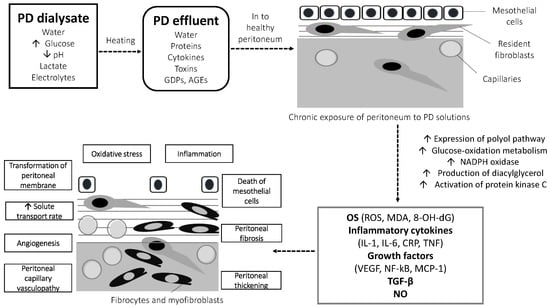
Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress
Abstract
:1. Introduction
2. OS in PD: The Composition of PD Solutions Is the Main Culprit
3. High-Glucose, Glucose Degradation Products, and Advanced Glycation End-Products
4. The Effect of Acidic pH and Lactate Buffer on Peritoneal OS
5. Other PD-Specific Factors Related with OS
6. Approaches to Ameliorate OS in PD and Preserve the Integrity of PM
7. Conclusions
Funding
Conflicts of Interest
Abbreviations/Nomenclature
| Abbreviation | Definition |
| 8-OH-dG | 8-hydroxy-2’-deoxyguanosine |
| AGEs | Advanced glycation end-products |
| AlaGln | Alanyl-glutamine |
| CAPD | Continuous ambulatory peritoneal dialysis |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| CSR | Cytoprotective cellular stress responses |
| CV | Cardiovascular |
| ESRD | End-stage renal disease |
| GDPs | Glucose degradation products |
| HD | Hemodialysis |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte chemotactic peptide-1 |
| MDA | Malondialdehyde |
| NAC | N-acetylcysteine |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOS | Nitric oxide synthase |
| OS | Oxidative stress |
| PD | Peritoneal dialysis |
| PM | Peritoneal membrane |
| PMC | Peritoneal mesothelial cell |
| RAGE | Receptor for AGEs |
| ROS | Reactive oxygen species |
| RRF | Residual renal function |
| RRT | Renal replacement therapy |
| TBARS | Thiobarbituric acid reactive substances |
| TGF-β | Tumor growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
References
- Jain, A.K.; Blake, P.; Cordy, P.; Garg, A.X. Global trends in rates of peritoneal dialysis. J. Am. Soc. Nephrol. 2012, 23, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.K.-T.; Chow, K.M.; Van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Mortier, S.; Lameire, N.H. What happens to the peritoneal membrane in long-term peritoneal dialysis? Perit. Dial. Int. 2001, 2021, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3494867. [Google Scholar] [CrossRef] [Green Version]
- Brunkhorst, R.; Mahiout, A. Pyruvate neutralizes peritoneal dialysate cytotoxicity: Maintained integrity and proliferation of cultured human mesothelial cells. Kidney Int. 1995, 48, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Chugh, S.; Chaudhry, S.; Ryan, T.; Margetts, P.J. Peritoneal Membrane Injury and Peritoneal Dialysis. Adv. Nephrol. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.T.; Chen, H.W.; Hsiao, H.H.; Chen, H.C. Heat shock response protects human peritoneal mesothelial cells from dialysate-induced oxidative stress and mitochondrial injury. Nephrol. Dial. Transplant. 2009, 24, 1799–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, H.; Lee, H.B. Effect of high glucose on peritoneal mesothelial cell biology. Perit. Dial. Int. 2000, 20, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, L.S.; Aguilera, A.; Selgas, R.; Ramírez-Huesca, M.; Pérez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; del Peso, G.; Sánchez-Tomero, J.A.; Jiménez-Heffernan, J.A. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: Role of vascular endothelial growth factor. Am. J. Kidney Dis. 2005, 46, 938–948. [Google Scholar] [CrossRef]
- Aguilera, A.; Loureiro, J.; Gónzalez-Mateo, G.; Selgas, R.; López-Cabrera, M. The mesothelial to mesenchymal transition a pathogenic and therapeutic key for peritoneal membrane failure. In The Latest in Peritoneal Dialysis; Peralta, A.A., Ed.; IntechOpen Limited: London, UK, 2013; Volume 21, pp. 21–37. [Google Scholar]
- López-Cabrera, M. Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv. Med. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Jagirdar, R.M.; Bozikas, A.; Zarogiannis, S.G.; Bartosova, M.; Schmitt, C.P.; Liakopoulos, V. Encapsulating Peritoneal Sclerosis: Pathophysiology and Current Treatment Options. Int. J. Mol. Sci. 2019, 20, 5765. [Google Scholar] [CrossRef] [Green Version]
- Gotloib, L. Mechanisms of cell death during peritoneal dialysis. In Peritoneal Dialysis-From Basic Concepts to Clinical Excellence; Karger Publishers: Basel, Switzerland, 2009; Volume 163, pp. 35–44. [Google Scholar]
- Simon, F.; Tapia, P.; Armisen, R.; Echeverria, C.; Gatica, S.; Vallejos, A.; Pacheco, A.; Sanhueza, M.E.; Alvo, M.; Segovia, E. Human peritoneal mesothelial cell death induced by high-glucose hypertonic solution involves Ca2+ and Na+ ions and oxidative stress with the participation of PKC/NOX2 and PI3K/Akt pathways. Front. Physiol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Gotloib, L. Mechanisms of cell death during peritoneal dialysis. A role for osmotic and oxidative stress. Contrib. Nephrol. 2009, 163, 35–44. [Google Scholar] [CrossRef]
- Gastaldello, K.; Husson, C.; Dondeyne, J.P.; Vanherweghem, J.L.; Tielemans, C. Cytotoxicity of mononuclear cells as induced by peritoneal dialysis fluids: Insight into mechanisms that regulate osmotic stress-related apoptosis. Perit. Dial. Int. 2008, 28, 655–666. [Google Scholar] [CrossRef]
- Lai, K.N.; Ho, S.K.; Leung, J.; Tang, S.C.; Chan, T.M.; Li, F.K. Increased survival of mesothelial cells from the peritoneum in peritoneal dialysis fluid. Cell Biol. Int. 2001, 25, 445–450. [Google Scholar] [CrossRef]
- Hung, K.Y.; Liu, S.Y.; Yang, T.C.; Liao, T.L.; Kao, S.H. High-dialysate-glucose-induced oxidative stress and mitochondrial-mediated apoptosis in human peritoneal mesothelial cells. Oxid. Med. Cell. Longev. 2014, 2014, 642793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, Y.; Sugimoto, T.; Ichikawa, Y.; Akatsuka, A.; Miyata, T.; Nangaku, M.; Tagawa, H.; Kurokawa, K. Glucose dialysate induces mitochondrial DNA damage in peritoneal mesothelial cells. Perit. Dial. Int. 2002, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Stocklauser-Farber, K.; Rosen, P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: Role of nitric oxide synthase? Free Radic. Biol. Med. 1999, 27, 752–763. [Google Scholar] [CrossRef]
- Gunal, A.I.; Celiker, H.; Ustundag, B.; Akpolat, N.; Dogukan, A.; Akcicek, F. The effect of oxidative stress inhibition with trimetazidine on the peritoneal alterations induced by hypertonic peritoneal dialysis solution. J. Nephrol. 2003, 16, 225–230. [Google Scholar]
- Catalan, M.P.; Santamaria, B.; Reyero, A.; Ortiz, A.; Egido, J.; Ortiz, A. 3,4-di-deoxyglucosone-3-ene promotes leukocyte apoptosis. Kidney Int. 2005, 68, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Mortier, S.; Faict, D.; Schalkwijk, C.G.; Lameire, N.H.; De Vriese, A.N.S. Long-term exposure to new peritoneal dialysis solutions: Effects on the peritoneal membrane. Kidney Int. 2004, 66, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Bartosova, M.; Schaefer, B.; Bermejo, J.L.; Tarantino, S.; Lasitschka, F.; Macher-Goeppinger, S.; Sinn, P.; Warady, B.A.; Zaloszyc, A.; Parapatics, K. Complement Activation in Peritoneal Dialysis–Induced Arteriolopathy. J. Am. Soc. Nephrol. 2018, 29, 268–282. [Google Scholar] [CrossRef]
- Ko, K.I.; Park, K.S.; Lee, M.J.; Doh, F.M.; Kim, C.H.; Koo, H.M.; Oh, H.J.; Park, J.T.; Han, S.H.; Kang, S.-W. Increased dialysate MCP-1 is associated with cardiovascular mortality in peritoneal dialysis patients: A prospective observational study. Am. J. Nephrol. 2014, 40, 291–299. [Google Scholar] [CrossRef]
- Kumano, K.; Schiller, B.; Hjelle, T.; Moran, J. Effects of osmotic solutes on fibronectin mRNA expression in rat peritoneal mesothelial cells. Blood Purif. 1996, 14, 165–169. [Google Scholar] [CrossRef]
- Kang, D.-H.; Hong, Y.-S.; Lim, H.J.; Choi, J.-H.; Han, D.-S.; Yoon, K.-I. High glucose solution and spent dialysate stimulate the synthesis of transforming growth factor-beta1 of human peritoneal mesothelial cells: Effect of cytokine costimulation. Perit. Dial. Int. 1999, 19, 221–230. [Google Scholar] [CrossRef]
- Lopez-Anton, M.; Lambie, M.; Lopez-Cabrera, M.; Schmitt, C.P.; Ruiz-Carpio, V.; Bartosova, M.; Schaefer, B.; Davies, S.; Stone, T.; Jenkins, R. miR-21 promotes fibrogenesis in peritoneal dialysis. Am. J. Pathol. 2017, 187, 1537–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int. J. Mol. Sci. 2019, 20, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.; Chen, L.; Hömme, M.; Hackert, T.; Gross, M.-L.; Hoffmann, G.F.; Schaefer, F.; Schmitt, C.P. Expression and function of matrix Gla protein in human peritoneal mesothelial cells. Nephrol. Dial. Transplant. 2010, 25, 3213–3221. [Google Scholar] [CrossRef] [Green Version]
- Combet, S.; Miyata, T.; Moulin, P.; Pouthier, D.; Goffin, E.; Devuyst, O. Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J. Am. Soc. Nephrol. 2000, 11, 717–728. [Google Scholar] [PubMed]
- Vostalova, J.; Galandakova, A.; Strebl, P.; Zadrazil, J. [Oxidative stress in patients on regular hemodialysis and peritoneal dialysis]. Vnitr. Lek. 2012, 58, 466–472. [Google Scholar] [PubMed]
- Witowski, J.; Topley, N.; Jorres, A.; Liberek, T.; Coles, G.A.; Williams, J.D. Effect of lactate-buffered peritoneal dialysis fluids on human peritoneal mesothelial cell interleukin-6 and prostaglandin synthesis. Kidney Int. 1995, 47, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topley, N.; Mackenzie, R.; Petersen, M.; Beavis, M.; Williams, D.; Thomas, N.; Faict, D.; Peluso, F.; Coles, G.; Davies, M. In vitro testing of a potentially biocompatible continuous ambulatory peritoneal dialysis fluid. Nephrol. Dial. Transplant. 1991, 6, 574–581. [Google Scholar] [CrossRef]
- Yamaji, Y.; Nakazato, Y.; Oshima, N.; Hayashi, M.; Saruta, T. Oxidative stress induced by iron released from transferrin in low pH peritoneal dialysis solution. Nephrol. Dial. Transplant. 2004, 19, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Ogata, S.; Naito, T.; Yorioka, N.; Kiribayashi, K.; Kuratsune, M.; Kohno, N. Effect of lactate and bicarbonate on human peritoneal mesothelial cells, fibroblasts and vascular endothelial cells, and the role of basic fibroblast growth factor. Nephrol. Dial. Transplant. 2004, 19, 2831–2837. [Google Scholar] [CrossRef] [Green Version]
- Mortier, S.; Faict, D.; Lameire, N.H.; De Vriese, A.S. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int. 2005, 67, 1559–1565. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Bloch, J.; Hömme, M.; Schaefer, J.; Hackert, T.; Philippin, B.; Schwenger, V.; Schaefer, F.; Schmitt, C.P. Buffer-dependent regulation of aquaporin-1 expression and function in human peritoneal mesothelial cells. Pediatr. Nephrol. 2012, 27, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Zareie, M.; Keuning, E.D.; ter Wee, P.M.; Schalkwijk, C.G.; Beelen, R.H.; van den Born, J. Improved biocompatibility of bicarbonate/lactate-buffered PDF is not related to pH. Nephrol. Dial. Transplant. 2006, 21, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, C.P.; Nau, B.; Gemulla, G.; Bonzel, K.E.; Hölttä, T.; Testa, S.; Fischbach, M.; John, U.; Kemper, M.J.; Sander, A. Effect of the dialysis fluid buffer on peritoneal membrane function in children. Clin. J. Am. Soc. Nephrol. 2013, 8, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eich, G.; Bartosova, M.; Tischer, C.; Wlodkowski, T.T.; Schaefer, B.; Pichl, S.; Kraewer, N.; Ranchin, B.; Vondrak, K.; Liebau, M.C. Bicarbonate buffered peritoneal dialysis fluid upregulates angiopoietin-1 and promotes vessel maturation. PLoS ONE 2017, 12, e0189903. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.C.; Jeong, T.K.; Lee, S.C.; Kim, S.W.; Kim, N.H.; Lee, K.Y. Nitric oxide is a marker of peritonitis in patients on continuous ambulatory peritoneal dialysis. Adv. Perit. Dial. 1998, 14, 173–179. [Google Scholar]
- Roumeliotis, S.; Eleftheriadis, T.; Liakopoulos, V. Is oxidative stress an issue in peritoneal dialysis? Semin. Dial. 2019, 32, 463–466. [Google Scholar] [CrossRef]
- Tarng, D.C.; Wen Chen, T.; Huang, T.P.; Chen, C.L.; Liu, T.Y.; Wei, Y.H. Increased oxidative damage to peripheral blood leukocyte DNA in chronic peritoneal dialysis patients. J. Am. Soc. Nephrol. 2002, 13, 1321–1330. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.N.; Leung, J.C. Inflammation in peritoneal dialysis. Nephron Clin. Pract. 2010, 116, c11–c18. [Google Scholar] [CrossRef] [Green Version]
- Bartosova, M.; Schaefer, B.; Vondrak, K.; Sallay, P.; Taylan, C.; Cerkauskiene, R.; Dzierzega, M.; Milosevski-Lomic, G.; Büscher, R.; Zaloszyc, A. Peritoneal dialysis vintage and glucose exposure but not peritonitis episodes drive peritoneal membrane Transformation during the first years of PD. Front. Physiol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Furuya, R.; Kumagai, H.; Odamaki, M.; Takahashi, M.; Miyaki, A.; Hishida, A. Impact of residual renal function on plasma levels of advanced oxidation protein products and pentosidine in peritoneal dialysis patients. Nephron Clin. Pract. 2009, 112, c255–c261. [Google Scholar] [CrossRef]
- Outerelo, M.C.; Gouveia, R.; e Costa, F.T.; Ramos, A. Intraperitoneal pressure has a prognostic impact on peritoneal dialysis patients. Perit. Dial. Int. 2014, 34, 652–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, I.D.; Cizman, B.; Mundt, K.; Wu, L.; Childers, R.; Mell, R.; Prichard, S. Relationship between drain volume/fill volume ratio and clinical outcomes associated with overfill complaints in peritoneal dialysis patients. Perit. Dial. Int. 2011, 31, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Díaz, V.P.; Ballesteros, S.S.; García, E.H.; Casado, E.D.; Callejo, I.H.; Perales, C.F. Intraperitoneal pressure in peritoneal dialysis. Nefrología (Engl. Ed.) 2017, 37, 579–586. [Google Scholar]
- Sanchez, N.C.; Tenofsky, P.L.; Dort, J.M.; Shen, L.Y. What is normal intra-abdominal pressure?/Discussion. Am. Surg. 2001, 67, 243. [Google Scholar] [PubMed]
- Enoch, C.; Aslam, N.; Piraino, B. Automated Peritoneal Dialysis Symposium: Intra-abdominal Pressure, Peritoneal Dialysis Exchange Volume, and Tolerance in APD. In Seminars in Dialysis; Blackwell Science Inc.: Malden, MA, USA, 2002; pp. 403–406. [Google Scholar]
- Cueto-Manzano, A.M.; Rojas-Campos, E.; Martinez-Ramirez, H.R.; Valera-Gonzalez, I.; Medina, M.; Monteon, F.; Ruiz, N.; Becerra, M.; Palomeque, M.A.; Cortes-Sanabria, L. Can the inflammation markers of patients with high peritoneal permeability on continuous ambulatory peritoneal dialysis be reduced on nocturnal intermittent peritoneal dialysis? Perit. Dial. Int. 2006, 26, 341–348. [Google Scholar] [CrossRef]
- Holmes, C.; Mujais, S. Glucose sparing in peritoneal dialysis: Implications and metrics. Kidney Int. 2006, 70, S104–S109. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y.; Seo, E.Y.; Lee, H.B.; Ha, H. Glucose-based peritoneal dialysis solution suppresses adiponectin synthesis through oxidative stress in an experimental model of peritoneal dialysis. Perit. Dial. Int. 2012, 32, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.; Leung, J.; Chan, L.; Li, F.; Tang, S.; Lam, M.; Tse, K.; Yip, T.; Chan, T.; Wieslander, A. Differential expression of receptors for advanced glycation end-products in peritoneal mesothelial cells exposed to glucose degradation products. Clin. Exp. Immunol. 2004, 138, 466–475. [Google Scholar] [CrossRef]
- Yung, S.; Lui, S.L.; Ng, C.K.; Yim, A.; Ma, M.K.; Lo, K.Y.; Chow, C.C.; Chu, K.H.; Chak, W.L.; Lam, M.F. Impact of a low-glucose peritoneal dialysis regimen on fibrosis and inflammation biomarkers. Perit. Dial. Int. 2015, 35, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Szeto, C.C.; Chow, K.M.; Lam, C.W.; Leung, C.B.; Kwan, B.C.; Chung, K.Y.; Law, M.C.; Li, P.K. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products--a 1-year randomized control trial. Nephrol. Dial. Transplant. 2007, 22, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Erixon, M.; Wieslander, A.; Lindén, T.; Carlsson, O.; Forsbäck, G.; Svensson, E.; Jönsson, J.Å.; Kjellstrand, P. How to avoid glucose degradation products in peritoneal dialysis fluids. Perit. Dial. Int. 2006, 26, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.P.; Aufricht, C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr. Nephrol. 2017, 32, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Szeto, C.C.; Johnson, D.W. Low GDP Solution and Glucose-Sparing Strategies for Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 30–42. [Google Scholar] [CrossRef]
- Htay, H.; Johnson, D.W.; Wiggins, K.J.; Badve, S.V.; Craig, J.C.; Strippoli, G.F.; Cho, Y. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst. Rev. 2018, 10, 1–131. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Topley, N.; Craig, K.J.; Mackenzie, R.K.; Pischetsrieder, M.; Lage, C.; Passlick-Deetjen, J.; Group, E.B.T. The Euro-Balance Trial: The effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004, 66, 408–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.W.; Brown, F.G.; Clarke, M.; Boudville, N.; Elias, T.J.; Foo, M.W.; Jones, B.; Kulkarni, H.; Langham, R.; Ranganathan, D. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: The balANZ trial. Nephrol. Dial. Transplant. 2012, 27, 4445–4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, K.; Hayes, A.; Cho, Y.; Cass, A.; Clarke, M.; Johnson, D.W. Economic Evaluation of Neutral-pH, Low–Glucose Degradation Product Peritoneal Dialysis Solutions Compared With Standard Solutions: A Secondary Analysis of the balANZ Trial. Am. J. Kidney Dis. 2015, 65, 773–779. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K. Neutral pH and low–glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef]
- Ueda, Y.; Miyata, T.; Goffin, E.; Yoshino, A.; Inagi, R.; Ishibashi, Y.; Izuhara, Y.; Saito, A.; Kurokawa, K.; Van Ypersele De Strihou, C. Effect of dwell time on carbonyl stress using icodextrin and amino acid peritoneal dialysis fluids. Kidney Int. 2000, 58, 2518–2524. [Google Scholar] [CrossRef] [Green Version]
- Gotloib, L.; Wajsbrot, V.; Shostak, A. Icodextrin-induced lipid peroxidation disrupts the mesothelial cell cycle engine. Free Radic. Biol. Med. 2003, 34, 419–428. [Google Scholar] [CrossRef]
- Gotloib, L.; Wajsbrot, V.; Shostak, A. Mesothelial dysplastic changes and lipid peroxidation induced by 7.5% icodextrin. Nephron 2002, 92, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S. Effect of icodextrin-based peritoneal dialysis solution on peritoneal membrane. Adv. Perit. Dial. 2005, 21. [Google Scholar]
- Moriishi, M.; Kawanishi, H. Icodextrin and intraperitoneal inflammation. Perit. Dial. Int. 2008, 28, S96–S100. [Google Scholar] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumeliotis, S.; Roumeliotis, A.; Gorny, X.; Mertens, P. Could antioxidant supplementation delay progression of cardiovascular disease in end-stage renal disease patients? Curr. Vasc. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid. Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shostak, A.; Gotloib, L.; Kushnier, R.; Wajsbrot, V. Protective effect of pyruvate upon cultured mesothelial cells exposed to 2 mM hydrogen peroxide. Nephron 2000, 84, 362–366. [Google Scholar] [CrossRef]
- Shostak, A.; Wajsbrot, V.; Gotloib, L. High glucose accelerates the life cycle of the in vivo exposed mesothelium. Kidney Int. 2000, 58, 2044–2052. [Google Scholar] [CrossRef]
- Wu, Y.T.; Wu, Z.L.; Jiang, X.F.; Li, S.; Zhou, F.Q. Pyruvate improves neutrophilic nitric oxide generation in peritoneal dialysis solutions. Art. Organs 2005, 29, 976–980. [Google Scholar] [CrossRef]
- Diaz-Buxo, J.A.; Gotloib, L. Agents that modulate peritoneal membrane structure and function. Perit. Dial. Int. 2007, 27, 16–30. [Google Scholar] [CrossRef]
- Aufricht, C.; Endemann, M.; Bidmon, B.; Arbeiter, K.; Mueller, T.; Regele, H.; Herkner, K.; Eickelberg, O. Peritoneal dialysis fluids induce the stress response in human mesothelial cells. Perit. Dial. Int. 2001, 21, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Lichtenauer, A.M.; Salzer, E.; Lechner, M.; Kuster, L.; Bergmeister, K.; Rizzi, A.; Mayer, B. Alanyl–glutamine dipeptide restores the cytoprotective stress proteome of mesothelial cells exposed to peritoneal dialysis fluids. Nephrol. Dial. Transplant. 2012, 27, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Gruber, K.; Lichtenauer, A.M.; Kuster, L.; Csaicsich, D.; Gleiss, A.; Alper, S.L.; Aufricht, C. Addition of Alanyl-Glutamine to dialysis fluid restores peritoneal cellular stress responses–A First-In-Man Trial. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, R.; Boehm, M.; Unterwurzacher, M.; Wagner, A.; Parapatics, K.; Majek, P.; Mueller, A.C.; Lichtenauer, A.; Bennett, K.L.; Alper, S.L. Effects of alanyl-glutamine treatment on the peritoneal dialysis effluent proteome reveal pathomechanism-associated molecular signatures. Mol. Cell. Proteom. 2018, 17, 516–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vychytil, A.; Herzog, R.; Probst, P.; Ribitsch, W.; Lhotta, K.; Machold-Fabrizii, V.; Wiesholzer, M.; Kaufmann, M.; Salmhofer, H.; Windpessl, M. A randomized controlled trial of alanyl-glutamine supplementation in peritoneal dialysis fluid to assess impact on biomarkers of peritoneal health. Kidney Int. 2018, 94, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Wiesenhofer, F.M.; Herzog, R.; Boehm, M.; Wagner, A.; Unterwurzacher, M.; Kasper, D.C.; Alper, S.L.; Vychytil, A.; Aufricht, C.; Kratochwill, K. Targeted metabolomic profiling of peritoneal dialysis effluents shows anti-oxidative capacity of alanyl-glutamine. Front. Physiol. 2019, 9, 1961. [Google Scholar] [CrossRef] [Green Version]
- Boehm, M.; Herzog, R.; Klinglmüller, F.; Lichtenauer, A.M.; Wagner, A.; Unterwurzacher, M.; Beelen, R.H.; Alper, S.L.; Aufricht, C.; Kratochwill, K. The peritoneal surface proteome in a model of chronic peritoneal dialysis reveals mechanisms of membrane damage and preservation. Front. Physiol. 2019, 10, 472. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. https://doi.org/10.3390/biom10050768
Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules. 2020; 10(5):768. https://doi.org/10.3390/biom10050768
Chicago/Turabian StyleRoumeliotis, Stefanos, Evangelia Dounousi, Marios Salmas, Theodoros Eleftheriadis, and Vassilios Liakopoulos. 2020. "Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress" Biomolecules 10, no. 5: 768. https://doi.org/10.3390/biom10050768
APA StyleRoumeliotis, S., Dounousi, E., Salmas, M., Eleftheriadis, T., & Liakopoulos, V. (2020). Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules, 10(5), 768. https://doi.org/10.3390/biom10050768