Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back
Abstract
1. Introduction
2. N7- and N9-Sugar Cytokinin Conjugates
2.1. Cytokinin 7- and 9-Glucosides
2.2. Cytokinin 9-Ribosides
2.2.1. IsCK 9-Ribosides
2.2.2. ArCK 9-Ribosides
2.3. Purine 9-(2′-Deoxyribosides) Cytokinin Conjugates
2.4. Purine N9-Arabinosides and Their Precursors
2.5. Cytokinin Disaccharide Conjugates
3. Non-Sugar N9-Substituted Cytokinins
3.1. 9-Alanyl Derivatives
3.2. Synthetic 9-Substituted Alkyl, Cycloalkyl, and Halogenoalkyl CK Derivatives
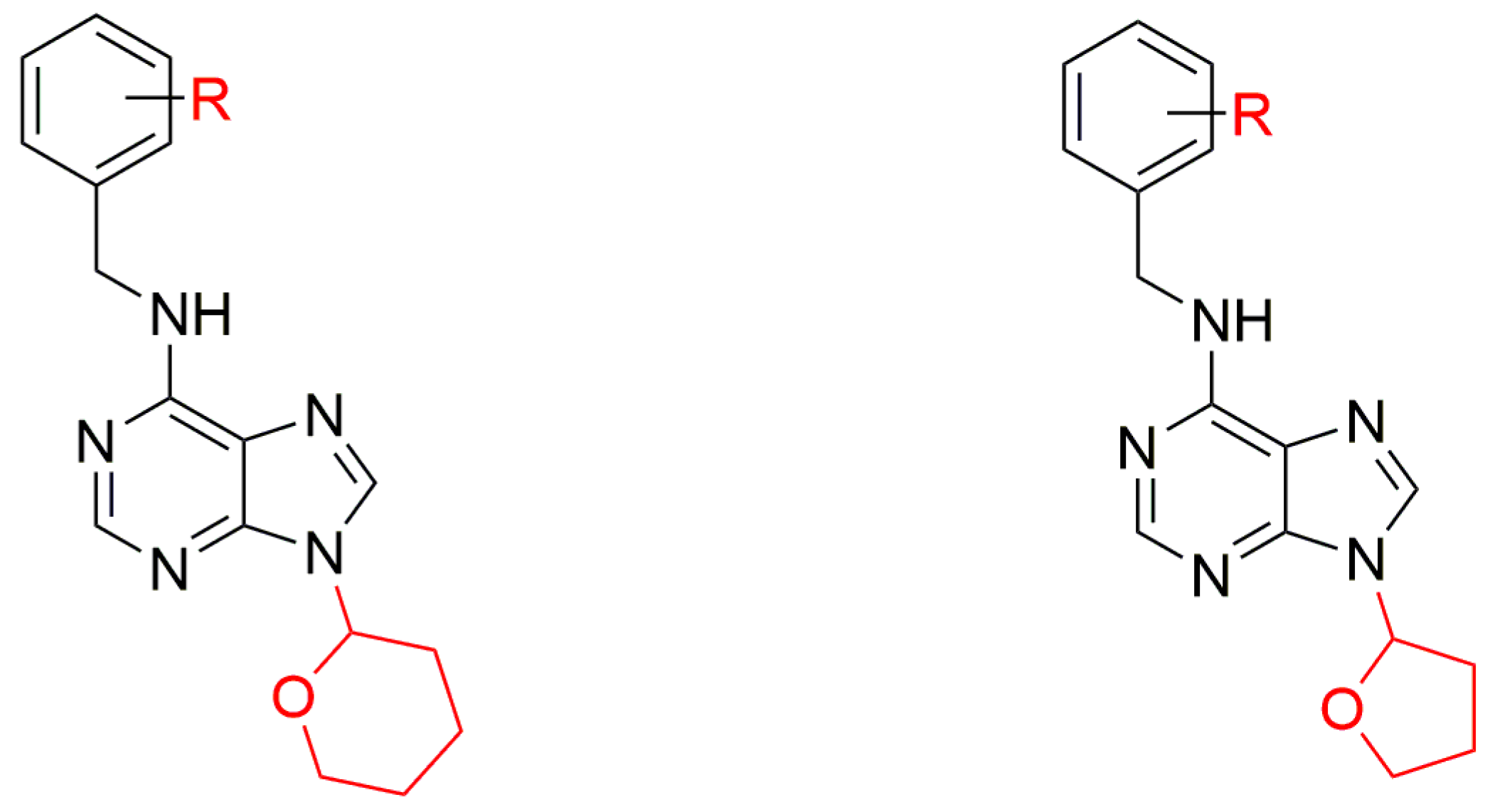
3.3. 9-(Tetrahydropyran-2-yl) and 9-(Tetrahydrofuran-2-yl)ated CKs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk Complexities between Auxin, Cytokinin, and Ethylene in Arabidopsis Root Development: From Experiments to Systems Modeling, and Back Again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef]
- Miller, C.O.; Skoog, F.; Von Saltza, M.H.; Strong, F.M. Kinetin, a Cell Division Factor from Deoxyribonucleic Acid. J. Am. Chem. Soc. 1955, 77, 1392. [Google Scholar] [CrossRef]
- Miller, C.O.; Skoog, F.; Okumura, F.S.; Von Saltza, M.H.; Strong, F.M. Isolation, Structure and Synthesis of Kinetin, a Substance Promoting Cell Division. J. Am. Chem. Soc. 1956, 78, 1375–1380. [Google Scholar] [CrossRef]
- Moyo, M.; Bairu, M.W.; Amoo, S.O.; Van Staden, J. Plant biotechnology in South Africa: Micropropagation research endeavours, prospects and challenges. S. Afr. J. Bot. 2011, 77, 996–1011. [Google Scholar] [CrossRef][Green Version]
- Barciüewski, J.; Rattan, S.I.S.; Siboska, G.; Clark, B.F.C. Kinetin—45 years on. Plant Sci. 1999, 148, 37–45. [Google Scholar] [CrossRef]
- Davies, P. Plant Hormones: Biosynthesis, Signal Transduction, Action! 3rd ed.; Springer: Maastricht, The Netherlands, 2010; ISBN 978-1-4020-2685-0. [Google Scholar]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Cortleven, A.; Schmülling, T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- D’Aloia, M.; Bonhomme, D.; Bouché, F.; Tamseddak, K.; Ormenese, S.; Torti, S.; Coupland, G.; Périlleux, C. Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J. 2011, 65, 972–979. [Google Scholar] [CrossRef]
- Brandes, H.; Kende, H. Studies on Cytokinin-Controlled Bud Formation in Moss Protonemata. Plant Physiol. 1968, 43, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Sakakibara, H.; Taniguchi, M.; Sugiyama, T. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: Implication of cytokinin species that induces gene expression of maize response regulator. Plant Cell Physiol. 2001, 42, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; Ehneß, R. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 2000, 32, 359–367. [Google Scholar] [CrossRef]
- Argueso, C.T.; Ferreira, F.J.; Kieber, J.J. Environmental perception avenues: The interaction of cytokinin and environmental response pathways. Plant Cell Environ. 2009, 32, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinins: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Osugi, A.; Sakakibara, H. Q and A: How do plants respond to cytokinins and what is their importance? BMC Biol. 2015, 13, 102. [Google Scholar] [CrossRef]
- Schoor, S.; Farrow, S.; Blaschke, H.; Lee, S.; Perry, G.; von Schwartzenberg, K.; Emery, N.; Moffatt, B. Adenosine kinase contributes to cytokinin interconversion in arabidopsis. Plant Physiol. 2011, 157, 659–672. [Google Scholar] [CrossRef]
- Hill, K.; Schaller, G.E. Enhancing plant regeneration in tissue culture. Plant Signal. Behav. 2013, 8, e25709. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2006, 143, 1442–1451. [Google Scholar] [CrossRef]
- Mok, D.W.S.; Mok, M.C. Cytokinin Metabolism and Action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef]
- Bastos de Almeida, W.A.; Silva Santana, G.; Pinheiro Martinelli Rodriguez, A.; Pereira de Carvalho Costa, M.A. Optimization of a protocol for the micropropagation of pineapple. Rev. Bras. Frutic. 2002, 24, 296–300. [Google Scholar] [CrossRef]
- Boudabous, M.; Mars, M.; Marzougui, N.; Ferchichi, A. Micropropagation of apple (Malus domestica L. cultivar Douce de Djerba) through in vitro culture of axillary buds. Acta Bot. Gall. 2010, 157, 513–524. [Google Scholar] [CrossRef]
- Goswami, K.; Sharma, R.; Singh, P.K.; Singh, G. Micropropagation of seedless lemon (Citrus limon L. cv. Kaghzi Kalan) and assessment of genetic fidelity of micropropagated plants using RAPD markers. Physiol. Mol. Biol. Plants 2013, 19, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Peixe, A.; Raposo, A.; Lourenço, R.; Cardoso, H.; Macedo, E. Coconut water and BAP successfully replaced zeatin in olive (Olea europaea L.) micropropagation. Sci. Hortic. 2007, 113, 1–7. [Google Scholar] [CrossRef]
- Hristova, L.; Damyanova, E.; Doichinova, Z.; Kapchina-Toteva, V. Effect Of 6-benzylaminopurine on micropropagation of artemisia chamaemelifolia Vill. (asteraceae). Bulg. J. Agric. Sci. 2013, 19, 57–60. [Google Scholar]
- Ružić, D.V.; Vujović, T.I. The effects of cytokinin types and their concentration on in vitro multiplication of sweet cherry cv. Lapins (Prunus avium L.). Hortic. Sci. 2008, 35, 12–21. [Google Scholar] [CrossRef]
- Grendysz, J.; Danuta, K.; Jacek, W. Influence of micropropagation with addition of kinetin on development of a willow (Salix viminalis L.). World Sci. J. 2017, 70, 201–215. [Google Scholar]
- Hesar, A.A.; Kaviani, B.; Tarang, A.; Zanjani, S.B. Effect of different concentrations of kinetin on regeneration of ten weeks (Matthiola incana). Plant Omics 2011, 4, 236–238. [Google Scholar]
- Hanur, V.S. In-Vitro Organogenesis in Tomato (Solanum Lycopersicum) Using Kinetin. Adv. Plants Agric. Res. 2016, 4, 397–401. [Google Scholar] [CrossRef]
- Lameira, O.A.; Pinto, J. In vitro propagation of Cordia verbenacea (Boraginaceae). Rev. Bras. Plant Med. Botucatu 2006, 8, 102–104. [Google Scholar]
- Naing, A.H.; Kim, S.H.; Chung, M.Y.; Park, S.K.; Kim, C.K. In vitro propagation method for production of morphologically and genetically stable plants of different strawberry cultivars. Plant Methods 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Amer, A.; Omar, H. In-vitro propagation of the multipurpose Egyptian medicinal plant Pimpinella anisum. Egypt Pharm. J. 2019, 18, 254–262. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Manokari, M.; Ravindran, C.P. Micropropagation, Micromorphological Studies, and in Vitro Flowering in Rungia pectinata L. Scientifica 2016. [Google Scholar] [CrossRef]
- Kelta, A.; Hajare, S.T.; Banjaw, A. Studies on in vitro Micropropagation in Banana. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3366–3375. [Google Scholar] [CrossRef]
- Melyan, G.; Sahakyan, A.; Harutyunyan, A. Micropropagation of grapevine (Vitis vinifera L.) seedless cultivar “Parvana” through lateral bud development. Vitis—J. Grapevine Res. 2015, 54, 253–255. [Google Scholar]
- Balajaru, K.; Agastian, P.; Preetamraj, J.P.; Arokiyaraj, S.; Ignacimuthu, S. Micropropagation of Vitex agnus-castus, (Verbenaceae)—A valuable medicinal plant. In Vitro Cell. Dev. Biol.—Plant 2008, 44, 436–441. [Google Scholar] [CrossRef]
- Pelegrini, L.L.; Ribas, L.L.F.; Zanette, F.; Koehler, H.S. Micropropagation of Ocotea porosa (Nees & Martius) Barroso. Afr. J. Biotechnol. 2011, 10, 1527–1533. [Google Scholar] [CrossRef]
- Galuszka, P.; Popelková, H.; Werner, T.; Frébortová, J.; Pospíšilová, H.; Mik, V.; Köllmer, I.; Schmülling, T.; Frébort, I. Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Werbrouck, S.; Strnad, M.; Onckelen, H.; Debergh, P. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Podlešáková, K.; Zalabák, D.; Čudejková, M.; Plíhal, O.; Szüčová, L.; Doležal, K.; Spíchal, L.; Strnad, M.; Galuszka, P. Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS ONE 2012, 7, e39293. [Google Scholar] [CrossRef]
- Bairu, M.W.; Jain, N.; Stirk, W.A.; Doležal, K.; Van Staden, J. Solving the problem of shoot-tip necrosis in Harpagophytum procumbens by changing the cytokinin types, calcium and boron concentrations in the medium. S. Afr. J. Bot. 2009, 75, 122–127. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; van der Jeugt, B.; Dewitte, W.; Prinsen, E.; Van Onckelen, H.A.; Debergh, P.C. The metabolism of benzyladenine in Spathiphyllum floribundum “Schott Petite” in relation to acclimatisation problems. Plant Cell Rep. 1995, 14, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Cary, A.J.; Liu, W.; Howell, S.H. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995, 107, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Doležal, K.; Popa, I.; Hauserová, E.; Spíchal, L.; Chakrabarty, K.; Novák, O.; Kryštof, V.; Voller, J.; Holub, J.; Strnad, M. Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorgan. Med. Chem. 2007, 15, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Vylíčilová, H.; Husičková, A.; Spíchal, L.; Srovnal, J.; Doležal, K.; Plíhal, O.; Plíhalová, L. C2-substituted aromatic cytokinin sugar conjugates delay the onset of senescence by maintaining the activity of the photosynthetic apparatus. Phytochemistry 2016, 122, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Plíhalová, L.; Vylíčilová, H.; Doležal, K.; Zahajská, L.; Zatloukal, M.; Strnad, M. Synthesis of aromatic cytokinins for plant biotechnology. Biotechnol. 2016, 33, 614–624. [Google Scholar] [CrossRef]
- Plíhal, O.; Szüčová, L.; Galuszka, P. N9-substituted aromatic cytokinins with negligible side effects on root development are an emerging tool for in vitro culturing. Plant Signal. Behav. 2013, 8, e24392. [Google Scholar] [CrossRef]
- Kieber, J.J. Cytokinins. Arabidopsis Book. 2002, 1, e0063. [Google Scholar] [CrossRef]
- Letham, D.S.; Gollnow, B. Regulators of cell division in plant tissues. XXX. Cytokinin metabolism in relation to radish cotyledon expansion and senescence. J. Plant Growth Regul. 1985, 4, 129–145. [Google Scholar] [CrossRef]
- Holub, J.; Hanuš, J.; Hanke, D.E.; Strnad, M. Biological activity of cytokinins derived from Ortho- and Meta-hydroxybenzyladenine. Plant Growth Regul. 1998, 26, 109–115. [Google Scholar] [CrossRef]
- Palni, L.M.; Summons, R.; Letham, D. Mass Spectrometric Analysis of Cytokinins in Plant Tissues: V. Identification of the Cytokinin Complex of Datura Innoxia Crown Gall Tissue. Plant Physiol. 1983, 72, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Spíchal, L.; Rakova, N.Y.; Riefler, M.; Mizuno, T.; Romanov, G.A.; Strnad, M.; Schmülling, T. Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol. 2004, 45, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Hošek, P.; Hoyerová, K.; Kiran, N.S.; Dobrev, P.I.; Zahajská, L.; Filepová, R.; Motyka, V.; Müller, K.; Kamínek, M. Distinct metabolism of N-glucosides of isopentenyladenine and trans-zeatin determines cytokinin metabolic spectrum in Arabidopsis. New Phytol. 2020, 225, 2423–2438. [Google Scholar] [CrossRef] [PubMed]
- Sayavedra-Soto, L.A.; Durley, R.C.; Trione, E.J.; Morris, R.O. Identification of cytokinins in young wheat spikes (Triticum aestivum cv. Chinese Spring). J. Plant Growth Regul. 1988, 7, 169–178. [Google Scholar] [CrossRef]
- Werner, T.; Hanuš, J.; Holub, J.; Schmülling, T.; Van Onckelen, H.; Strnad, M. New cytokinin metabolites in IPT transgenic Arabidopsis thaliana plants. Physiol. Plant. 2003, 118, 127–137. [Google Scholar] [CrossRef]
- Hou, B.; Lim, E.K.; Higgins, G.S.; Bowles, D.J. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 47822–47832. [Google Scholar] [CrossRef]
- Entsch, B.; Letham, D.S. Enzymic glucosylation of the cytokinin, 6-benzylaminopurine. Plant Sci. Lett. 1979, 14, 205–212. [Google Scholar] [CrossRef]
- Šmehilová, M.; Dobrůšková, J.; Novák, O.; Takáč, T.; Galuszka, P. Cytokinin-specific glycosyltransferases possess different roles in cytokinin homeostasis maintenance. Front. Plant Sci. 2016, 7, 1264. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, B.; Dong, R.R.; Hou, B.K. AtUGT76C2, an Arabidopsis cytokinin glycosyltransferase is involved in drought stress adaptation. Plant Sci. 2015, 236, 157–167. [Google Scholar] [CrossRef]
- Lao, J.; Oikawa, A.; Bromley, J.R.; McInerney, P.; Suttangkakul, A.; Smith-Moritz, A.M.; Plahar, H.; Chiu, T.Y.; González Fernández-Niño, S.M.; Ebert, B.; et al. The plant glycosyltransferase clone collection for functional genomics. Plant J. 2014, 79, 517–529. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.M.; Kojima, M.; Sakakibara, H.; Hou, B.K. N-glucosyltransferase UGT76C2 is involved in cytokinin homeostasis and cytokinin response in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 2200–2213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.M.; Kojima, M.; Sakakibara, H.; Hou, B.K. Glucosyltransferase UGT76C1 finely modulates cytokinin responses via cytokinin N-glucosylation in Arabidopsis thaliana. Plant Physiol. Biochem. 2013, 65, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gajdošová, S.; Spíchal, L.; Kamínek, M.; Hoyerová, K.; Novák, O.; Dobrev, P.I.; Galuszka, P.; Klíma, P.; Gaudinová, A.; Žižková, E.; et al. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J. Exp. Bot. 2011, 62, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Argueso, C.T. Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann. Bot. 2017, 119, 725–735. [Google Scholar] [CrossRef]
- Skoog, F.; Hamzi, H.Q.; Szweykowska, A.M.; Leonard, N.J.; Carraway, K.L.; Fujii, T.; Helgeson, J.P.; Loeppky, R.N. Cytokinins: Structure/activity relationships. Phytochemistry 1967, 6, 1169–1192. [Google Scholar] [CrossRef]
- Leonard, N.J.; Hecht, S.M.; Skoog, F.; Schmitz, R.Y. Cytokinins: Synthesis, mass spectra, and biological activity of compounds related to zeatin. Biochemistry 1969, 63, 175–182. [Google Scholar] [CrossRef]
- Fleysher, M.H. N6-Substituted Adenosines: Synthesis, Biological Activity, and Some Structure-Activity Relationships. J. Med. Chem. 1972, 15, 187–191. [Google Scholar] [CrossRef]
- Schmitz, R.Y.; Skoog, F.; Playtis, A.J.; Leonard, N.J. Cytokinins: Synthesis and Biological Activity of Geometric and Position Isomers of Zeatin. Plant Physiol. 1972, 50, 702–705. [Google Scholar] [CrossRef]
- Laloue, M.; Terrine, C.; Guern, J. Cytokinins: Metabolism and Biological Activity of N6-(Δ2-Isopentenyl)adenosine and N6-(Δ2-Isopentenyl)adenine in Tobacco Cells and Callus. Plant Physiol. 1977, 59, 478–483. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Kojima, M.; Yamaya, T.; Sakakibara, H. Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol. 2004, 134, 1654–1661. [Google Scholar] [CrossRef]
- Kamínek, M.; Pačes, V.; Corse, J.; Challice, J. Effect of Stereospecifîc Hydroxylation of N6-(Δ2-Isopentenyl)adenosine on Cytokinin Activity. Planta 1979, 145, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, J.; Bayley, A.D.; Upfold, S.J.; Drewes, F.E. Cytokinins in Cut Carnation Flowers. VIII. Uptake, Transport and Metabolism of Benzyladenine and the Effect of Benzyladenine Derivatives on Flower Longevity. J. Plant Physiol. 1990, 135, 703–707. [Google Scholar] [CrossRef]
- Radhika, V.; Ueda, N.; Tsuboi, Y.; Kojima, M.; Kikuchi, J.; Kudo, T.; Sakakibara, H. Methylated Cytokinins from the Phytopathogen Rhodococcus fascians Mimic Plant Hormone Activity. Plant Physiol. 2015, 169, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Pertry, I.; Vaclavikova, K.; Depuydt, S.; Galuszka, P.; Spichal, L.; Temmerman, W.; Stes, E.; Schmulling, T.; Kakimoto, T.; Van Montagu, M.C.E.; et al. Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proc. Natl. Acad. Sci. USA 2009, 106, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Pertry, I.; Vaclavikova, K.; Gemrotova, M.; Spichal, L.; Galuszka, P.; Depuydt, S.; Temmerman, W.; Stes, E.; De Keyser, A.; Riefler, M.; et al. Rhodococcus fascians impacts plant development through the dynamic fas-mediated production of a cytokinin mix. Mol. Plant Microbe Interact. 2010, 23, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Fujii, T.; Iacobellis, N.; Riva, I.S.; Sisto, A.; Surico, G. Structure-activity relationship of zeatin cytokinins produced by plant pathogenic Pseudomonades. Phytochemistry 1991, 30, 3505–3510. [Google Scholar] [CrossRef]
- Fujii, T.; Ohba, M.; Kawamura, H.; Nakashio, Y.; Honda, K.; Matsubara, S. Purines. LXII. Both enantiomers of N6-(1,3-dimethyl-2-butenyl)adenine and their 9-β-D-ribofuranosides: Synthesis and cytokinin activity. Chem. Pharm. Bull. 1994, 42, 1045–1049. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, 1–7. [Google Scholar] [CrossRef]
- Lomin, S.N.; Krivosheev, D.M.; Steklov, M.Y.; Osolodkin, D.I.; Romanov, G.A. Receptor Properties and Features of Cytokinin Signaling. Acta Nat. 2012, 4, 31–45. [Google Scholar] [CrossRef]
- Osugi, A.; Kojima, M.; Takebayashi, Y.; Ueda, N.; Kiba, T.; Sakakibara, H. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat. Plants 2017, 3, 17112. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Conesa, C.M.; Saez, A.; Navarro-Neila, S.; Garcia-Mina, J.M.; Zamarreño, A.M.; Baigorri, R.; Swarup, R.; del Pozo, J.C. Role of cis-zeatin in root responses to phosphate starvation. New Phytol. 2019, 224, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.E.; Mok, M.C.; Mok, D.W.S. Zeatin Metabolism in Fruits of Phaseolus. Plant Physiol. 1985, 79, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Letham, D.S. The biosynthesis and metabolism of cytokinins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1983, 34, 163–197. [Google Scholar] [CrossRef]
- Singh, S.; Palni, L.M.S.; Letham, D.S. Cytokinin Biochemistry in Relation to Leaf Senescence V. Endogenous Cytokinin Levels and Metabolism of Zeatin Riboside in Leaf Discs from Green and Senescent Tobacco (Nicotiana rustica) Leaves. J. Plant Physiol. 1992, 139, 279–283. [Google Scholar] [CrossRef]
- Scott, I.M.; Martin, G.C.; Horgan, R.; Heald, J. Mass spectrometric measurement of zeatin glycoside levels in Vinca rosea L. crown gall tissue. Planta 1982, 154, 273–276. [Google Scholar] [CrossRef]
- Duke, C.C.; Letham, D.S.; Parker, C.W.; MacLeod, J.K.; Summons, R.E. The complex of O-glucosylzeatin derivatives formed in Populus species. Phytochemistry 1979, 18, 819–824. [Google Scholar] [CrossRef]
- Aremu, A.O.; Plačková, L.; Gruz, J.; Bíba, O.; Šubrtová, M.; Novák, O.; Doležal, K.; Van Staden, J. Accumulation pattern of endogenous cytokinins and phenolics in different organs of 1-year-old cytokinin pre-incubated plants: Implications for conservation. Plant Biol. 2015, 17, 1146–1155. [Google Scholar] [CrossRef]
- Von Schwartzenberg, K.; Núñez, M.F.; Blaschke, H.; Dobrev, P.I.; Novák, O.; Motyka, V.; Strnad, M. Cytokinins in the bryophyte Physcomitrella patens: Analyses of activity, distribution, and cytokinin oxidase/dehydrogenase overexpression reveal the role of extracellular cytokinins. Plant Physiol. 2007, 145, 786–800. [Google Scholar] [CrossRef]
- Kiran, N.S.; Benková, E.; Reková, A.; Dubová, J.; Malbeck, J.; Palme, K.; Brzobohatý, B. Retargeting a maize β-glucosidase to the vacuole—Evidence from intact plants that zeatin-O-glucoside is stored in the vacuole. Phytochemistry 2012, 79, 67–77. [Google Scholar] [CrossRef]
- Fusseder, A.; Ziegler, P. Metabolism and compartmentation of dihydrozeatin exogenously supplied to photoautotrophic suspension cultures of Chenopodium rubrum. Planta 1988, 173, 104–109. [Google Scholar] [CrossRef]
- Horgan, R.; Hewett, E.W.; Horgan, J.M.; Purse, J.; Wareing, P.F. A new cytokinin from Populus x robusta. Phytochemistry 1975, 14, 1005–1008. [Google Scholar] [CrossRef]
- Chaves das Neves A new cytokinin from fruits of Zantedeschia-Aethiopica. Tetrahedron 1980, 21, 4387–4390. [CrossRef]
- Ge, L.; Yong, J.W.H.; Goh, N.K.; Chia, L.S.; Tan, S.N.; Ong, E.S. Identification of kinetin and kinetin riboside in coconut (Cocos nucifera L.) water using a combined approach of liquid chromatography-tandem mass spectrometry, high performance liquid chromatography and capillary electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 829, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, L.; Jones, L.H.; Oropeza, C.; Vláčil, D.; Strnad, M. Endogenous isoprenoid and aromatic cytokinins in different plant parts of Cocos nucifera (L.). Plant Growth Regul. 2003, 39, 205–215. [Google Scholar] [CrossRef]
- Kamínek, M.; Vaněk, T. Cytokinin activities of N6-benzyladenosine derivatives hydroxylated on the side-chain phenyl ring. J. Plant Growth Regul. 1987, 113–120. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Aguirreolea, J.; Martínková, H.; Hanuš, J.; Strnad, M. Aromatic cytokinins in micropropagated potato plants. Plant Physiol. Biochem. 2002, 40, 217–224. [Google Scholar] [CrossRef]
- Vasudevan, R.; van Staden, J. Cytokinin and explant types influence in vitro plant regeneration of Leopard Orchid (Ansellia africana Lindl.). Plant Cell Tissue Organ Cult. 2011, 107, 123–129. [Google Scholar] [CrossRef]
- Podwyszynska, M.; Wegrzynowicz-Lesiak, E.; Dolezal, K.; Krekule, J.; Strnad, M.; Saniewski, M. New cytokinins—Meta-methoytopolins in micropropagation of Cotinus Coggygria Scop. ‘Royal Purple’. Propag. Ornam. PLANTS 2012, 12, 220–228. [Google Scholar]
- Ördög, V.; Stirk, W.A.; Van Staden, J.; Novák, O.; Strnad, M. Endogenous cytokinins in three genera of microalgae from the chlorophyta. J. Phycol. 2004, 40, 88–95. [Google Scholar] [CrossRef]
- Ivanova, M.; Novák, O.; Strnad, M.; Van Staden, J. Endogenous cytokinins in shoots of Aloe polyphylla cultured in vitro in relation to hyperhydricity, exogenous cytokinins and gelling agents. Plant Growth Regul. 2006, 50, 219–230. [Google Scholar] [CrossRef]
- Tarkowská, D.; Doležal, K.; Tarkowski, P.; Åstot, C.; Holub, J.; Fuksová, K.; Schmülling, T.; Sandberg, G.; Strnad, M. Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus x canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography/frit-fast atom bombardment mass spectrometry. Physiol. Plant. 2003, 117, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, I.; Van Cauter, S.; Werbrouck, S.P.O.; Doležal, K. New aromatic cytokinins can make the difference. Acta Hortic. 2006, 725 I, 265–270. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell. Tissue Organ Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Amoo, S.O.; Finnie, J.F.; van Staden, J. The role of meta-topolins in alleviating micropropagation problems. Plant Growth Regul. 2011, 63, 197–206. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Finnie, J.F.; Van Staden, J. The role of meta-topolins on the photosynthetic pigment profiles and foliar structures of micropropagated “Williams” bananas. J. Plant Physiol. 2012, 169, 1530–1541. [Google Scholar] [CrossRef]
- Matsubara, S. Structure-activity relationships of cytokinins. Phytochemistry 1980, 19, 2239–2253. [Google Scholar] [CrossRef]
- Murvanidze, N.; Doležal, K.; Werbrouck, S.P.O. Fluorine containing topolin cytokinins for Phalaenopsis Amabilis (L.) blume micropropagation. Propag. Ornam. Plants 2019, 19, 48–51. [Google Scholar]
- Durán-Medina, Y.; Díaz-Ramírez, D.; Marsch-Martínez, N. Cytokinins on the move. Front. Plant Sci. 2017, 8, 146. [Google Scholar] [CrossRef]
- Bryksová, M. Preparation and biological activity of the new cytokinin derivatives. Ph.D. Thesis, Palacký University Olomouc, Olomouc, Czech republic.
- Wan, Z.K.; Binnun, E.; Wilson, D.P.; Lee, J. A highly facile and efficient one-step synthesis of N6-adenosine and N6-2′-deoxyadenosine derivatives. Org. Lett. 2005, 7, 5877–5880. [Google Scholar] [CrossRef]
- Kobayashi, H.; Morisaki, N.; Tago, Y.; Hashimoto, Y.; Iwasaki, S.; Kawachi, E.; Nagata, R.; Shudo, K. Identification of a major cytokinin in coconut milk. Experientia 1995, 51, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Miernyk, J.A.; Blaydes, D.F. Short-Term Metabolism of Radioactive Kinetin during Lettuce Seed Germination. Physiol. Plant. 1977, 39, 4–8. [Google Scholar] [CrossRef]
- Pietraface, W.J.; Blaydes, D.F. Activity and metabolism of 9-substituted cytokinins during lettuce seed germination. Physiol. Planetarum 1981, 53, 249–254. [Google Scholar] [CrossRef]
- Zatloukal, M.; Dolezal, K.; Voller, J.; Spichal, L.; Strnad, M. Substitution Derivatives of N-Benzyladenosine-5′-monophosphate, Methods of Preparation Thereof, Use Thereof as Medicaments, and Therapeutic Preparations Containing these Compounds. WO 2011134444, EP2563801 (24.9.2014).
- Voller, J.; Zatloukal, M.; Lenobel, R.; Doležal, K.; Béreš, T.; Kryštof, V.; Spíchal, L.; Niemann, P.; Džubák, P.; Hajdúch, M.; et al. Anticancer activity of natural cytokinins: A structure-activity relationship study. Phytochemistry 2010, 71, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; pp. 120–121. ISBN 0-8153-3577-6. [Google Scholar]
- Inoue, Y.; Ling, F.; Kimura, A. 2′-Deoxyribosylzeatin: A Novel Inhibitor for DNA Polymerase I of Escherichia coli. Agric. Biol. Chem. 1991, 55, 629–631. [Google Scholar] [CrossRef]
- Matušková, V.; Zatloukal, M.; Voller, J.; Grúz, J.; Pěkná, Z.; Briestenská, K.; Mistríková, J.; Spíchal, L.; Doležal, K.; Strnad, M. New aromatic 6-substituted 2′-deoxy-9-(β)-D-ribofuranosylpurine derivatives as potential plant growth regulators. Bioorgan. Med. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Pohjala, L.; Barai, V.; Azhayev, A.; Lapinjoki, S.; Ahola, T. A luciferase-based screening method for inhibitors of alphavirus replication applied to nucleoside analogues. Antivir. Res. 2008, 78, 215–222. [Google Scholar] [CrossRef]
- Tararov, V.I.; Tijsma, A.; Kolyachkina, S.V.; Oslovsky, V.E.; Neyts, J.; Drenichev, M.S.; Leyssen, P.; Mikhailov, S.N. Chemical modification of the plant isoprenoid cytokinin N 6-isopentenyladenosine yields a selective inhibitor of human enterovirus 71 replication. Eur. J. Med. Chem. 2015, 90, 406–413. [Google Scholar] [CrossRef]
- Drenichev, M.S.; Oslovsky, V.E.; Sun, L.; Tijsma, A.; Kurochkin, N.N.; Tararov, V.I.; Chizhov, A.O.; Neyts, J.; Pannecouque, C.; Leyssen, P.; et al. Modification of the length and structure of the linker of N6-benzyladenosine modulates its selective antiviral activity against enterovirus 71. Eur. J. Med. Chem. 2016, 111, 84–94. [Google Scholar] [CrossRef]
- Dutta, S.P.; Mittelman, A.; Chen, C.M.; Chheda, G.B. Synthesis and biological-activities of some analogs of N-6-(Δ2-isopentenyl)adenosine. J. Carbohydrates Nucleosides Nucleotides 1978, 5, 47–57. [Google Scholar]
- Hansske, F.; Madej, D.; Robins, M.J. 2′ And 3′-ketonucleosides and their arabino and xylo reduction products. Tetrahedron 1984, 40, 125–135. [Google Scholar] [CrossRef]
- Reist, E.J.; Benitez, A.; Goodman, L.; Baker, B.R.; Lee, W.W. Potential Anticancer Agents.1 LXXVI. Synthesis of Purine Nucleosides of β-D-Arabinofuranose. J. Org. Chem. 1962, 27, 3274–3279. [Google Scholar] [CrossRef]
- Secrist III, J.A.; Shortnacy, A.T.; Montgomery, J.A. Synthesis and Biological Evaluations of Certain 2-Halo-2′-Substituted Derivatives of 9-β-D-Arabinofuranosyladenine. J. Med. Chem. 1988, 31, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, G.; Bergogne, M.C.; Imbach, J.L. Obtaining of arabinofurannonucleosides using the chemical transformation of certain xylofurannonucleosides. Nucleosides Nucleotides 1984, 3, 265–275. [Google Scholar] [CrossRef]
- Krenitsky, T.A.; Koszalka, G.W.; Tuttle, J.V.; Rideout, J.L.; Elion, G.B. An enzymic synthesis of purine D-Arabinonucleosides. Carbohydr. Res. 1981, 97, 139–146. [Google Scholar] [CrossRef]
- Koszalka, G.W.; Averett, D.R.; Fyfe, J.A.; Roberts, G.B.; Spector, T.; Biron, K.; Krenitsky, T.A. 6-N-substituted derivatives of adenine arabinoside as selective inhibitors of varicella-zoster virus. Antimicrob. Agents Chemother. 1991, 35, 1437–1443. [Google Scholar] [CrossRef][Green Version]
- Kaneko, M.; Kimura, M.; Nishimura, T.S.B. Synthesis of N6-substituted or 8-substituted 9-(β-D-arabinofuranosyl)-adenines and their anti-viral activities against Herpes-Simplex and Vaccinia viruses. Chem. Pharm. Bull. 1977, 25, 2482–2489. [Google Scholar] [CrossRef]
- Ikehara, M.; Kaneko, M.; Ogiso, Y. Cleavage of 8,2′-anhydro-8-oxy-9-β-D-arabinofuranosyladenine with hydrogen sulfide and its interconversion with 8,5′-cyclonucleoside. Tetrahedron Lett. 1970, 11, 4673–4676. [Google Scholar] [CrossRef]
- Ikehara, M.; Ogiso, Y. Studies of nucleosides and nucleotides-LIV. Purine cyclonucleosides. 19. Further investigations on the cleavage of the 8,2′-O-anhydro linkage. A new synthesis of 9-β-D-arabinofuranosyladenine. Tetrahedron 1972, 28, 3695–3704. [Google Scholar] [CrossRef]
- Bryksová, M.; Dabravolski, S.; Kučerová, Z.; Zavadil Kokáš, F.; Špundová, M.; Plíhalová, L.; Takáč, T.; Grúz, J.; Hudeček, M.; Hloušková, V.; et al. Aromatic cytokinin arabinosides promote PAMP-like responses and positively regulate leaf longevity. ACS Chem. Biol. 2020. Submitted. [Google Scholar]
- Taylor, J.S.; Koshioka, M.; Pharis, R.P.; Sweet, G.B. Changes in Cytokinins and Gibberellin-Like Substances in Pinus radiata Buds during Lateral Shoot Initiation and the Characterization of Ribosyl Zeatin and a Novel Ribosyl Zeatin Glycoside. Plant Physiol. 1984, 74, 626–631. [Google Scholar] [CrossRef]
- Morris, J.W.; Doumas, P.; Morris, R.O.; Zaerr, J.B. Cytokinins in vegetative and reproductive buds of Pseudotsuga menziesii. Plant Physiol. 1990, 93, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Horgan, K.J.; Reynolds, P.H.S.; Norris, G.E.; Jameson, P.E. Novel cytokinins: The predominant forms in mature buds of Pinus radiata. Physiol. Plant. 2001, 112, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Horgan, K.J.; Reynolds, P.H.S.; Jameson, P.E. Cytokinins and bud morphology in Pinus radiata. Physiol. Plant. 2003, 117, 264–269. [Google Scholar] [CrossRef]
- Blakesley, D.; Lenton, J.R.; Horgan, R. Benzyladenine ribosylglucoside: A metabolite of benzyladenine in Gerbera jamesonii. Phytochemistry 1991, 30, 387–388. [Google Scholar] [CrossRef]
- Auer, C.A.; Cohen, J.D. Identification of a benzyladenine disaccharide conjugate produced during shoot organogenesis in Petunia leaf explant. Plant Physiol. 1993, 102, 541–545. [Google Scholar] [CrossRef]
- Cortizo, M.; Cuesta, C.; Centeno, M.L.; Rodríguez, A.; Fernández, B.; Ordás, R. Benzyladenine metabolism and temporal competence of Pinus pinea cotyledons to form buds in vitro. J. Plant Physiol. 2009, 166, 1069–1076. [Google Scholar] [CrossRef]
- Zhang, H.; Horgan, K.J.; Reynolds, P.H.S.; Jameson, P.E. 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol. 2010, 30, 514–526. [Google Scholar] [CrossRef][Green Version]
- Tahir, M.; Banyal, R. Clonal forestry: An effective technique for increasing the productivity of plantations. SKUAST J. Res. 2017, 19, 22–28. [Google Scholar]
- Fox, J.E.; Sood, C.K.; Buckwalter, B.; McChesney, J.D. The metabolism and biological activity of a 9-substituted cytokinin. Plant Physiol. 1971, 47, 275–281. [Google Scholar] [CrossRef]
- Zhang, R.; Letham, D.S. Cytokinin biochemistry in relation to leaf senescence. III. The senescence-retarding activity and metabolism of 9-substituted 6-benzylaminopurines in soybean leaves. J. Plant Growth Regul. 1989, 8, 181–197. [Google Scholar] [CrossRef]
- Corse, J.; Pacovsky, R.S.; Lyman, M.L.; Brandon, D.L. Biological activity of several 9-nonglycosidic-substituted natural cytokinins. J. Plant Growth Regul. 1989, 8, 211–223. [Google Scholar] [CrossRef]
- Szüčová, L.; Spíchal, L.; Doležal, K.; Zatloukal, M.; Greplová, J.; Galuszka, P.; Kryštof, V.; Voller, J.; Popa, I.; Massino, F.J.; et al. Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives. Bioorganic Med. Chem. 2009, 17, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Duke, C.; Macleod, J.; Summons, R.; Letham, D.; Parker, C. The Structure and Synthesis of Cytokinin Metabolites. II. Lupinic Acid and O-β-d-Glucopyranosylzeatin From Lupinus angustifolius. Aust. J. Chem. 1978, 31, 1291–1301. [Google Scholar] [CrossRef]
- Entsch, B.; Parker, C.W.; Letham, D.S. An enzyme from lupin seeds forming alanine derivatives of cytokinins. Phytochemistry 1983, 22, 375–381. [Google Scholar] [CrossRef]
- Palni, L.M.S.; Palmer, M.V.; Letham, D.S. The stability and biological activity of cytokinin metabolites in soybean callus tissue. Planta 1984, 160, 242–249. [Google Scholar] [CrossRef]
- Mok, D.W.S.; Mok, M.C. Cytokinins: Chemistry, Activity, and Function; CRC Press: Boca Raton, FL, USA, 1994; ISBN 0849362520. [Google Scholar]
- Kuhnle, J.A.; Fuller, G.; Corse, J.; Mackey, B.E. Antisenescent Activity of Natural Cytokinins. Physiol. Plant. 1977, 41, 14–21. [Google Scholar] [CrossRef]
- Kuroda, M.; Oaiawa, T.; Imagawa, H. Changes in chloroplast peroxidase activities in relation to chlorophyll loss in barley leaf segments. Physiol. Plant. 1990, 80, 555–560. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Varga, A.; Bruinsma, J. Effects of different cytokinins on the senescence of detached oat leaves. Planta 1973, 111, 91–93. [Google Scholar] [CrossRef]
- Eisinger, W. Role of Cytokinins in Carnation Flower Senescence. Plant Physiol. 1977, 59, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Mik, V.; Szüčová, L.; Šmehilová, M.; Zatloukal, M.; Doležal, K.; Nisler, J.; Grúz, J.; Galuszka, P.; Strnad, M.; Spíchal, L. N9-substituted derivatives of kinetin: Effective anti-senescence agents. Phytochemistry 2011, 72, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Mik, V.; Szüčová, L.; Spíchal, L.; Plíhal, O.; Nisler, J.; Zahajská, L.; Doležal, K.; Strnad, M. N9-Substituted N6-[(3-methylbut-2-en-1-yl)amino]purine derivatives and their biological activity in selected cytokinin bioassays. Bioorg. Med. Chem. 2011, 19, 7244–7251. [Google Scholar] [CrossRef] [PubMed]
- Robins, M.J.; Hall, R.H.; Thedford, R. N6-(Δ2-Isopenteny1) adenosine. A Component of the Transfer Ribonucleic Acid of Yeast and of Mammalian Tissue, Methods of Isolation, and Characterization. Biochemistry 1967, 6, 1837–1848. [Google Scholar] [CrossRef]
- Nisler, J.; Zatloukal, M.; Popa, I.; Doležal, K.; Strnad, M.; Spíchal, L. Cytokinin receptor antagonists derived from 6-benzylaminopurine. Phytochemistry 2010, 71, 823–830. [Google Scholar] [CrossRef]
- Spíchal, L.; Werner, T.; Popa, I.; Riefler, M.; Schmülling, T.; Strnad, M. The purine derivative PI-55 blocks cytokinin action via receptor inhibition. FEBS J. 2009, 276, 244–253. [Google Scholar] [CrossRef]
- Johnston, G.F.S.; Jeffcoat, B. Effects of some growth regulators on tiller bud elongation in cereals. New Phytol. 1977, 79, 239–245. [Google Scholar] [CrossRef]
- Letham, D.S. Regulators of Cell Division in Plant Tissues: VI. The effects of zeatin and other stimulants of cell division on apple fruit development. N. Z. J. Agric. Res. 1969, 12, 1–20. [Google Scholar] [CrossRef]
- Weaver, R.J.; van Overbeek, J.; Pool, R.M. Induction of Fruit Set in Vitis vinifera L. by a Kinin. Nature 1965, 206, 952–953. [Google Scholar] [CrossRef]
- Arena, M.E.; Pastur, G.J.M. Adventitious shoot induction from leaf explants of Ribes magellanicum cultured in vitro. Sci. Hortic. 1997, 72, 73–79. [Google Scholar] [CrossRef]
- Falck, J.R.; Li, D.R.; Bejot, R.; Mioskowski, C. An economic and practical synthesis of the 2-tetrahydrofuranyl ether protective group. Tetrahedron Lett. 2006, 47, 5111–5113. [Google Scholar] [CrossRef] [PubMed]
- Amoo, S.O.; Aremu, A.O.; Moyo, M.; Szüčová, L.; Doležal, K.; Van Staden, J. Physiological effects of a novel aromatic cytokinin analogue in micropropagated Aloe arborescens and Harpagophytum procumbens. Plant Cell. Tissue Organ Cult. 2014, 116, 17–26. [Google Scholar] [CrossRef]
- Amoo, S.O.; Aremu, A.O.; Moyo, M.; Sunmonu, T.O.; Plíhalová, L.; Doležal, K.; Van Staden, J. Physiological and biochemical effects of a tetrahydropyranyl-substituted meta-topolin in micropropagated Merwilla plumbea. Plant Cell. Tissue Organ Cult. 2015, 121, 579–590. [Google Scholar] [CrossRef]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Plant growth regulator induced phytochemical and antioxidant variations in micropropagated and acclimatized Eucomis autumnalis subspecies autumnalis (Asparagaceae). Acta Physiol. Plant. 2014, 36, 2467–2479. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated “Williams” banana. Acta Physiol. Plant. 2012, 36, 2265–2273. [Google Scholar] [CrossRef]
- Aremu, A.O.; Plačková, L.; Bairu, M.W.; Novák, O.; Szüčová, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. Endogenous cytokinin profiles of tissue-cultured and acclimatized “Williams” bananas subjected to different aromatic cytokinin treatments. Plant Sci. 2014, 214, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zahajská, L.; Nisler, J.; Voller, J.; Gucký, T.; Pospíšil, T.; Spíchal, L.; Strnad, M. Preparation, characterization and biological activity of C8-substituted cytokinins. Phytochemistry 2017, 135, 115–127. [Google Scholar] [CrossRef]
- Taddei, D.; Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. Synthesis and full characterisation of 6-chloro-2-iodopurine, a template for the functionalisation of purines. Org. Biomol. Chem. 2004, 2, 665–670. [Google Scholar] [CrossRef]
- Robins, R.K.; Godefroi, E.F.; Taylor, E.C.; Lewis, L.R.; Jackson, A. Purine Nucleosides. I. The Synthesis of Certain 6-Substituted-9-(tetrahydro-2-pyxanyl)- purines as Models of Purine Deoxynucleosides. J. Am. Chem. Soc. 1961, 83, 2574–2579. [Google Scholar] [CrossRef]
- MJNolsoee, J.; Gundersen, L.-L.; Rise, F. Synthesis of 8-Halopurines by Reaction of Lithiated Purines with Appropriate Halogen Donors. Synth. Commun. 1998, 28, 4303–4315. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Spíchal, L.; Grúz, J.; Kadlecová, A.; Voller, J.; Svobodová, A.R.; Vostálová, J.; Ulrichová, J.; Doležal, K.; et al. New cytokinin derivatives possess UVA and UVB photoprotective effect on human skin cells and prevent oxidative stress. Eur. J. Med. Chem. 2018, 150, 946–957. [Google Scholar] [CrossRef] [PubMed]

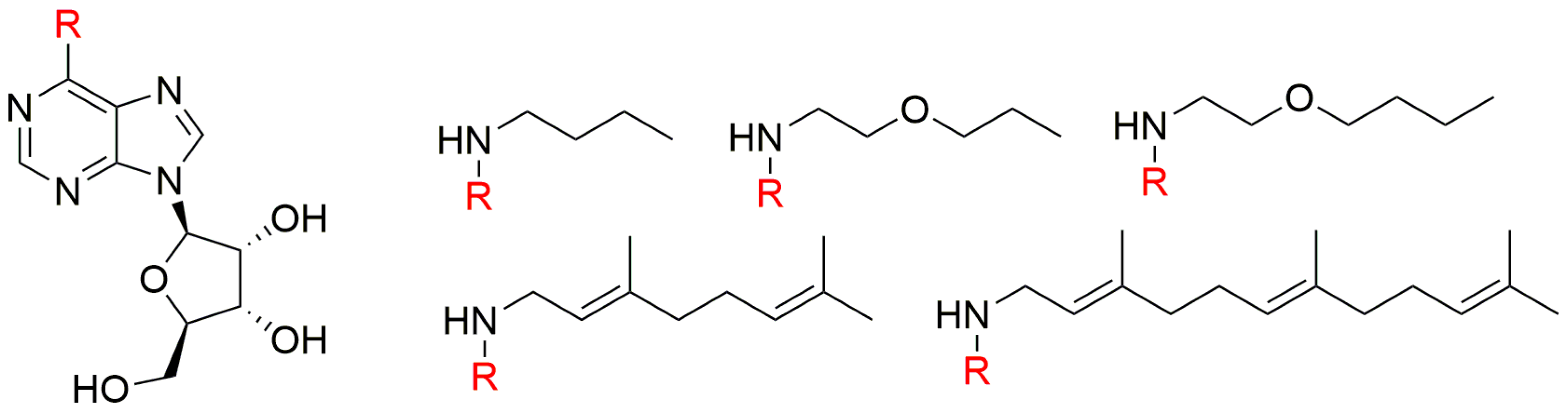

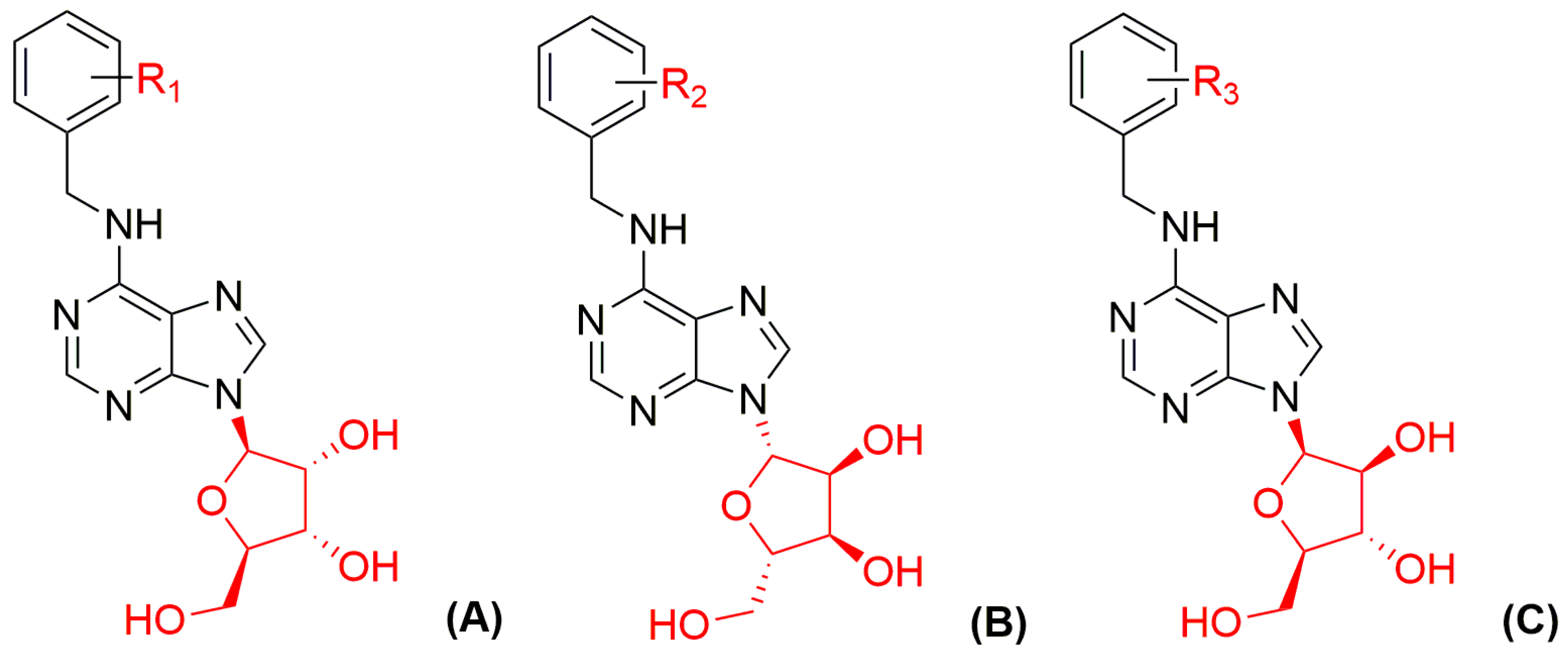







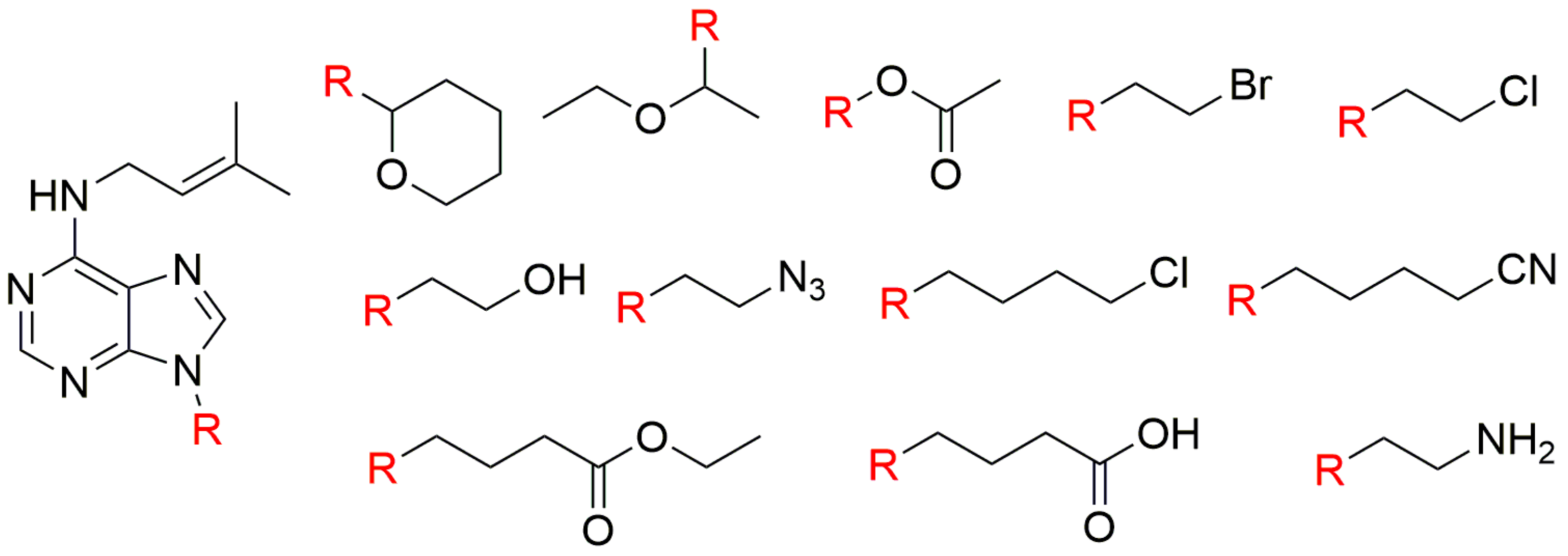
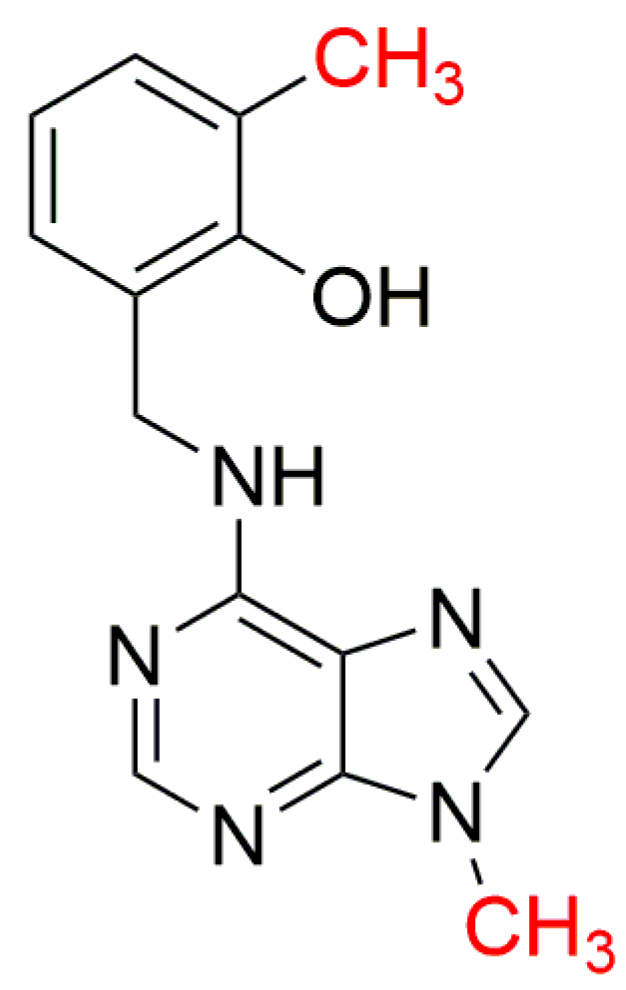
| Bioassay | Std. | Position of the Substituent on the Purine Ring | Ref. | |||
|---|---|---|---|---|---|---|
| N9 | N6 | C2 | C8 | |||
| Amaranthus caudatus betacyanin | BAP | β-D-ribofuranosyl- | 2-chlorobenzyl-, 3-chlorobenzyl-, 2-bromobenzyl-, 3-bromobenzyl-, 3-iodobenzyl-, 3,5-difluorobenzyl-, 2,4,5-trifluorobenzyl-, 2-chloro-4-fluorobenzyl-, 2-trifluoromethylbenzyl-, 3-trifluoromethoxybenzyl- | H | H | [45] |
| 2-fluorobenzyl-, 3-fluorobenzyl-, 4-fluorobenzyl-, 2-chlorobenzyl-, 3-chlorobenzyl-, 2-bromobenzyl-, 3-bromobenzyl-, 4-bromobenzyl-, 3-iodobenzyl- | Cl | H | [46] | |||
| 2′-deoxy-β-D-ribofuranosyl- | 3-hydroxybenzyl-, 2-fluorobenzyl-, 4-fluorobenzyl-, 2-chlorobenzyl, 3-chlorobenzyl-, 2-bromobenzyl-, 3-brombenzyl-, 2-methybenzyl-, 2-trifluoromethylbenzyl- | H | H | [120] | ||
| tetrahydropyran-2-yl | isopentenyl-, furfuryl- | H | 3-aminopropyl-, 4-aminobutyl-, methylsulfanyl-, dimethyl-, allyl- | [172] | ||
| benzyl- | H | H | [147] | |||
| tetrahydrofuran-2-yl | benzyl-, 2-methoxybenzyl-, 3-methoxybenzyl- | H | H | [147] | ||
| thiofen-2-yl- | Cl | H | [176] | |||
| iP | tetrahydropyran-2-yl, ethoxyethyl-, 2-bromoethyl-, 2-chloroethyl-, 4-ethoxy-4-oxobutyl- | isopentenyl- | H | H | [158] | |
| Senescence (WLS) | BAP | β-D-ribofuranosyl- | 2-fluorobenzyl-, 3-fluorobenzyl-, 4-fluorobenzyl-, 2-chlorobenzyl-, 4-chlorobenzyl-, 2-methylbenzyl-, 3-methybenzyl-, 2-methoxybenzyl-, 3-methoxybenzyl-, 3,4-dichlorobenzyl-, 2,3-dimethoxybenzyl-, 2,4-difluorobenzyl-, 3,5-difluorobenzyl-, 2,3,4-trifluorobenzyl-, 2,3,6-trifluorobenzyl-, 2-chloro-4-fluorobenzyl-, 3-chloro-4-fluorobenzyl-, 2-hydroxy-5-methylbenzyl-, 2-difluoromethoxybenzyl- | H | H | [45] |
| 2-fluorobenzyl-, 3-fluorobenzyl-, 4-fluorobenzyl-, 2-chlorobenzyl-, 3-chlorobenzyl-,4-chlorobenzyl-, 3-bromobenzyl-, 4-bromobenzyl- | Cl | H | [46] | |||
| β-D-arabinofuranosyl- | benzyl-, 2-fluorobenzyl-, 3-fluorobenzyl-, 4-fluorobenzyl-, 3-chlorobenzyl-, 2-methoxybenzyl-,3-methoxybenzyl-, 3-hydroxybenzyl-, 3-methylbenzyl-, 2,5-difluorobenzyl-, 3,5-difluorobenzyl- | H | H | [134] | ||
| 2′-deoxy-β-D-ribofuranosyl- | benzyl-, 2-hydroxybenzyl-, 3-hydroxybenzyl-, 4-hydroxybenzyl, 3-methoxybenzyl-, furfuryl- 2,5-dimethoxybenzyl-, 2-fluorobenzyl-, 3-fluorobenzyl-, 4-fluorobenzyl-, 2-chlorobenzyl-, 3-chlorobenzyl-, 4-chlorobenzyl-, 2-bromobenzyl-, 3-bromobenzyl-, 4-bromobenzyl-, 2-methylbenzyl-, 3-methylbenzyl-, 2-trifluoromethylbenzyl-, 3-trifluoromethylbenzyl-, | H | H | [120] | ||
| tetrahydropyran-2-yl | benzyl-, 3-hydroxybenzyl-, 2-methoxybenzyl-, | H | H | [147] | ||
| tetrahydrofuran-2-yl | benzyl-, 3-hydroxybenzyl- | H | H | [147] | ||
| tetrahydrofuran-2-yl-, thiofen-2-yl-, 5-methylthiofen-2-yl- | H | H | [176] | |||
| tetrahydrofurfuryl- | Cl | H | [176] | |||
| Kin | 2-bromoethyl-, 2-chloroethyl-, 4-chlorobutyl-, 1-ethoxyethyl-, tetrahydrofuran-2-yl | furfuryl- | H | H | [157] | |
| Tobaccocallus | BAP | β-D-ribofuranosyl- | 2-fluorobenzyl-, 4-fluorobenzyl-, 2-bromobenzyl-, 2-methoxybenzyl- | H | H | [45] |
| 2-flourobenzyl-, 3-fluorobenzyl-, 4-fluorobenzyl-, 2-chlorobenzyl-, 3-chlorobenzyl-, 4-bromobenzyl- | Cl | H | [46] | |||
| 2′-deoxy-β-D-ribofuranosyl- | benzyl-, 4-fluorobenzyl-, furfuryl- | H | H | [120] | ||
| tetrahydropyran-2-yl | isopentenyl-, furfuryl- | H | 2-aminoethyl-, 3-aminopropyl-, 4-aminobutyl-, 6-aminohexyl-, methoxy-, 2-hydroxyethyl- | [172] | ||
| benzyl- | H | H | [147] | |||
| tetrahydrofuran-2-yl | furfuryl-, thiofen-2-yl, 5-hydroxymethylfuran-2-yl- | H | H | [176] | ||
| furfuryl-, tetrahydrofurfuryl-, thiofen-2-yl- | Cl | H | [176] | |||
| iP | ethoxyethyl-, acetoxy-, 2-azidoethyl-, 4-chlorobutyl-, 3-cyanopropyl- | isopentenyl- | H | H | [158] | |
| Kin | 2-bromoethyl, 2-chloroethyl-, 1-ethoxyethyl-, tetrahydrofuran-2-yl | furfuryl- | H | H | [157] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vylíčilová, H.; Bryksová, M.; Matušková, V.; Doležal, K.; Plíhalová, L.; Strnad, M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules 2020, 10, 832. https://doi.org/10.3390/biom10060832
Vylíčilová H, Bryksová M, Matušková V, Doležal K, Plíhalová L, Strnad M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules. 2020; 10(6):832. https://doi.org/10.3390/biom10060832
Chicago/Turabian StyleVylíčilová, Hana, Magdaléna Bryksová, Vlasta Matušková, Karel Doležal, Lucie Plíhalová, and Miroslav Strnad. 2020. "Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back" Biomolecules 10, no. 6: 832. https://doi.org/10.3390/biom10060832
APA StyleVylíčilová, H., Bryksová, M., Matušková, V., Doležal, K., Plíhalová, L., & Strnad, M. (2020). Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules, 10(6), 832. https://doi.org/10.3390/biom10060832







