3.4 c |
| Aβ + 25 µM cHP4 | 71.2 ± 6.8 bc | 74.6 ± 3.2 d |
| Aβ + 50 µM cHP4 | 79.4 ± 6.2 c | 82.3 ± 3.5 e |
| Aβ + 100 µM cHP4 | 87.9 ± 6.6 d | 88.5 ± 3.6 f |
| Groups | TAC Level (mmolTrolox Equiv./L) | TOS Level (µmol H2O2 Equiv./L) |
|---|---|---|
| Control (−) | 4.8 ± 0.7 b | 2.1 ± 0.3 a |
| Control (+) | 12.7 ± 1.3 f | 5.7 ± 0.6 c |
| 20 µM Aβ1-42 | 2.6 ± 0.4 a | 5.1 ± 0.5 b |
| 0.1 µM cHP1 | 4.9 ± 0.6 b | 2.0 ± 0.3 a |
| 1 µM cHP1 | 5.2 ± 0.8 b | 1.8 ± 0.2 a |
| 10 µM cHP1 | 5.6 ± 0.7 c | 2.0 ± 0.3 a |
| 25 µM cHP1 | 6.1 ± 0.6 c | 2.3 ± 0.4 a |
| 50 µM cHP1 | 6.3 ± 0.5 c | 2.2 ± 0.2 a |
| 100 µM cHP1 | 6.5 ± 0.7 c | 2.0 ± 0.2 a |
| 0.1 µM cHP2 | 4.7 ± 0.5 b | 1.7 ± 0.1 a |
| 1 µM cHP2 | 4.9 ± 0.7 b | 2.0 ± 0.2 a |
| 10 µM cHP2 | 5.3 ± 0.6 b | 2.1 ± 0.2 a |
| 25 µM cHP2 | 5.4 ± 0.8 b | 2.3 ± 0.3 a |
| 50 µM cHP2 | 6.1 ± 0.6 c | 2.3 ± 0.4 a |
| 100 µM cHP2 | 6.4 ± 0.7 c | 2.4 ± 0.3 a |
| 0.1 µM cHP3 | 5.0 ± 0.3 b | 1.9 ± 0.2 a |
| 1 µM cHP3 | 5.3 ± 0.5 c | 2.0 ± 0.2 a |
| 10 µM cHP3 | 5.8 ± 0.4 c | 2.0 ± 0.2 a |
| 25 µM cHP3 | 6.7 ± 0.5 c | 2.2 ± 0.3 a |
| 50 µM cHP3 | 7.1 ± 0.8 d | 2.2 ± 0.3 a |
| 100 µM cHP3 | 7.6 ± 0.5 d | 2.4 ± 0.2 a |
| 0.1 µM cHP4 | 4.9 ± 0.7 b | 1.8 ± 0.1 a |
| 1 µM cHP4 | 5.5 ± 0.7b | 2.0 ± 0.3 a |
| 10 µM cHP4 | 6.4 ± 0.6 c | 2.0 ± 0.2 a |
| 25 µM cHP4 | 6.8 ± 0.4 c | 2.0 ± 0.2 a |
| 50 µM cHP4 | 7.7 ± 0.7 d | 1.9 ± 0.1 a |
| 100 µM cHP4 | 8.9 ± 0.9 e | 2.0 ± 0.2 a |
| Groups | TAC Level (mmolTrolox Equiv./L) | TOS Level (µmol H2O2 Equiv./L) | |
|---|---|---|---|
| Control (−) | 4.8 ± 0.7 d | 2.1 ± 0.3 a | |
| Control (+) | 12.7 ± 1.3 e | 5.7 ± 0.6 e | |
| 20 µM Aβ1-42 | 2.6 ± 0.4 a | 5.1 ± 0.5 d | |
| Aβ1-42 plus cHP1 | 0.1 µM | 2.5 ± 0.5 a | 5.0 ± 0.7 d |
| 1 µM | 2.6 ± 0.4 a | 4.8 ± 0.5 d | |
| 10 µM | 2.7 ± 0.6 a | 4.7 ± 0.5 d | |
| 25 µM | 2.8 ± 0.5 a | 4.4 ± 0.6 d | |
| 50 µM | 3.0 ± 0.7 ab | 4.0 ± 0.5 c | |
| 100 µM | 3.2 ± 0.8 ab | 3.9 ± 0.4 c | |
| Aβ1-42 plus cHP2 | 0.1 µM | 2.7 ± 0.5 a | 4.8 ± 0.6 d |
| 1 µM | 2.8 ± 0.5 a | 4.7 ± 0.7 d | |
| 10 µM | 2.8 ± 0.5 a | 4.5 ± 0.5 d | |
| 25 µM | 3.0 ± 0.7 ab | 4.4 ± 0.4 d | |
| 50 µM | 3.1 ± 0.7 ab | 4.3 ± 0.6 d | |
| 100 µM | 3.2 ± 0.8 ab | 4.0 ± 0.4 c | |
| Aβ1-42 plus cHP3 | 0.1 µM | 2.7 ± 0.6 a | 4.7 ± 0.3 d |
| 1 µM | 2.9 ± 0.5 a | 4.6 ± 0.4 d | |
| 10 µM | 3.1 ± 0.7 ab | 4.5 ± 0.5 d | |
| 25 µM | 3.4 ± 0.7 b | 4.2 ± 0.4 d | |
| 50 µM | 3.5 ± 0.6 b | 3.9 ± 0.6 c | |
| 100 µM | 3.8 ± 0.8 c | 3.6 ± 0.5 c | |
| Aβ1-42 plus cHP4 | 0.1 µM | 2.7 ± 0.6 a | 4.6 ± 0.7 d |
| 1 µM | 2.9 ± 0.5 a | 4.3 ± 0.6 d | |
| 10 µM | 3.5 ± 0.7 b | 4.0 ± 0.5 c | |
| 25 µM | 3.7 ± 0.6 b | 3.6 ± 0.4 c | |
| 50 µM | 3.9 ± 0.8 c | 3.0 ± 0.4 b | |
| 100 µM | 4.6 ± 1.0 d | 2.7 ± 0.3 b | |
| Gene | Aβ1-42 | Aβ1-42 plus CHP4 |
|---|---|---|
| ACADVL | −0.77 | 3.83 |
| AKT1 | 0.15 | 0.08 |
| ADM2 | −0.43 | 3.68 |
| BCL2 | −0.94 | 4.67 |
| BCL2L1 | −0.77 | 5.12 |
| CASP8 | 3.45 | 2.89 |
| CASP9 | 2.91 | 0.83 |
| CYP2D6 | −0.45 | 1.75 |
| DNAJB9 | −0.63 | 2.35 |
| ERCC1 | 10.25 | 2.55 |
| FASLG | 1.14 | 0.64 |
| HSPA1A | 3.66 | 0.16 |
| METAP2 | −0.71 | 3.13 |
| PARP2 | 7.93 | 2.06 |
| SLC7A11 | −0.38 | 13.14 |
| UHRF1 | −0.54 | 3.12 |
| Groups | SCEs/Cell | MN/2000 Cells | Nuclear Division Index (NDI) | |
|---|---|---|---|---|
| Control (-) | 4.6 ± 0.7 a | 0.8 ± 0.1 a | 1.5 ± 0.3 b | |
| Control (+) | 11.4 ± 0.9 b | 3.9 ± 0.5 b | 1.0 ± 0.2 a | |
| cHP4 | 0.1 µM | 4.3 ± 0.4 a | 0.7 ± 0.2 a | 1.4 ± 0.2 b |
| 1 µM | 4.1 ± 0.7 a | 0.8 ± 0.2 a | 1.4 ± 0.1 b | |
| 10 µM | 4.5 ± 0.5 a | 0.9 ± 0.2 a | 1.4 ± 0.2 b | |
| 25 µM | 4.3 ± 0.7 a | 0.8 ± 0.2 a | 1.4 ± 0.3 b | |
| 50 µM | 4.7 ± 0.6 a | 0.9 ± 0.2 a | 1.3 ± 0.2 b | |
| 100 µM | 4.6 ± 0.5 a | 0.8 ± 0.2 a | 1.3 ± 0.2 b | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkez, H.; Cacciatore, I.; Arslan, M.E.; Fornasari, E.; Marinelli, L.; Di Stefano, A.; Mardinoglu, A. Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates. Biomolecules 2020, 10, 737. https://doi.org/10.3390/biom10050737
Turkez H, Cacciatore I, Arslan ME, Fornasari E, Marinelli L, Di Stefano A, Mardinoglu A. Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates. Biomolecules. 2020; 10(5):737. https://doi.org/10.3390/biom10050737
Chicago/Turabian StyleTurkez, Hasan, Ivana Cacciatore, Mehmet Enes Arslan, Erika Fornasari, Lisa Marinelli, Antonio Di Stefano, and Adil Mardinoglu. 2020. "Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates" Biomolecules 10, no. 5: 737. https://doi.org/10.3390/biom10050737
APA StyleTurkez, H., Cacciatore, I., Arslan, M. E., Fornasari, E., Marinelli, L., Di Stefano, A., & Mardinoglu, A. (2020). Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates. Biomolecules, 10(5), 737. https://doi.org/10.3390/biom10050737








