The Effect of the Photoperiod on the Fatty Acid Profile and Weight in Hatchery-Reared Underyearlings and Yearlings of Atlantic Salmon Salmo salar L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Description
2.2. Lipid Extraction and Lipid Classes Analysis
2.3. Fatty Acid Analysis
2.4. Statistical Analysis
3. Results
3.1. Weight Gain
3.2. Total Lipids and Lipid Classes
3.3. Fatty Acids Profile
4. Discussion
4.1. Weight Gain in the Hatchery-Reared Underyearlings and Yearlings under Different Photoperiods
4.2. The Total Lipid Content and Change of Lipid Classes and Fatty Acids in the Hatchery-Reared Underyearlings and Yearlings under Different Photoperiods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Björnsson, B.T. The biology of salmon growth hormone: From daylight to dominance. Fish Physiol. Biochem. 1997, 17, 9–24. [Google Scholar] [CrossRef]
- Handeland, S.O.; Porter, M.; Björnsson, B.T.; Stefansson, S.O. Osmoregulation and growth in a wild and a selected strain of Atlantic salmon smolts on two photoperiod regimes. Aquaculture 2003, 222, 29–43. [Google Scholar] [CrossRef]
- Sundell, K.S.; Sundh, H. Intestinal fluid absorption in anadromous salmonids: Importance of tight junctions and aquaporins. Front. Physiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipsmark, C.K.; Sørensen, K.J.; Madsen, S.S. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J. Exp. Biol. 2010, 213, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosdat, A.; Metailler, R.; Desbruyeres, E.; Huelvan, C. Comparison of brown trout (Salmo trutta) reared in fresh water and sea water to freshwater rainbow trout (Oncorhynchus mykiss): I. Growth and nitrogen balance. Aquat. Living Resour. 1997, 10, 157–167. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bell, J.G.; Dick, J.R.; Henderson, R.J.; McGhee, F.; Michell, D.; Morris, P.C. Polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation and the effects of dietary linseed and rapeseed oils. Fish Physiol. Biochem. 2000, 23, 59–73. [Google Scholar] [CrossRef]
- Jutfelt, F.; Olsen, R.; Erik, R.; Björnsson, B.T.; Sundell, K. Parr–smolt transformation and dietary vegetable lipids affect intestinal nutrient uptake, barrier function and plasma cortisol levels in Atlantic salmon. Aquaculture 2007, 273, 298–311. [Google Scholar] [CrossRef]
- Nemova, N.N.; Nefedova, Z.A.; Murzina, S.A.; Veselov, A.E.; Ripatti, P.O.; Pavlov, D.S. The influence of environmental living conditions on the dynamics of fatty acids in juvenile Atlantic salmon (Salmo salar L.). Rus. J. Ecol. 2015, 3, 206–212. [Google Scholar]
- Villarreal, C.A.; Thorpe, J.E.; Miles, M.S. Influence of photoperiod on growth changes in juvenile Atlantic salmon, Salmo salar L. J. Fish Biol. 1988, 33, 15–30. [Google Scholar] [CrossRef]
- Taylor, J.F.; North, B.P.; Porter, M.J.R.; Bromage, N.R.; Migaud, H. Photoperiod can be used to enhance growth and improve feeding efficiency in farmed rainbow trout, Oncorhynchus mykiss. Aquaculture 2006, 256, 216–234. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Sautin, E.Y. The problem of regulation of adaptive changes in lipogenesis, lipolysis and lipid transport in fish. Biol. Bull. Rev. 1989, 107, 131–149. [Google Scholar]
- Nesterov, V.D. The Behavior of Juvenile Atlantic Salmon Salmo Salar during Downhill Migration. Ph.D. Thesis, Russian Federal Research Institute of Fisheries and Oceanography, Specialty 03.00.10 (Ichthyology), Moskow, Russia, 1985. [Google Scholar]
- Rabinovich, A.L. The temperature dependence of the conformational properties of oligomeric chains of natural lipids: Computer simulation. Biophysics 2008, 53, 426–433. [Google Scholar]
- Pavlov, D.S.; Nemova, N.N.; Nefedova, Z.A.; Ruokolainen, T.R.; Vasivieva, O.B.; Kirillov, P.I.; Kirillova, E.A. Lipid status of juvenile of mykizhi Parasalmo mykiss and coho salmon Oncorhynchus kisutch. J. Ichthyol. 2010, 50, 120–129. [Google Scholar]
- Kazakov, R.V.; Veselov, A.E. Patterns of smoltification of Atlantic salmon. In Atlantic Salmon; Kazakov, R.V., Ed.; Nauka: Saint Petersburg, Russia, 1998; pp. 195–241. [Google Scholar]
- Shustov, Y.A. Ecological Aspects of the Behavior of Juvenile Salmon Fish in River Conditions; Shustov, Y.A., Ed.; Nauka: Saint-Petersburg, Russia, 1995; p. 161. [Google Scholar]
- Shustov, Y.A.; Belyakova, E.E.; Baryshev, I.A. Specific features of the feeding of juvenile Atlantic salmon (Salmo salar L.) in the subarctic varzuga river and its small tributaries (Kola Peninsula). Inland Water Biol. 2012, 5, 288–292. [Google Scholar] [CrossRef]
- Atlantic Salmon Ecology; Aas, Ø.; Klemetsen, A.; Einum, S.; Skurdal, J. (Eds.) Blackwell Publishing Ltd.: Oxford, UK, 2011. [Google Scholar]
- Skilbrei, O.T.; Hansen, T.; Stefansson, S.O. Effects of decreases in photoperiod on growth and bimodality in Atlantic salmon Salmo salar L. Aquac Res. 1997, 28, 43–49. [Google Scholar] [CrossRef]
- Thorpe, J.E.; Adams, C.E.; Miles, M.S.; Keay, D.S. Some influences of photoperiod and temperature on opportunity for growth in juvenile Atlantic salmon, Salmo salar L. Aquaculture 1989, 82, 119–126. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloan-Syanley, G.H. A simple method for the isolation and purification of total lipids from animal tissue (for brain, liver and muscle). J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Olsen, R.E.; Henderson, R.J. The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J. Exp. Mar. Biol. Ecol. 1989, 129, 189–197. [Google Scholar] [CrossRef]
- Hellwig, J. Definig Parameters for A Reproducible TLC-separation of Phospholipids Using ADC 2. Diploma Thesis, University of Applied Sciences Northwestern Switzerland (FHNW), Windisch, Switzerland, 2005. [Google Scholar]
- Jamieson, G.R. GLC-identification techniques for longchain unsaturated fatty acids. J. Chromatogr. Sci. 1975, 13, 491–497. [Google Scholar] [CrossRef]
- Nemova, N.N.; Nefedova, Z.A.; Pekkoeva, S.N.; Voronin, V.P.; Ruokolainen, T.R.; Murzina, S.A. Effect of the photoperiod on lipid spectrum of young Atlantic Salmon Salmo salar L. Rus. J. Physiol. 2020, 106. [Google Scholar] [CrossRef]
- Langdon, J.S. Smoltification Physiology in the Culture of Salmonids. In Recent Advances in Aquaculture; Muir, J.F., Roberts, R.J., Eds.; Springer: Boston, MA, USA, 1985; pp. 79–118. [Google Scholar]
- Peterson, H.H. Adults returns to date from hatchery reared one-year old smolts. In Atlantic Salmon Foundation; Smith, M.V., Carter, W.M., Eds.; The International Atlantic Salmon Foundation: NY, USA, 1973; pp. 219–226. [Google Scholar]
- Muir, J.; Roberts, R. Recent Advances in Aquaculture; Routledge: New York, NY, USA, 2019; p. 280. [Google Scholar]
- Churova, M.V.; Shulgina, N.; Kuritsyn, A.; Krupnova, M.Y.; Nemova, N.N. Muscle-specific gene expression and the metabolic enzyme activities in Atlantic salmon Salmo salar L. fry reared under different photoperiod regimes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 239, 110330. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, S.O.; Bjömsson, B.T.; Hansen, T.; Haux, C.; Taranger, G.L.; Saunders, R.L. Growth, parr–smolt transformation, and changes in growth hormone of Atlantic salmon (Salmo salar) reared under different photoperiods. Can. J. Fish Aquat. Sci. 1991, 48, 2100–2108. [Google Scholar] [CrossRef]
- Johnston, I.A.; Manthri, S.; Smart, A.; Campbell, P.; Nickell, D.; Alderson, R. Plasticity of muscle fibre number in seawater stages of Atlantic salmon in response to photoperiod manipulation. J. Exp. Biol. 2003, 206, 3425–3435. [Google Scholar] [CrossRef] [Green Version]
- Gaignon, J.L.; Quémener, L. Influence of early thermic and photoperiodic control on growth and smoltification in Atlantic salmon (Salmo salar). Aquat. Living Resour. 1992, 5, 185–195. [Google Scholar] [CrossRef]
- Sonmez, A.Y.; Hisar, O.; Hisar, S.A.; Alak, G.; Aras, M.S.; Yanik, T. The effects of different photoperiod regimes on growth, feed conversion rate and survival of rainbow trout (Oncorhynchus mykiss) fry. J. Anim. Vet. Adv. 2009, 8, 760–763. [Google Scholar]
- Ayala, M.D.; Abellán, E.; Arizcun, M.; García-Alcázar, A.; Navarro, F.; Blanco, A.; López-Albors, O.M. Muscle development and body growth in larvae and early post-larvae of shi drum, Umbrinacirrosa L., reared under different larval photoperiod: Muscle structural and ultrastructural study. Fish Physiol. Biochem. 2013, 39, 807–827. [Google Scholar] [CrossRef]
- Murzina, S.A.; Nefedova, Z.A.; Pekkoeva, S.N.; Veselov, A.E.; Ruch’ev, M.A.; Nemova, N.N. Fatty Acid Status of Freshwater Resident and Anadromous Forms of Young Brown Trout (Salmo trutta L.). Contemp. Probl. Ecol. 2018, 11, 320–324. [Google Scholar] [CrossRef]
- Nefedova, Z.A.; Murzina, S.A.; Veselov, A.E.; Pekkoeva, S.N.; Ruokolainen, T.R.; Ruch’ev, M.A.; Nemova, N.N. The biochemical variability of the lipid status of juveniles of the brown trout Salmo trutta L. inhabiting rivers belonging to the watershed area of the White Sea. Biol. Bull. 2017, 44, 50–54. [Google Scholar] [CrossRef]
- Nefedova, Z.A.; Murzina, S.A.; Pekkoeva, S.N.; Voronin, V.P.; Nemova, N.N. Comparative characteristics of the fatty-acid composition of lipids in factory and wild juveniles of Atlantic salmon Salmo salar L. Contemp. Probl. Ecol. 2020, 13, 156–161. [Google Scholar] [CrossRef]
- Nefedova, Z.A.; Murzina, S.A.; Pekkoeva, S.N.; Nemova, N.N. Comparative Analysis of the Fatty Acid Profiles of Smolts of the Brown Trout Salmo trutta L. and Atlantic Salmon Salmo salar L. during Smoltification (Indera River, White Sea Basin). Biol. Bull. 2018, 45, 126–131. [Google Scholar] [CrossRef]
- Carta, G.; Angioni, E.; Murru, E.; Melis, M.P.; Spada, S.; Banni, S. Modulation of lipid metabolism and vitamin A by conjugated linoleic acid. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; Henderson, R.J.; Tocher, D.R.; McGhee, F.; Dick, J.R.; Porter, A.; Smullen, R.P.; Sargent, J.R. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J. Nutr. 2002, 132, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Mommsen, T.P.; Plisetskaya, E.M. Insulin in fishes and agnathans: History, structure, and metabolic regulation. Rev. Aquat. Sci. 1991, 4, 225–259. [Google Scholar]
- Ruchin, A.B. The influence of the photoperiod on the growth, physiological and hematological parameters of juvenile Siberian sturgeon Acipenser baerii. Biol. Bull. 2007, 6, 698–704. [Google Scholar]
- Nefedova, Z.A.; Murzina, S.A.; Pekkoeva, S.N.; Veselov, A.E.; Nemova, N.N. The effect of lipid and fatty acid status on the processes of primary settlement and the formation of phenotypic groups of Atlantic salmon Salmo salar L. juvenile. Trans. KarRC RAS 2016, 6, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Pekkoeva, S.N.; Murzina, S.A.; Nefedova, Z.A.; Ruokolainen, T.R.; Veselov, A.E.; Nemova, N.N. Comparative characteristics of the lipid status of juvenile young Atlantic salmon Salmo salar L. of the Varzuga river (Kola Peninsula). Trans. KarRC RAS 2018, 4, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Conceicao, L. Success Factors for Fish Larval Production; John Wiley & Sons: Oxford, UK, 2018; p. 512. [Google Scholar]
- Murzina, S.A.; Nefedova, Z.A.; Pekkoeva, S.N.; Veselov, A.E.; Baryshev, I.A.; Ripatti, P.O.; Nemova, N.N. The content of fatty acids in the feed objects of juvenile salmon fish of the rivers of the Onega Lake basin. Inland Water Biol. 2019, 1, 65–72. [Google Scholar]
- Rollin, X.; Peng, J.; Pham, D.; Ackman, R.G.; Larondelle, Y. The effects of dietary lipid and strain difference on polyunsaturated fatty acid composition and conversion in anadromous and landlocked salmon (Salmo salar L.) parr. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 349–366. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and conditions in fish: The influence of lipids on membrane competency and immune response. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: Heidelberg, Dordrecht; London, UK; New York, NY, USA, 2009; pp. 237–257. [Google Scholar]
- Pavlov, D.S.; Nefedova, Z.A.; Veselov, A.E.; Nemova, N.N.; Ruokolainen, T.R.; Vasilieva, O.B.; Ripatti, P.O. Lipid status of fingerlings of the Atlantic salmon Salmo salar from different microbiotopes of the Varzuga River. J. Ichthyol. 2008, 48, 648–654. [Google Scholar] [CrossRef]
- Pavlov, D.S.; Savvaitova, K.A. On the problem of ratio of anadromy and residence in salmonids (Salmonidae). J. Ichthyol. 2008, 48, 778–791. [Google Scholar] [CrossRef]
- Ecological and Biochemical Status of Juvenile Atlantic Salmon Salmo Salar L. from Some Rivers of the White Sea Basin; Nemova, N.N. (Ed.) RIO KarRC RAS: Petrozavodsk, Russia, 2016; p. 205. [Google Scholar]
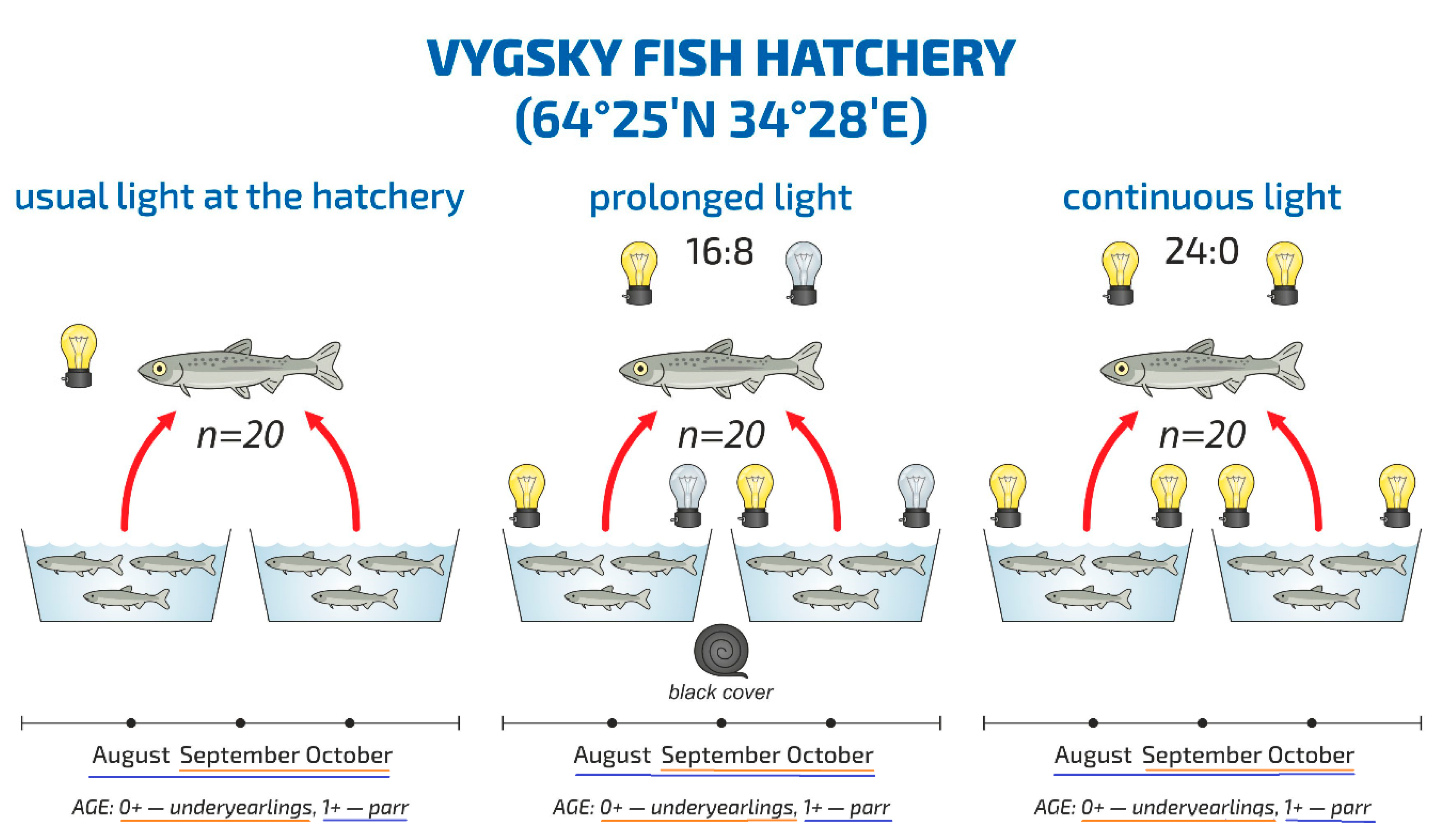




| Time Period | July–10th of August | 10th of August–10th of September | 10th of September–the Entire October |
|---|---|---|---|
| Light regime | 24 h Natural light regime Sunrise and sunset at the location of the Vygsky fish hatchery and certain latitude can be found here: https://sunsetsunrisetime.com/sun/belomorsk | from 8 a.m. till 5 p.m. Natural light regime from 5 a.m. till 8 p.m. The hatchery’s light was turned (artificial light) | 24 h The hatchery’s light was turned (artificial light) |
| Month | September (September 5) | October (October 5) | ||||
|---|---|---|---|---|---|---|
| Light regime | Control (HL) | LD 16:8 | LD 24:0 | Control (HL) | LD 16:8 | LD 24:0 |
| Group | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Weight, g (average ----------- min-max) | 2.5 ± 0.1 ------------ 1.9–3.1 | 2.5 ± 0.1 ----------- 1.9–3.0 | 2.6 ± 0.2 ----------- 1.6–4.3 | 3.7 ± 0.0 A ------------ 3.0–4.7 | 3.5 ± 0.1 A ------------ 2.6–4.9 | 3.7 ± 0.03 A ------------ 2.5–5.5 |
| Length, cm (average ----------- min-max) | 6.1 ± 0.1 ----------- 5.8–6.5 | 6.1 ± 0.1 ----------- 5.5–6.4 | 6.2 ± 0.1 ----------- 5.4–7.3 | 7.2 ± 0.1 A ------------ 6.7–7.7 | 7.0 ± 0.1 A ------------ 6.4–7.9 | 7.1 ± 0.1 A ------------ 6.2–8.2 |
| Condition factor (CF, %) | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 A | 1.0 ± 0.0 A | 1.0 ± 0.0 A |
| TL, % dry weight | 27.1 ± 0.3 | 26.8 ± 0.9 | 26.6 ± 0.4 | 32.8 ± 0.9 A | 34.2 ± 0.8 A | 32.7 ± 0.9 A |
| Month | August (August 8) | September (September 5) | October (October 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Light regime | Control (HL) | LD 16:8 | LD 24:0 | Control (HL) | LD 16:8 | LD 24:0 | Control (HL) | LD 16:8 | LD 24:0 |
| Group | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Weight, g (average ----------- min-max) | 21.6 ± 0.8 ----------- 16.4–25.8 | 16.6 ± 0.8 1 ------------ 10.8–20.5 | 19.1 ± 0.5 1,2 ------------- 15.1–22.2 | 26.7 ± 0.4 A ------------ 15.5–39.7 | 26.7 ± 0.4 A -------------- 17.6–37.7 | 25.3 ± 0.3 A ------------- 18.2–31.3 | 28.1 ± 0.3 A -------------- 16.9–53.0 | 27.5 ± 0.6 A ------------- 19.5–44.1 | 29.6 ± 0.3 A ------------- 22.0–41.8 |
| Length, cm (average ----------- min-max) | 11.9 ± 0.1 ----------- 11.0–12.6 | 11.0 ± 0.2 1 ------------- 9.5–12.0 | 11.1 ± 0.1 1 ------------- 10.5–11.8 | 13.0 ± 0.3 A ------------ 10.7–15.1 | 13.0 ± 0.2 A ------------ 11.3–14.4 | 12.8 ± 0.2 A ------------ 11.3–13.9 | 13.5 ± 0.1 A ------------- 11.4–16.2 | 13.4 ± 0.2 A ------------ 11.9–15.7 | 13.8 ± 0.1 AB --------------- 12.6–15.4 |
| Condition factor (CF) | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.2 1,2 | 1.2 ± 0.0 A | 1.2 ± 0.0 A | 1.2 ± 0.0 A | 1.1 ± 0.0 AB | 1.1 ± 0.0 AB | 1.1 ± 0.0 AB |
| TL, % dry weight | 26.7 ± 1.0 | 29.5 ± 0.8 1 | 30.0 ± 0.7 1 | 26.2 ± 0.8 | 28.6 ± 0.9 1 | 30.2 ± 0.8 1 | 18.6 ± 1.1 AB | 17.9 ± 1.3 AB | 15.9 ± 1.0 AB1 |
| Month | September (September 5) | October (October 5) | ||||
|---|---|---|---|---|---|---|
| Light regime | Control (HL) | LD 16:8 | LD 24:0 | Control (HL) | LD 16:8 | LD 24:0 |
| Group | 1 | 2 | 3 | 1 | 2 | 3 |
| Fatty acids | % of total FA | |||||
| 14:0 | 4.2 ± 0.0 | 4.1 ± 0.0 | 4.4 ± 0.1 1,2 | 4.3 ± 0.2 | 4.1 ± 0.0 | 4.2 ± 0.1 |
| 16:0 | 16.4 ± 0.1 | 16.4 ± 0.1 | 17.1 ± 0.3 | 16.8 ± 0.6 | 16.0 ± 0.1 A | 16.1 ± 0.1 |
| 18:0 | 3.9 ± 0.1 | 4.0 ± 0.0 | 4.1 ± 0.1 | 3.9 ± 0.1 | 3.8 ± 0.1 | 3.7 ± 0.0 A |
| ∑ SFA | 26.5 ± 0.1 | 26.4 ± 0.1 | 27.4 ± 0.5 | 27.0 ± 0.9 | 26.1 ± 0.2 | 26.0 ± 0.2 A |
| 16:1(n-7) | 5.3 ± 0.1 | 5.2 ± 0.0 | 5.1 ± 0.1 | 5.3 ± 0.2 | 5.1 ± 0.0 | 5.1 ± 0.0 |
| 18:1(n-9) | 19.7 ± 0.5 | 20.3 ± 0.4 | 19.7 ± 0.4 | 19.9 ± 0.6 | 19.6 ± 0.2 | 18.5 ± 0.3 1,2 |
| 18:1(n-7) | 3.0 ± 0.0 | 3.3 ± 0.2 | 3.2 ± 0.0 1 | 3.4 ± 0.1 A | 3.1 ± 0.0 1 | 3.4 ± 0.2 |
| 20:1(n-9) | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.5 ± 0.1 | 5.5 ± 0.2 | 5.2 ± 0.1 | 5.3 ± 0.1 |
| 22:1(n-11) | 6.1 ± 0.1 | 6.0 ± 0.0 1 | 6.3 ± 0.12 | 5.7 ± 0.2 | 5.6 ± 0.1 A | 5.7 ± 0.1 A |
| ∑ MUFA | 42.5 ± 0.4 | 43.2 ± 0.3 | 43.1 ± 0.7 | 43.0 ± 1.4 | 41.6 ± 0.1 A | 40.9 ± 0.3 A1 |
| 18:2(n-6) | 5.5 ± 0.1 | 5.5 ± 0.1 | 5.3 ± 0.2 | 5.3 ± 0.1 | 5.4 ± 0.1 | 5.5 ± 0.0 2 |
| 20:4(n-6) | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 A | 0.6 ± 0.0 A |
| ∑ (n-6) PUFA | 7.8 ± 0.2 | 7.5 ± 0.0 | 7.3 ± 0.2 | 7.5 ± 0.1 | 7.5 ± 0.1 | 7.7 ± 0.0 2 |
| 18:3(n-3) | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.0 A |
| 18:4(n-3) | 1.5 ± 0.1 | 1.4 ± 0.0 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.0 | 1.5 ± 0.0 A2 |
| 20:5(n-3) | 3.4 ± 0.2 | 3.0 ± 0.1 | 2.9 ± 0.2 | 3.1 ± 0.3 | 3.4 ± 0.1 A | 3.5 ± 0.1 A2 |
| 22:5(n-3) | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.0 A | 1.4 ± 0.0 |
| 22:6(n-3) | 13.1 ± 0.2 | 13.6 ± 0.2 | 13.1 ± 0.5 | 13.2 ± 1.4 | 14.9 ± 0.2 A | 15.2 ± 0.3 A |
| ∑ (n-3) PUFA | 21.8 ± 0.4 | 21.8 ± 0.2 | 21.0 ± 0.9 | 21.4 ± 2.2 | 23.7 ± 0.2 A | 24.4 ± 0.4 A |
| ∑ PUFA | 31.0 ± 0.5 | 30.4 ± 0.2 | 29.4 ± 1.2 | 30.0 ± 2.3 | 32.3 ± 0.3 A | 33.1 ± 0.4 A |
| Others * | 8.3 | 8.0 | 7.9 | 8.2 | 8.2 | 8.1 |
| ∑ (n-3)/∑ (n-6) | 2.8 ± 0.1 | 2.9 ± 0.02 | 2.9 ± 0.1 | 2.8 ± 0.3 | 3.2 ± 0.0 A | 3.2 ± 0.1 A |
| 16:0/18:1(n-9) | 0.8 ± 0.0 | 0.8 ± 0.02 | 0.9 ± 0.0 2 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.0 2 |
| Month | August (August 8) | September (September 5) | October (October 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Light regime | Control (HL) | LD 16:8 | LD 24:0 | Control (HL) | LD 16:8 | LD 24:0 | Control (HL) | LD 16:8 | LD 24:0 |
| Group | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Fatty acids | % of total FA | ||||||||
| 14:0 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.1 ± 0.1 A | 3.0 ± 0.1 A | 3.0 ± 0.0 A | 2.8 ± 0.1 A | 2.7 ± 0.1 A | 2.7 ± 0.1 AB |
| 16:0 | 15.0 ± 0.4 | 14.7 ± 0.3 | 15.2 ± 0.3 | 13.8 ± 0.3 | 13.4 ± 0.4 | 13.2 ± 0.2 A | 13.6 ± 0.4 A | 13.0 ± 0.3 A | 13.5 ± 0.2 A |
| 18:0 | 3.6 ± 0.1 | 3.5 ± 0.1 | 3.8 ± 0.0 2 | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.3 ± 0.0 A | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.1 B |
| ∑ SFA | 23.8 ± 0.6 | 23.5 ± 0.4 | 24.2 ± 0.4 | 21.8 ± 0.5 A | 21.4 ± 0.6 | 21.0 ± 0.0 A | 21.5 ± 0.5 A | 20.8 ± 0.6 A | 21.7 ± 0.3 A |
| 16:1(n-7) | 4.2 ± 0.2 | 4.3 ± 0.1 | 4.5 ± 0.2 | 4.0 ± 0.1 | 3.8 ± 0.1 A | 3.8 ± 0.1 A | 3.6 ± 0.1 A | 3.5 ± 0.1 A | 3.4 ± 0.1 AB |
| 18:1(n-9) | 26.7 ± 0.9 | 26.4 ± 0.6 | 28.3 ± 1.1 | 29.6 ± 0.8 | 30.0 ± 0.8 A | 30.3 ± 0.4 | 29.6 ± 0.2 | 30.0 ± 0.3A | 28.2 ± 0.6 B2 |
| 18:1(n-7) | 3.4 ± 0.1 | 3.3 ± 0.0 | 3.6 ± 0.1 2 | 3.3 ± 0.0 | 3.4 ± 0.0 | 3.4 ± 0.0 1 | 3.3 ± 0.1 | 3.2 ± 0.0 B | 3.1 ± 0.1 A |
| 20:1(n-9) | 4.5 ± 0.1 | 4.6 ± 0.1 | 4.7 ± 0.1 | 4.8 ± 0.1 | 4.7 ± 0.1 | 4.8 ± 0.0 | 4.5 ± 0.2 | 4.4 ± 0.1 | 4.2 ± 0.1 AB |
| 22:1(n-11) | 3.9 ± 0.1 | 4.0 ± 0.1 | 4.1 ± 0.1 | 3.9 ± 0.1 | 3.8 ± 0.2 | 3.7 ± 0.1 A | 3.5 ± 0.2 | 3.5 ± 0.1 A | 3.2 ± 0.1 AB |
| ∑ MUFA | 45.3 ± 0.5 | 45.2 ± 0.3 | 47.9 ± 0.7 1,2 | 48.0 ± 0.5 A | 48.0 ± 0.5 A | 48.4 ± 0.3 | 46.9 ± 0.4 | 46.8 ± 0.2 A | 44.2 ± 0.5 AB1,2 |
| 18:2(n-6) | 9.5 ± 0.3 | 9.5 ± 0.3 | 9.3 ± 0.3 | 10.1 ± 0.3 | 10.4 ± 0.2 | 10.5 ± 0.1 A | 10.0 ± 0.2 | 10.5 ± 0.3 | 10.0 ± 0.2 B |
| 20:4(n-6) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 2 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 AB | 0.6 ± 0.0 B1 |
| ∑ (n-6) PUFA | 11.8 ± 0.4 | 11.8 ± 0.3 | 11.5 ± 0.3 | 12.4 ± 0.3 | 12.7 ± 0.3 | 12.9 ± 0.1 A | 12.4 ± 0.3 | 12.8 ± 0.3 | 12.4 ± 0.2 A |
| 18:3(n-3) | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.6 ± 0.2 | 2.6 ± 0.1 | 2.8 ± 0.0 A | 2.6 ± 0.1 | 2.8 ± 0.2 | 2.6 ± 0.1 |
| 18:4(n-3) | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.1 ± 0.0 1,2 | 1.2 ± 0.0 A | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.0 ± 0.1 A | 1.0 ± 0.1 | 1.0 ± 0.0 B |
| 20:5(n-3) | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.3 ± 0.2 2 | 2.4 ± 0.1 | 2.3 ± 0.1 A | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.6 ± 0.0 B | 2.8 ± 0.2 B1 |
| 22:5(n-3) | 1.2 ± 0.1 | 1.2 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.0 | 1.1 ± 0.0 A | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 | 1.1 ± 0.1 |
| 22:6(n-3) | 9.3 ± 0.3 | 9.6 ± 0.3 | 7.6 ± 0.5 1,2 | 8.5 ± 0.3 | 8.7 ± 0.3 | 8.4 ± 0.2 | 10.2 ± 0.2 | 10.1 ± 0.2 B | 12.1 ± 0.3 AB2 |
| ∑ (n-3) PUFA | 18.2 ± 0.4 | 18.6 ± 0.4 | 15.6 ± 0.7 1.2 | 17.0 ± 0.3 A | 17.1 ± 0.4 A | 17.0 ± 0.3 | 18.5 ± 1.6 | 18.8 ± 0.4 B | 20.9 ± 0.5 AB1,2 |
| ∑ PUFA | 30.8 ± 0.4 | 31.3 ± 0.3 | 27.9 ± 0.5 1,2 | 30.1 ± 0.2 | 30.5 ± 0.5 | 30.6 ± 0.3 A | 31.6 ± 1.8 | 32.4 ± 0.7 | 34.0 ± 0.3 AB |
| Others * | 7.6 | 7.8 | 6.9 | 7.0 | 6.9 | 7.1 | 7.5 | 7.1 | 7.1 |
| ∑ (n-3)/∑ (n-6) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.0 | 1.5 ± 0.1 | 1.5 ± 0.0 B | 1.7 ± 0.1 AB2 |
| 16:0/18:1(n-9) | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 A | 0.4 ± 0.0 A | 0.5 ± 0.0 A | 0.4 ± 0.0 A | 0.5 ± 0.0 B1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemova, N.N.; Nefedova, Z.A.; Pekkoeva, S.N.; Voronin, V.P.; Shulgina, N.S.; Churova, M.V.; Murzina, S.A. The Effect of the Photoperiod on the Fatty Acid Profile and Weight in Hatchery-Reared Underyearlings and Yearlings of Atlantic Salmon Salmo salar L. Biomolecules 2020, 10, 845. https://doi.org/10.3390/biom10060845
Nemova NN, Nefedova ZA, Pekkoeva SN, Voronin VP, Shulgina NS, Churova MV, Murzina SA. The Effect of the Photoperiod on the Fatty Acid Profile and Weight in Hatchery-Reared Underyearlings and Yearlings of Atlantic Salmon Salmo salar L. Biomolecules. 2020; 10(6):845. https://doi.org/10.3390/biom10060845
Chicago/Turabian StyleNemova, Nina N., Zinaida A. Nefedova, Svetlana N. Pekkoeva, Viktor P. Voronin, Natalia S. Shulgina, Maria V. Churova, and Svetlana A. Murzina. 2020. "The Effect of the Photoperiod on the Fatty Acid Profile and Weight in Hatchery-Reared Underyearlings and Yearlings of Atlantic Salmon Salmo salar L." Biomolecules 10, no. 6: 845. https://doi.org/10.3390/biom10060845





