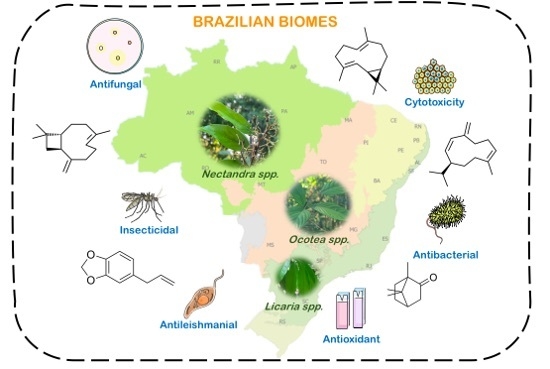
Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes
Abstract
:1. Introduction
2. Distribution of Main Compound Classes in Essential Oil Samples
3. Volatile Profiles
3.1. Oils Rich in Monoterpene Hydrocarbons
3.2. Oils Rich in Sesquiterpene Hydrocarbons
3.3. Oils Rich in Oxygenated Sesquiterpenoids
3.4. Oils Rich in Sesquiterpene Hydrocarbons and Oxygenated Sesquiterpenes
3.5. Oils Rich in Phenylpropanoids and Monoterpenes
3.6. Oils Rich in Phenylpropanoids and Sesquiterpenes
3.7. Oils Rich in Benzenoids
4. Occurrence of Different Chemical Profiles
5. Seasonal Variation in the Volatile Constituents
6. Biological Activities
6.1. Antibacterial Activity
6.2. Antifungal Activity
6.3. Cardiovascular Activity
6.4. Reduction of Motor and Anesthetic Activity
6.5. Antioxidant Activity
6.6. Cytotoxic Activity
6.7. Toxicological Activity
6.8. Leishmanicidal Activity
6.9. Antichemotactic Activity
6.10. Other Activities
7. Chemical Composition-Geographic Distribution Correlation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Van Der Werff, H. A Key to the Genera of Lauraceae in the New World. Ann. Mo. Bot. Gard. 1991, 78, 377. [Google Scholar] [CrossRef]
- Gonçalves, R.D.A.; Pinheiro, A.B.; De Oliveira, M.A.; Nascimento, R.T.D.; Rosalem, P.F.; Garcia, V.L.; Martins, A.R. Anatomical characters and chemical profile of leaves of three species in Lauraceae family. Rev. Bras. Farm. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Available online: http://www.ibge.gov.br (accessed on 24 April 2020).
- De Miranda, E.; De Carvalho, C.A.; Martinho, P.R.R.; Oshiro, O.T. Contribuições do geoprocessamento à compreensão do mundo rural e do desmatamento no bioma Amazônia. COLOQUIO 2019, 17, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, M.N.; Campos, F.I. Bioma cerrado: Relevância no cenário hídrico brasileiro. In Proceedings of the IX Simpósio Nacional de ciência e Meio Ambiente—SNCMA, Anápolis, Brazil, 23–26 October 2018; Volume 2, pp. 399–409. [Google Scholar]
- Prado, D.E. As caatingas da América do Sul. In Ecologia e conservação da Caatinga; Leal, I.R., Marcelo, T., Silva, J.M.C., Eds.; Editora Universitária UFPE: Recife, Brazil, 2003; pp. 3–74. ISBN 857315215. [Google Scholar]
- De Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and Evolution of Flowering Plants of the Caatinga Domain; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 23–63. [Google Scholar]
- Dantas, M.D.S.; Almeida, N.V.; Medeiros, I.D.S.; Da Silva, M.D. Diagnóstico da vegetação remanescente de Mata Atlântica e ecossistemas associados em espaços urbanos. J. Environ. Anal. Prog. 2017, 2, 87. [Google Scholar] [CrossRef] [Green Version]
- Konig, F.; Gonçalves, C.E.P.; Aguiar, A.R.; Silva, A.C.F. Bioma Pampa: Interações entre micro-organismos e espécies vegetais nativas. Agrárias 2014, 37, 3–9. [Google Scholar] [CrossRef]
- Santos, S.; Da Silva, L.G. Mapeamento por imagens de sensoriamento remoto evidencia o bioma Pampa brasileiro sob ameaça. Bol. de Geogr. 2012, 29, 49–57. [Google Scholar] [CrossRef]
- Quinet, A.; Kutschenko, D.C.; Barrosa, F.S.M.; Moraes, M.M.V.; Fernandez, E.P.; Messina, T. Lauraceae. In Livro vermelho da flora do Brasil, 1st ed.; Martinelli, G., Moraes, M.A., Eds.; Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2013; pp. 591–606. ISBN 978-85-88742-58-1. [Google Scholar]
- Lauraceae in Flora do Brasil 2020 em Construção. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB143 (accessed on 20 April 2020).
- Marques, C.A. Importância econômica da família Lauraceae Lindl. Floran 2001, 8, 195–206. [Google Scholar]
- Maia, J.G.S.; Ramos, L.S.; Luz, A.I.R. Estudo do óleo essencial do puxuri por cromatografia de gás /espectrometria de massa (CG/EM). Acta Amaz. 1985, 15, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Carlini, E.L.D.A.; De Oliveira, A.; De Oliveira, G. Psychopharmacological effects of the essential oil fraction and of the hydrolate obtained from the seeds of Licaria puchury-major. J. Ethnopharmacol. 1983, 8, 225–236. [Google Scholar] [CrossRef]
- Graça, R.R. Licaria Puchury-Major (MART.) Kosterm. Ph.D. Thesis, Universidade Federal do Amazonas, Manaus, Brazil, 2015. [Google Scholar]
- Alves, E.O.; Mota, J.H.; Soares, T.S.; Vieira, M.; Da Silva, C.B. Levantamento etnobotânico e caracterização de plantas medicinais em fragmentos florestais de Dourados-MS. Ciência e Agrotecnologia 2008, 32, 651–658. [Google Scholar] [CrossRef]
- Fosberg, F.R.; Schultes, R.E.; Raffauf, R.F. The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia. Taxon 1991, 40, 157. [Google Scholar] [CrossRef]
- Petroni, L.M.; Huffman, M.A.; Rodrigues, E. Medicinal plants in the diet of woolly spider monkeys (Brachyteles arachnoides, E. Geoffroy, 1806)—A bio-rational for the search of new medicines for human use? Rev. Bras. de Farm. 2017, 27, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Chernoviz, P.L.N. Formulário e Guia Médico, 9th ed.; Casa do Autor: Paris, France, 1874. [Google Scholar]
- Yamaguchi, M.; Filho, B.; Cortez, D.; Ueda-Nakamura, T.; Nakamura, C. Programa de Pós-graduação em Ciências Farmacêuticas Universidade Estadual de Maringá PR Brazil Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorifera (Lauraceae). Planta Med. 2008, 74, 507–514. [Google Scholar] [CrossRef]
- Ferraz, E.D.O.; Vieira, M.A.R.; Ferreira, M.I.; Junior, A.F.; Marques, M.; Minatel, I.O.; Albano, M.; Sambo, P.; Lima, G.P.P. Seasonality effects on chemical composition, antibacterial activity and essential oil yield of three species of Nectandra. PLoS ONE 2018, 13, e0204132. [Google Scholar] [CrossRef]
- Amaral, L.D.P.; Tondolo, J.; Schindler, B.; Da Silva, D.T.; Pinheiro, C.G.; Longhi, S.J.; Mallmann, C.A.; Heinzmann, B.M. Seasonal influence on the essential oil production of Nectandra megapotamica (Spreng.) Mez. Braz. Arch. Boil. Technol. 2015, 58, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Alcântara, J.M.; Yamaguchi, K.K.D.L.; Junior, V.F.D.V.; Lima, E.S. Composição química de óleos essenciais de espécies de Aniba e Licaria e suas atividades antioxidante e antiagregante plaquetária. Química Nova 2010, 33, 141–145. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrande, E.H.A.; Santos, A.S.; Silva, M.H.L.; Maia, J.G.S. Constituintes Voláteis de Espécies de Lauraceae com Ocorrência na Floresta Nacional de Caxiuanã—Melgaço-PA; CBO 014- Estação Científica Ferreira Penna: Belém, Brazil, 2003; pp. 1–3. [Google Scholar]
- Da Silva, J.K.R.; Gomes, M.V.S.; Maia, J.G.S.; Dosoky, N.S.; Setzer, W.N. Chemical composition and in vitro biological activities of essential oil chemotypes of Licaria rigida (Kosterm.) Kosterm. (Lauraceae). Int. J. Appl. Res. Nat. Prod. 2016, 9, 1–9. [Google Scholar]
- Bosquiroli, L.S.S.; Ferreira, A.C.D.S.; Farias, K.D.S.; Da Costa, E.C.; Matos, M.D.F.C.; Kadri, M.C.T.; Rizk, Y.S.; Alves, F.M.; Perdomo, R.T.; Carollo, C.A.; et al. In vitro antileishmania activity of sesquiterpene-rich essential oils from Nectandra species. Pharm. Boil. 2017, 55, 2285–2291. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.K.R.; Andrade, E.H.D.A.; Mourão, R.H.V.; Maia, J.G.S.; Dosoky, N.S.; Setzer, W.N. Chemical Profile and in vitro Biological Activities of Essential Oils of Nectandra puberula and N. cuspidata from the Amazon. Nat. Prod. Commun. 2017, 12, 131–134. [Google Scholar] [CrossRef]
- Érica, R.C.; Louro, G.M.; Simionatto, S.; Vasconcelos, N.G.; Cardoso, C.A.; Mallmann, V.; Da Silva, R.C.; Matos, M.D.F.; Pizzuti, L.; Santiago, E.F.; et al. Chemical Composition, Antitumoral and Antibacterial Activities of Essential Oils from Leaves and Stem Bark of Nectandra lanceolata (Lauraceae). J. Essent. Oil Bear. Plants 2017, 20, 1184–1195. [Google Scholar] [CrossRef]
- Danielli, L.J.; Pippi, B.; Soares, K.D.; Duarte, J.A.; Maciel, A.J.; Machado, M.M.; Oliveira, L.F.S.; Bordignon, S.A.; Fuentefria, A.M.; Apel, M.A. Chemosensitization of filamentous fungi to antifungal agents using Nectandra Rol. ex Rottb. species essential oils. Ind. Crop. Prod. 2017, 102, 7–15. [Google Scholar] [CrossRef]
- Tondolo, J.; Amaral, L.D.P.; Simões, L.N.; Garlet, Q.I.; Schindler, B.; Oliveira, T.M.; Da Silva, B.F.; Gomes, L.D.C.; Baldisserotto, B.; Mallmann, C.A.; et al. Anesthesia and transport of fat snook Centropomus parallelus with the essential oil of Nectandra megapotamica (Spreng.) Mez. Neotropical Ichthyol. 2013, 11, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Grecco, S.D.S.; Martins, E.G.A.; Girola, N.; De Figueiredo, C.R.; Matsuo, A.L.; Soares, M.G.; Bertoldo, B.D.C.; Sartorelli, P.; Lago, J. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm. Boil. 2014, 53, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, C.S.B.; De Oliveira, L.F.; Szabo, E.M.; Souza, Ângela, M.; Dias, J.F.G.; Miguel, M.D.; Miguel, O.G. Chemical composition, antioxidant and biological activity of Ocotea bicolor Vattimo-Gil (LAURACEAE) essential oil. Braz. J. Pharm. Sci. 2018, 53, 53. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, D.F.; Dias, C.S.; Barbosa-Filho, J.M.; Agra, M.D.F.; Martins, R.; Silva, T.M.; Da-Cunha, E.V.; Silva, M.S.; Craveiro, A.A. Composition and Molluscicidal Activity of the Essential Oil from the Stem Bark of Ocotea bracteosa (Meisn.) Mez. J. Essent. Oil Res. 2007, 19, 482–484. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Da Trindade, R.; Moreira, E.C.D.O.; Maia, J.G.S.; Dosoky, N.S.; Miller, R.S.; Cseke, L.J.; Setzer, W.N. Chemical Diversity, Biological Activity, and Genetic Aspects of Three Ocotea Species from the Amazon. Int. J. Mol. Sci. 2017, 18, 1081. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, M.M.; Da Camara, C.A.G.; Da Silva, M.M. Comparative toxicity of essential oil and blends of selected terpenes of Ocotea species from Pernambuco, Brazil, against Tetranychus urticae Koch. Anais da Academia Brasileira de Ciências 2017, 89, 1417–1429. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Cunha, R.M.; Dias, C.S.; Athayde-Filho, P.F.; Da Silva, M.S.; Da-Cunha, E.V.L.; Machado, M.I.L.; Craveiro, A.A.; De Medeiros, I.A.; Ii, I. GC-MS analysis and cardiovascular activity of the essential oil of Ocotea duckei. Rev. Bras. de Farm. 2008, 18, 37–41. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, M.M.; Da Camara, C.A.; De Araujo, C.A. Chemical composition of essential oil from leaves of Ocotea limae Vattimo Gil. and Ocotea gardneri (Meisn.) Mez. growing wild in Atlantic forest of North-Eastern Brazil. Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas 2017, 16, 585–592. [Google Scholar] [CrossRef]
- Botelho, P.S.; Moraes, M.M.; Nevez, I.A.; Neves, R.C.S.; Ribeiro, N.C.; Born, F.S.; Camara, C.A.G. Composição química e Ação Repelente do óleo essencial Ocotea gardneri (Meisn) Mez. sobre o ácaro rajado Tetranychus urticae Koch. In In Proceedings of the IX-Jornada de Ensino Pesquisa e Extensão, UFRPE, Recife, Brazil, 24–31 October 2009; pp. 1–3. [Google Scholar]
- Dias, C.S.; Coutinho, D.F.; Martins, R.M.; Silva, T.M.S.; Craveiro, A.A.; Agra, M.F.; Barbosa-Filho, J.M. Análise por CG-EM e atividade moluscicida do óleo essencial das folhas de Ocotea gadneri (Meisn.) Mez (Lauraceae). In Proceedings of the 29a Reunião Anual da Sociedade Brasileira de Química, Centro de Convenções do Hotel Monte Real Resort, Águas de Lindóia, Brazil, 19–22 May 2006; pp. 1–2. [Google Scholar]
- Costa, R.G.; Da Cruz, R.A.; Rocha, L.; Santos, M.G.; Da Silva, A.J.R. Chemical Composition and Toxicity of Ocotea notata (Nees) Mez Essential Oil. J. Essent. Oil Bear. Plants 2010, 13, 455–459. [Google Scholar] [CrossRef]
- De Araujo, A.J.; Lordello, A.L.L.; Maia, B.H.L.N.S. Análise Comparativa Dos Óleos Essenciais De Folhas E Galhos De Ocotea puberula (Lauraceae). Visão Acadêmica 2001, 2, 81–84. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Alcantara, J.M.; Lima, E.S.; Da Veiga-Junior, V.F. Chemical Composition and Platelet Aggregation Activity of Essential Oils of Two Species of the Genus Ocotea (Lauraceae). J. Essent. Oil Bear. Plants 2013, 16, 518–523. [Google Scholar] [CrossRef]
- Silva, D.T.; Bianchini, N.H.; Muniz, M.F.; Heinzmann, B.M.; Labidi, J. Chemical composition and inhibitory effects of Nectandra grandiflora leaves essential oil against wood decay fungi. Drewno 2016, 59. [Google Scholar] [CrossRef]
- Garlet, Q.; Pires, L.; Silva, D.; Spall, S.; Gressler, L.; Bürger, M.; Baldisserotto, B.; Heinzmann, B.M. Effect of (+)-dehydrofukinone on GABAA receptors and stress response in fish model. Braz. J. Med Boil. Res. 2016, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romoff, P.; Ferreira, M.J.P.; Padilla, R.; Toyama, D.O.; Fávero, O.A.; Lago, J. Chemical composition of volatile oils from leaves of Nectandra megapotamica Spreng. (Lauraceae). Química Nova 2010, 33, 1119–1121. [Google Scholar] [CrossRef] [Green Version]
- Farias, K.D.S.; Delatte, T.; Arruda, R.D.C.D.O.; Alves, F.M.; Silva, D.B.; Beekwilder, J.; Carollo, C.A. In depth investigation of the metabolism of Nectandra megapotamica chemotypes. PLoS ONE 2018, 13, e0201996. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.D.L.; Da Silva, D.T.; Garlet, Q.I.; Da Cunha, M.A.; Mallmann, C.A.; Baldisserotto, B.; Longhi, S.J.; Pereira, A.M.S.; Heinzmann, B.M. Anesthetic activity of Brazilian native plants in silver catfish (Rhamdia quelen). Neotropical Ichthyol. 2013, 11, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, D.T.; Pinheiro, C.G.; Bianchini, N.H.; Batista, B.F.; Diefenthaeler, J.; Muniz, M.D.F.B.; Heinzmann, B.M. Microbiological damage influences the content, chemical composition and the antifungal activity of essential oils in a wild-growing population of Ocotea lancifolia (Schott) Mez. J. Essent. Oil Res. 2018, 30, 265–277. [Google Scholar] [CrossRef]
- Figueiredo, A.; Nascimento, L.M.; Lopes, L.G.; Giglioti, R.; Albuquerque, R.D.; Santos, M.G.; Falcão, D.Q.; Nogueira, J.A.; Rocha, L.; Chagas, A.C.S. First report of the effect of Ocotea elegans essential oil on Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2018, 252, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Leporatti, M.L.; Pintore, G.; Foddai, M.; Chessa, M.; Piana, A.; Petretto, G.L.; Masia, M.D.; Mangano, G.; Nicoletti, M. Chemical, biological, morphoanatomical and antimicrobial study of Ocotea puchury-major Mart. Nat. Prod. Res. 2013, 28, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Mossi, A.J.; Zanella, C.A.; Kubiak, G.; Lerin, L.A.; Cansian, R.L.; Frandoloso, F.S.; Prá, V.D.; Mazutti, M.A.; Costa, J.A.V.; Treichel, H. Essential oil of Ocotea odorifera: An alternative against Sitophilus zeamais. Renew. Agric. Food Syst. 2013, 29, 161–166. [Google Scholar] [CrossRef]
- Cansian, R.L.; Mossi, A.J.; Paroul, N.; Toniazzo, G.; Zboralski, F.; Prichoa, F.C.; Kubiak, G.B.; Lerin, L.A. Atividade antioxidante e antimicrobiana de extratos de canela-sassafrás (Ocotea odorifera (vell.) rowher). Perspectiva 2010, 34, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Alcoba, A.E.T.; Andrade, P.M.; Melo, D.C.; Miranda, M.L.D.; Magalhães, L.G. Bioatividades do óleo essencial das folhas de Ocotea odorifera (Lauraceae). In Proceedings of the 69a Reunião Anual da SBPC, UFMG, Belo Horizonte, Brazil, 16–22 July 2017; pp. 1–3. [Google Scholar]
- Silva, J.; Carmo, D.F.M.D.; Reis, Érika, M.; Machado, G.M.C.; Leon, L.; Da Silva, B.O.; Ferreira, J.L.P.; Amaral, A.C.F. Chemical and biological evaluation of essential oils with economic value from Lauraceae species. J. Braz. Chem. Soc. 2009, 20, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, S.G.; Mar, J.M.; Da Silva, L.S.; França, L.P.; Machado, M.; Tadei, W.P.; Bezerra, J.D.A.; Dos Santos, A.L.; Sanches, E.A. Bioactivity of Licaria puchury-major Essential Oil Against Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. Rec. Nat. Prod. 2018, 12, 229–238. [Google Scholar] [CrossRef]
- Sanches, E.A.; Trovati, G.; Chierice, G.O. Chemical Analysis of the Essential Oil Extracted from the Seeds of Licaria puchury-major. J. Essent. Oil Res. 2008, 20, 191–192. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Bianchini, N.H.; Amaral, L.D.P.; Longhi, S.J.; Heinzmann, B.M. Análise do efeito da sazonalidade sobre o rendimento do óleo essencial das folhas de Nectandra grandiflora Nees. Revista Árvore 2015, 39, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Castellani, D.C.; Casali, V.W.D.; Souza, A.L.; Cecon, P.R.; Cardoso, C.A.; Marques, V.B. Produção de óleo essencial em canela (Ocotea odorifera Vell.) e guaçatonga (Casearia sylvestris Swartz) em função da época de colheita. Rev. Bras. Pl. Med. 2006, 8, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Apel, M.A.; Lima, M.E.L.; Souza, A.; Cordeiro, I.; Young, M.C.M.; Sobral, M.E.; Suffredini, I.B.; Moreno, P.R.H. Screening of the biological activity from essential oils of native species from the Atlantic rain forest (São Paulo–Brazil). Pharmacol. Online 2006, 3, 376–383. [Google Scholar]
- Masia, M.D.; Deidda, S.; Deriu, G.M.; Deriu, M.G.; Chessa, M.; Petretto, G.L.; Foddai, M.; Maida, G.; Pintore, G.; Piana, A. Antimicrobial Activities of Essential Oils against Common Hospital Fungi Species. Open J. Prev. Med. 2014, 4, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.T.; Amaral, L.D.P.; Pinheiro, C.G.; Pires, M.M.; Schindler, B.; Garlet, Q.I.; Benovit, S.C.; Baldisserotto, B.; Longhi, S.J.; Kotzian, C.B.; et al. Larvicidal Activity of Brazilian Plant Essential Oils Against Coenagrionidae Larvae. J. Econ. Èntomol. 2014, 107, 1713–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Species | Collection Site | Date | Plant Part | Extraction Type | Major Components | References |
|---|---|---|---|---|---|---|
| L. canella | Manaus, AM | October, 2007 | Leaf | HD | Profile I, dry season: benzyl benzoate (69.70%), α-copaene (4.99%), and α-phellandrene (4.20%) | [56] |
| L. canella | Manaus, AM | February, 2008 | Leaf | HD | Profile I, rainy season: benzyl benzoate (73.00%), α-copaene (4.51%), and α-phellandrene (3.33%) | [56] |
| L. martiniana | Belém, PA | March, 2008 | Leaf | HD | Profile I: β-caryophyllene (41.70%), β-selinene (7.90%), and linalool isovalerate (5.90%) | [25] |
| L. martiniana | Belém, PA | March, 2008 | Stem | HD | Profile I: β-caryophyllene (21.40%), spathulenol (11.50%), and linalool (6.50%) | [25] |
| L. puchury-major | Belém, PA | Not reported | Seed | SD | Profile I: safrole (51.30%), 1,8-cineole (25.50%), and α-terpineol (8.60%) | [16] |
| L. puchury-major | Belém, PA | Not reported | Seed | HD | Profile II: safrole (38.80%), 1,8-cineole (21.70%), and limonene (8.27%) | [57] |
| L. puchury-major | Belém, PA | Not reported | Seed | SD | Profile II: safrole (36.11%), 1,8-cineole (21.12%), and limonene (12.20%) | [15] |
| L. puchury-major | Manaus, AM | July, 2002 | Seed | HD | Profile III: safrole (58.40%), dodecanoic acid (13.70%), and α-terpineol (8.40%) | [58] |
| L. puchury-major | Borba, AM | June, 2006 | Leaf | HD | Profile I: safrole (39.40%), 1,8-cineole (27.60%), and sabinene (8.50%) | [52] |
| L. rigida | Melgaço, PA | Not reported | Leaf | HD | Profile I: β-caryophyllene (59.40%), caryophyllene oxide (12.10%), and α-humulene (7.80%) | [26] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile I: β-caryophyllene (76.09%), α-humulene (6.61%), and viridiflorene (4.65%) | [27] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile II: δ-cadinene (10.53%), β-caryophyllene (9.73%), β-bourbonene (9.44%), and α-copaene (8.89%) | [27] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile III: 6-methoxyelemicin (51.86%), β-caryophyllene (15.33%), and selin-11-en-4α-ol (9.68%) | [27] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Twig | HD | Profile I: caryophyllene oxide (29.88%), 14-hydroxy-9-epi-β-caryophyllene (10.28%), and β-caryophyllene (8.92%) | [27] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Twig | HD | Profile II: 6-methoxyelemicin (63.31%), selin-11-en-4α-ol (23.99%), α-selinene (2.45%), and terpinen-4-ol (2.31%) | [27] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Branch | HD | Profile I: γ-cadinene (12.04%), terpinen-4-ol (10.67%), selin-11-en-4α-ol (7.67%), and ledol (6.68%) | [27] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Not reported | Branch | HD | Profile II: 6-methoxyelemycin (39.55%), selin-11-en-4α-ol (21.82%), and terpinen-4-ol (9.97%) | [27] |
| N. amazonum | Cáceres, MS | Not reported | Leaf | HD | Profile I: β-caryophyllene (28.50%), intermedeol (16.20%), and germacrene B (14.80%) | [28] |
| N. barbellata | Ribeirão Grande, SP | Not reported | Leaf | HD | Profile I: δ-cadinene (11.42%), β-caryophyllene (9.79%), and α-muurolol (7.56%) | [3] |
| N. cuspidata | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile I: β-caryophyllene (26.90%), bicyclogermacrene (16.00%), and spathulenol (5.20%) | [29] |
| N. gardneri | Campo Grande, MS | Not reported | Stem bark | HD | Profile I: intermedeol (58.20%), α-amorphene (8.00%), agarospirol (4.00%), germacrene D (3.50%) and α-elemene (3.50%) | [28] |
| N. grandiflora | Botocatu, SP | Not reported | Leaf | HD | Profile I, spring, summer, fall and winter: iso-bicyclogermacrenal (39.10%, 27.80%, 39.60%, 29.60%), spathulenol (13.30%, 18.50%, 11.10%, 20.10%), rosadiene (11.60%, 16.60%, 11.20%, 15.10%) | [23] |
| N. grandiflora | Jaguari, RS | Not reported | Leaf | HD | Profile II: dehydrofukinone (26.85%), valencene (6.89%), kaurene (6.03%), and selin-11-en-4-α-ol (5.34%) | [45] |
| N. grandiflora | Jaguari, RS | October-november, 2013 | Leaf | HD | Profile III: dehydrofukinone (24.70%), bicyclogermacrene (5.93%), and kaurene (5.49%) | [46] |
| N. hihua | Maracaju, MS | Not reported | Leaf | HD | Bicyclogermacrene (28.10%), germacrene D (13.80%), and β-caryophyllene (9.0%) | [28] |
| N. lanceolata | Barracão, RS | Not reported | Leaf | HD | Profile I: β-caryophyllene (32.50%), bicyclogermacrene (27.80%), and spathulenol (11.80%) | [31] |
| N. lanceolata | Mundo Novo, MS | February–march, 2013 | Leaf | HD | Profile I: bicyclogermacrene (18.20%), spathulenol (16.90%), and β-caryophyllene (12.45%) | [30] |
| N. lanceolata | Mundo Novo, MS | February–march, 2013 | Stem bark | HD | Profile I: guaiol (13.2%), cubenol (7.50%),γ-cadinene (7.5%), and α-eudesmol (7.0%) | [30] |
| N. lanceolata | Botocatu, SP | Not reported | Leaf | HD | Profile II, fall (May), winter (August): iso-bicyclogermacrenal (41.8%; 30.0%), spathulenol (11.9%; 20.2%), rosadiene (3.1%; 6.1%) | [23] |
| Spring (November), summer (February): iso-bicyclogermacrenal (34.1%; 34.3%), bicyclogermacrene (12.1%; 4.8%), spathulenol (7.6%; 15.9%) | ||||||
| N. leucantha | Ecological Park of Pereque, Cubatão, SP | December, 2012 | Leaf | HD | Profile I: bicyclogermacrene (28.44%), germacrene A (7.34%), α-pinene (6.59%), and spathulenol (5.82%) | [33] |
| N. megapotamica | Santa Maria-RS | November, 2010–September, 2011 | Leaf (young) | HD | Profile I, spring, summer, fall and winter: α-pinene (33.23%, 28.3%, 21.46% and 17.46%), β-pinene (17.8%, 15.43%, 13.86% and 10.36%), bicyclogermacrene (15.4%, 32.93%, 26.83% and 23.1%), germacrene D (6.4%, 10.43%, 9.4% and 10.13%) | [24] |
| N. megapotamica | Santa Maria-RS | November, 2010–September, 2011 | Leaf (Adult) | HD | Profile I, spring, summer, fall and winter: α-pinene (36.86%, 34.86%, 24.86%, and 15.5%),β-pinene (18.76%, 20.23%, 15.96%, and 10.06%), bicyclogermacrene (17.96%, 25.5%, 22.1%, and 23.6%), germacrene D (3.53%, 6.36%, 7.83%, and 9.8%). | [24] |
| N. megapotamica | Santa Maria, RS | November, 2010 | Leaf (young) | HD | Profile I: bicyclogermacrene (46.47%), α-pinene (26.82%), germacrene D (9.61%), and β-pinene (7.95%) | [32] |
| N. megapotamica | Santa Maria, RS | November, 2010 | Leaf (adult) | HD | Profile I: bicyclogermacrene (34.56%), α-pinene (26.19%), β-pinene (12.30%), germacrene D (9.2%) | [32] |
| N. megapotamica | Barracão, RS | Not reported | Leaf | HD | Profile II: bicyclogermacrene (33.40%), germacrene D (16.8%), and limonene (14.1%) | [31] |
| N. megapotamica | Maracaju, MS | April, 2014 | Leaf | HD | Profile III: bicyclogermacrene (66.7%), germacrene D (18.2%), and elemicin (5.6%) | [48] |
| N. megapotamica | Ponta Porã, RS | April, 2014 | Leaf | HD | Profile IV: δ-elemene (32.2%), bicyclogermacrene (28.2%), and α-asarone (10.3%) | [48] |
| N. megapotamica | Ponta Porã, RS | April, 2014 | Leaf | HD | Profile IV: δ-elemene (37.9%), bicyclogermacrene (26.3%), and α-asarone (15.0%) | [48] |
| N. megapotamica | Campo Grande, MS | October, 2013 | Leaf | HD | Profile V: α-asarone (22.6%), δ-elemene (15.6%), and α-santalene (11.8%) | [48] |
| N. megapotamica | Campo Grande, MS | November, 2013 | Leaf | HD | Profile VI: elemicin (35.9%), bicyclogermacrene (24.8%), and δ-3-carene (10.9%) | [48] |
| N. megapotamica | Campo Grande, MS | November, 2013 | Leaf | HD | Profile VII: elemicin (52.7%), and bicyclogermacrene (8.9%), and α-pinene (5.7%) | [48] |
| N. megapotamica | São Paulo-SP | February and August, 2007 | Leaf | HD | Profile VIII, summer: α-bisabolol (68.55%) and δ-elemene (12.2%). | [47] |
| Profile VIII, winter: α-bisabolol (63.55%) and δ-elemene (22.55%). | ||||||
| N. megapotamica | São Paulo-SP | November, 2014 | Leaf | HD | Profile IX: α-bisabolol (59.7%), δ-elemene (13.8%), and iso-spathulenol (11.3%) | [48] |
| N. megapotamica | São Paulo-SP | November, 2014 | Leaf | HD | Profile X: α-bisabolol (84.3%), germacrene D (4.0%), and β-bisabolene (2.5%) | [48] |
| N. megapotamica | São Paulo-SP | November, 2014 | Leaf | HD | Profile XI: α-bisabolol (93.7%), β-ocimene (1.5%) and germacrene D (1.4%) | [48] |
| N. megapotamica | São Paulo-SP | November, 2014 | Leaf | HD | Profile XII: iso-spathulenol (26.8%), δ-elemene (23.8%), and β-bisabolene (13.3%) | [48] |
| N. megapotamica | São Paulo-SP | November, 2014 | Leaf | HD | Profile XIII: β-sesquiphellandrene (32.0%), β-bergamotene (19.0%), and α-bisabolol (8.9%) | [48] |
| N. megapotamica | Botocatu, SP | Not reported | Leaf | HD | Profile XIV, spring (November): α-pinene (18.2%), β-pinene (16.2%), α-phellandrene (10.0%) | [23] |
| Summer (February): bicyclogermacrene (14.80%), α-phellandrene (11.0%), α-pinene (10.1%), and β-pinene (9.6%) | ||||||
| Fall (May): α-pinene (25.1%), β-pinene (22.3%), and bicyclogermacrene (9.1%) | ||||||
| Winter (August): α-pinene (20.1%), β-pinene (18.5%), and bicyclogermacrene (10.6%) | ||||||
| N. megapotamica | Campo Grande, MS | Not reported | Stem bark | HD | Profile I elemicin (41.7%), α-asarone (19.7%), and α- pinene (8.5%) | [28] |
| N. megapotamica | Campo Grande, MS | Not reported | Stem bark | HD | Profile II: α-asarone (42.4%), α-cadinol (14.4%), and τ-cadinol (8.1%) | [28] |
| N. puberula | Santarém, PA | Not reported | Leaf | HD | Profile I: apiole (22.2%), β-caryophyllene (15.1%), and β-pinene (13.3%) | [29] |
| N. puberula | Santarém, PA | Not reported | Branch | HD | Profile I: apiole (28.1%), pogostol (19.8%), and guaiol (11.2%) | [29] |
| O. acutifolia | São Francisco de Assis, RS | May, 2011 | Leaf | HD | Profile I: caryophyllene oxide (56.9%), calarene epoxide (11.74%), and τ-elemene (8.17%) | [49] |
| O. bicolor | Curitiba, PR | August, 2015 | Leaf | HD | Profile I: δ-cadinene (7.39%), β-sesquiphellandrene (6.67%), β-elemene (5.41%), and α-cadinol (5.23%) | [34] |
| O. bracteosa | Santa Rita, PB | May, 2004 | Stem bark | HD | Profile I: δ-cadinene (12.4%), ledene (11.1%), and globulol (10.1%) | [35] |
| O. caniculata | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile I: β-selinene (20.3%), β-caryophyllene (18.9%), and 7-epi-α-selinene (14.3%) | [36] |
| O. caniculata | Caxiuanã National Forest, Melgaço, PA | Not reported | Branch | HD | Profile I: selin-11-en-4α-ol (20.6%), β-selinene (12.1%), and 7-epi-α-selinene (9.0%) | [36] |
| O. caudata | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile I: bicyclogermacrene (29.6%), germacrene D (19.9%), α-pinene (9.8%), and β-pinene (9.7%) | [36] |
| O. caudata | Caxiuanã National Forest, Melgaço, PA | Not reported | Branch | HD | Profile I: δ-cadinene (13.8%), germacrene D (8.9%), β-guaiene (8.3%), and α-muurolol (7.8%) | [36] |
| O. cujumary | Caxiuanã National Forest, Melgaço, PA | Not reported | Leaf | HD | Profile I: β-caryophyllene (22.2%), caryophyllene oxide (12.4%), 2-tridecanone (7.30%), and δ-cadinene (6.6%) | [36] |
| O. cujumary | Caxiuanã National Forest, Melgaço, PA | Not reported | Branch | HD | Profile I: 2-tridecanone (30.0%), limonene (20.5%), and β-caryophyllene (8.1%) | [36] |
| O. cymbarum | Melgaço, PA | Not reported | Stem bark | HD | Profile I: α-selinene (25.8%), δ-cadinene (18.6%), and terpinen-4-ol (9.0%) | [26] |
| O. duckei | Santa Rita, PB | March, 2005 | Leaf | SD | Profile I: β-caryophyllene (60.54%), α-humulene (4.63%), and δ-selinene (4.4%) | [38] |
| O. duckei | Santa Rita, PB | March, 2005 | Stem bark | SD | Profile I: β-eudesmol (27.51%), α-pinene (9.02%), limonene (6.65%), and borneol (6.18%) | [38] |
| O. duckei | Santa Rita, PB | March, 2005 | Fruit | SD | Profile I: limonene (30.12%), β-pinene (12.25%), and α-pinene (9.89%) | [38] |
| O. duckei | Santa Rita, PB | March, 2005 | Root | SD | Profile I: elemol (24.31%), β-elemene (16.69%), and β-eudesmol (13.44%) | [38] |
| O. duckei | Senhorzinho Cabral Forest, Camocim of São Félix, PE | September, 2010 | Leaf | HD | Profile II: β-caryophyllene (18.1%), valencene (17.6%), and elemol (6.8%) | [37] |
| O. elegans | Restinga de Jurubatiba National Park, Carapebus, RJ | November, 2014–january, 2015 | Leaf | HD | Profile I: sesquirosefuran (92.2%) | [51] |
| O. gardneri | Forest of Cruzina, Igarassú, PE | March, 2008 | Leaf | HD | Profile I: germacrene D (26.9%), bicyclogermacrene (21.7%), β-caryophyllene (6.1%), and germacrene B (4.9%) | [39] |
| O. gardneri | Igarassú, PE | Not reported | Leaf | HD | Profile I: germacrene D (26.96%), bicyclogermacrene (20.73%) and viridiflorol (5.52%) | [40] |
| O. gardneri | not reported | Not reported | Leaf | HD | Profile I: β-caryophyllene (29.28%), α-pinene (15.4%), kaurene (18.35%), and β-pinene (8.93%) | [41] |
| O. glomerata | Senhorzinho Cabral Forest, Camocim of São Félix, PE | September, 2010 | Leaf | HD | Profile I: aromadendrene (17.3%), β-caryophyllene (14.6%), α-pinene (6.90%), and γ-terpinene (6.40%) | [37] |
| O. indecora | Ribeirão Grande, SP | Not reported | Leaf | HD | Profile I: bicyclogermacrene (29.79%), valerianol (15.12%), β-pinene (11.41%), and spathulenol (11.16%) | [3] |
| O. lancifolia | Santa Maria, RS | April, 2013–march, 2014 | Leaf | HD | Profile I: Seasonal study: April, June, August: caryophyllene oxide (36.40–40.6%), allo-himachalol (6.2–8.0%), bulnesol (6.0–7.10%), and bicyclogermacrene (5.8–6.1%). | [50] |
| May: β-chenopodiol (20.9%), kaurene (11.9%), (Z)-nerolidyl acetate (9.3%), and caryophyllene oxide (7.0%). | ||||||
| July: β-chenopodiol (17.4%), (Z)-nerolidyl acetate (8.7%), α-guaiene (5.0%), and (E)-β-ocimene (4.9%). | ||||||
| September, October: caryophyllene oxide (42.2/46.4%), bicyclogermacrene (6.3/7.3%), allo-himachalol (5.7/5.9%), and calarene epoxide (5.5/6.7%). | ||||||
| November, January, February, March: caryophyllene oxide (38.6–42.2%), bicyclogermacrene (6.7–7.80%), allo-himachalol (5.9–7.4%) | ||||||
| O. lancifolia | Santa Maria, RS | April and May, 2013 | Inflorescences | HD | Profile I: seasonal study, April: caryophyllene oxide (34.9%), bicyclogermacrene (8.1%), and β-chenopodiol (6.0%) | [50] |
| May: β-chenopodiol (38.7%), α-guaiene (6.0%), and (Z)-nerolidyl acetate (4.5%) | ||||||
| O. lancifolia | Santa Maria, RS | July–november, 2013 | Fruit | HD | Profile I: seasonal study, July: β-chenopodiol (17.1%), β-ocimene (6.2%), and γ-muurolene (4.7%) | [50] |
| August, September: caryophyllene oxide (46.2%, 52.1%), bicyclogermacrene (8.9%, 9.9%), and β-ocimene (2.8%, 3.1%) | ||||||
| October: caryophyllene oxide (48.1%), bicyclogermacrene (6.7%), and (E)-iso-valencenol (3.8%) | ||||||
| November: caryophyllene oxide (27.9%), bicyclogermacrene (6.9%), and allo-himachalol (6.7%) | ||||||
| O. limae | Igarassú, PE | March, 2008 | Leaf | HD | Profile I: spathulenol (13.3%), β-caryophyllene (12.4%), bicyclogermacrene (11.3%), and germacrene D (10.9%) | [39] |
| O. longifólia | Melgaço, PA | Not reported | Stem bark | HD | Profile I: dillapiole (15.2%), δ-cadinene (20.0%), α-cubebene (6.5%), and α-copaene (5.1%) | [26] |
| O. nigrescens | Manaus, AM | March, 2008 | Leaf | HD | Profile I: β-caryophyllene (37.9%), β-pinene (6.9%), α-pinene (6.6%), and α-copaene (6.2%) | [44] |
| O. notata | Restinga de Jurubatiba National Park, Carapebus, RJ | November, 2006 | Leaf | SD | Profile I: β-caryophyllene (22.9%), germacrene A (22.7%), α-pinene (8.7%), and β-pinene (6.9%) | [42] |
| O. odorifera | Machado, MG | July, 2016 | Leaf | HD | Profile I: safrole (36.3%), γ-cadinene (6.6%), camphor (6.5%), and α-copaene (6.0%) | [55] |
| O. odorifera | Marcelino Ramos, RS | Not reported | Leaf | HD | Profile II: camphor (43.0%), safrole (42.0%), camphene (6.0%), limonene (3.0%) | [53] |
| O. odorifera | Marcelino Ramos, RS | Not reported | Leaf | HD | Profile II: safrole (40.23%), camphor (34.35%), limonene (7.42%), and camphene (5.02%) | [54] |
| O. puberula | Curitiba, PR | Not reported | Leaf | HD | Profile I: β-caryophyllene (31.0%), bicyclogermacrene (14.0%), β-elemene (9.7%), and longifolene (8.7%) | [43] |
| O. puberula | Curitiba, PR | Not reported | Branch | HD | Profile I: bicyclogermacrene (31.0%), β-caryophyllene (14.0%), β-pinene (7.9%), and β-elemene (5.3%) | [43] |
| O. splendens | Manaus, AM | March, 2008 | Leaf | HD | Profile I: β-caryophyllene (51.0%), caryophyllene oxide (9.9%), and α-humulene (6.2%) | [44] |
| Lauraceae Species | Collection Site | Plant Part | Major Components | Bioactivities | References |
|---|---|---|---|---|---|
| L. canella | Manaus, AM | Leaf | Benzyl benzoate (69.70%), α-copaene (4.99%), and α-phellandrene (4.20%) | Anti-leishmanial (Leishmania amazonensis, promastigotes, IC50 19.0 µg/mL), cytotoxic (mice BALB-c macrophage, IC50 6.20 µg/mL), toxicological (Artemia salina lethality, LC50: 5.25 μg/mL) | [56] |
| L. martiniana | Belém, PA | Leaf | L: β-caryophyllene (41.7%), β-selinene (7.90%), linalyl isovalerate (5.90%), and linalool (5.30%) | Antioxidant (DPPH method, EC50 > 1000 μg/mL), and antiplatelet activities (L: 4.24%, S: 36.95%) | [25] |
| S: β-caryophyllene (21.40%), spathulenol (11.50%), and linalool (6.50%) | |||||
| L. puchury-major | Belém, PA | Seeds | Profile I: safrole (51.30%), 1,8-cineole (25.50%), and α-terpinen-4-ol (8.60%) | Reduced motor activity in rats (50–100 mg/kg) and anesthetized mice (800 mg/kg) for < 1 h. | [16] |
| L. puchury-major | Belém, PA | Seeds | Profile I: safrole (38.80%), 1,8-cineole (21.70%), and limonene (8.27%) | Antioxidant (DPPH method, IC50 27.8 μg/mL), larvicidal (Aedes aegypti LC50 98.9 μg/mL; acaricide (Tetranychus urticae Koch, LC50 30.8 μg/mL; filter paper disks method, EO at 500 ppm), insecticidal Cerataphis lataniae, LC50 13.5 μg/mL, filter paper disks method, EO at 500 ppm) | [57] |
| L. puchury Mayor | Borba, AM | Not reported | Not reported | Antifungal, disc diffusion technique (Aspergillus fumigatus, Rhodotorula spp., Candida albicans, Fusarium spp., Alternaria spp.), no MIC values | [62] |
| L. puchury-major | Borba, AM | Leaf | Safrole (39.4%),1,8-cineole (27.60%), sabinene (8.50%), and α-terpineol (7.90%) | Antimicrobial (bacteria: Streptococcus agalactiae, Staphylococcus aureus; fungi: Rhodotorula spp., Candida spp., agar disc diffusion technique), no MIC values | [52] |
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Leaf | Profile I: β-caryophyllene (76.09%), α-humulene (6.61%), and viridiflorene (4.65%) (L-I). | Antibacterial (Escherichia coli, microbroth dilution method, MIC< 19.50 µg/mL to L-I, L-II, and L-III); Cytotoxic (MCF-7 mammary adenocarcinoma, MTT assay) IC50 66.50 μg/mL (L-II), IC50 158.60 μg/mL (L-III); Antioxidant (DPPH method, L-III 718.1 ± 106.5 mg.ET/mL); | [27] |
| Profile II: δ-cadinene (10.53%), β-caryophyllene (9.73%), β-bourbonene (9.44%), and α-copaene (8.89%) (L-II) | |||||
| Profile III: 6-methoxy-elemicin (51.86%), β-caryophyllene (15.33%), selin-11-en-4α-ol (9.68%) (L-III) | |||||
| L. rigida | Caxiuanã National Forest, Melgaço,PA | Twig | Profile I: caryophyllene oxide (29.88%), 14-hydroxy-9-epi-β-caryophyllene (10.28%), and β-caryophyllene (8.92%) (T-I) | Antibacterial (Escherichia coli, MIC < 19.50 µg/mL, microbroth dilution method to T-I, and T-II) | [27] |
| Profile II: 6-methoxy-elemicin (63.31%), selin-11-en-4α-ol (23.99%), and α-selinene (2.45%) (T-II). | |||||
| L. rigida | Caxiuanã National Forest, Melgaço, PA | Branch | Profile I: γ-cadinene (12.04%), terpinen-4-ol (10.67%), selin-11-en-4α-ol (7.67%), ledol (6.68%) (B-I). | Cytotoxic (MCF-7 mammary adenocarcinoma, MTT assay): IC50 110.70 μg/mL (B-I) and IC50 95.10 μg/mL (B-II). Antibacterial (Escherichia coli, MIC< 19.50 µg/mL, microbroth dilution method) | [27] |
| Profile II: 6-methoxy-elemicin (39.55%), selin-11-en-4α-ol (21.82%), and terpinen-4-ol (9.97%) (B-II). | |||||
| N. amazonum | Cáceres, MS | Leaf | β-caryophyllene (28.50%), intermediol (16.20%), and germacrene B (14.80%) | Anti-leishmanial (Leishmania infantum, amastigotes, IC50 31.90 µg/mL; L. amazonensis, amastigotes, IC50 22.10 µg/mL). Cytotoxic, fibroblast cells (NIH/3T3, IC50 58.0 µg/mL); sarcoma cells (J774.A1, IC50 29.40 µg/mL) | [28] |
| N. cuspidata | Caxiuanã National Forest, Melgaço, PA | Leaf | β-caryophyllene (26.9%), bicyclogermacrene (16.0%) and spathulenol (5.2%) | Antibacterial, (Escherichia coli, MIC 19.50 μg/mL; Bacillus cereus, MIC 312.50–625.0 μg/mL; Staphylococcus aureus, MIC 312.50–625.0 μg/mL; Staphylococcus epidermidis, MIC 625.0 μg/mL, microbroth dilution method), cytotoxic, MCF-7 breast tumor cells (IC50 117.10 μg/mL) | [29] |
| N. gardneri | Campo grande, MS | Stem bark | Intermediol (58.20%), α-amorphene (8.0%), agarospirol (4.0%), germacrene D (3.50%), α-elemene (3.50%) | Anti-leishmanial (Leishmania infantum, amastigotes, IC50 2.70 µg/mL; L. amazonensis, amastigotes, IC50 2.10 µg/mL). Cytotoxic, fibroblast cells (NIH/3T3, IC50 51.60 µg/mL); sarcoma cells (J774A.1, IC50 29.90 µg/mL) | [28] |
| N. grandiflora | Botocatu, SP | Leaf | Profile I, spring, summer, fall and winter: iso-bicyclogermacrenal (39.10%, 27.80%, 39.60%, 29.60%), spathulenol (13.30%, 18.50%, 11.10%, 20.10%), rosadiene (11.60%, 16.60%, 11.20%, 15.10%) | Antibacterial, resazurin-based assay: Escherichia coli (winter, MIC 6.50%; spring, MIC 4.25%; summer, MIC 10.10%; fall, MIC 10.10%), and Staphylococcus aureus (winter, MIC 1.90%; spring, MIC 1.80%; summer, MIC 1.90%; fall, MIC 3.0%) | [23] |
| N. grandiflora | Jaguari, RS | Leaf | Profile II: dehydrofukinone (26.85%), valencene (6.89%), kaurene (6.03%), 4,5-di-epi-aristolochene (5.41%) | Antifungal (Pycnoporus sanguineus, LC50 1.22 μL/mL; Gloeophyllum trabeum, LC50 0.39 μL/mL, radial growth technique) | [45] |
| N. grandiflora | Jaguari, RS | Leaf | Profile III: dehydrofukinone (24.70%), bicyclogermacrene (5.93%), and kaurene (5.49%) | Sustained sedative effect in silver catfish (Rhamdia quelen) for 12 h at 10–20 ug/mL | [46] |
| N. hihua | Maracaju, MS | Leaf | Bicyclogermacrene (28.10%), germacrene D (13.80%), β-caryophyllene (9.0%), 9-epi-β-caryophylene (7.0%) | Antileishmanial (Leishmania infantum, amastigotes, IC50 0.20 µg/mL; L. amazonenses, amastigotes, IC50 24.20 µg/mL). Cytotoxic, fibroblast cells (NIH/3T3, IC50 54.90 µg/mL); sarcoma cells (J774A.1, IC50 29.80 µg/mL) | [28] |
| N. lanceolata | Barracão, RS | Leaf | Profile I: β-caryophyllene (32.5%), bicyclogermacrene (27.8%), and spathulenol (11.8%) | Antifungal (Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis and Microsporum gypseum, MIC 250–500 μL/mL, microdilution method); antioxidant, DPPH method (250 µg/mL, above 50% inhibition); antichemotactic effect (leukocyte migration inhibition, 30.70–96.70%) | [31] |
| N. lanceolata | Novo Mundo, MS | Leaf and Bark | Profile II: bicyclogermacrene (18.20%), spathulenol (16.70%), and β-caryophyllene (12.45%). | Cytotoxic (K562 leukemia) TGI = 72.40 and 14.60 mg/mL; U251 glioma, TGI = 75.80 and 37.30 mg/mL. | [30] |
| Bark: Guaiol (13.20%), cubenol (7.60%), γ-cadinene (7.60%), α-pinene (6.90%) | |||||
| N. lanceolata | Botocatu, SP | Leaf | Fall and winter: iso-bicyclogermacrenal (41.80/30.0%), spathulenol (11.90/20.20%), rosadiene (3.10/6.10%) | Antibacterial, resazurin-based assay: Escherichia coli (winter, MIC 7.50%; spring, MIC 4.0%; summer, MIC 10.10%; fall, MIC 10.10%), and Staphylococcus aureus (winter, MIC 0.60%; spring, MIC 0.70%; summer, MIC 0.55%; fall, MIC 0.55%) | [23] |
| Spring and summer: iso-bicyclogermacrenal (34.10/34.30%), bicyclogermacrene (12.10/4.80%), spathulenol (7.60/15.90%) | |||||
| N. leucantha | Ecological Park of Pereque, Cubatão, SP | Leaf | Bicyclogermacrene (28.44%), germacrene A (7.34%), and α-pinene (6.59%) | Cytotoxic (B16F10-Nex2 murine melanoma, IC50 33 µg/mL; U87 human glioblastoma, IC50 75.95 µg/mL; HeLa human cervical carcinoma, IC50 60 µg/mL) | [33] |
| N. megapotamica | Cananéia, SP | Leaf | Not reported | Antibacterial (Escherichia coli, 20.20%; Staphylococcus aureus, 71.0%; Pseudomonas aeruginosa, 51.0%, microdilution method); anti-inflamatory, leukocyte migration assay (average distance of 16.20 ± 3.80 mm); cytotoxic (MCF-7 mammary adenocarcinoma, NCI lung great cells carcinoma, KM colon adenocarcinoma, SF glioblastoma, < 50.0%; PC-3 prostate carcinoma, 65.50%; RPMI multiple myeloma, 76.20%). EO at 3.125 µL/mL | [61] |
| N. megapotamica | Santa Maria, RS | Leaf and Bark | Not reported | Larvicidal activity against Coenagrionidae larvae (20%, and 60% mortality after 19 h, respectively),EO at 0.1 uL/mL | [63] |
| N. megapotamica | Santa Maria, RS | Leaf (young/old) | Profile I: bicyclogermacrene (46.5/34.6%), α-pinene (26.8/26.2%), β-pinene (7.9/12.3%), and germacrene D (9.6/9.1%) | Anesthetic potential to the fish species Centropomus parallelus (mild sedation at 30 μL/L [1.3–3.2 min], and deep anesthesia at 150 μL/L [5.6–8.0 min]) | [32] |
| N. megapotamica | Barracão, RS | Leaf | Profile II: Bicyclogermacrene (33.40%), germacrene D (16.80%) and limonene (14.10%) | Antifungal (Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis and Microsporum gypseum, MIC 250–500 μL/mL, microdilution method); antioxidant, DPPH method (250 µg/mL, above 40% inhibition); antichemotactic effect (leukocyte migration inhibition, 34.50–94.10%) | [31] |
| N. megapotamica | Botocatu, SP | Leaf | Profile XIV: spring (November): α-pinene (18.20%), β-pinene (16.20%), α-phellandrene (10.0%), and bicyclogermacrene (8.70%) | Antibacterial, resazurin-based assay: Escherichia coli (winter, MIC 2.25%; spring, MIC 5.50%; summer, MIC 6.50%; fall, MIC 6.75%), and Staphylococcus aureus (winter, MIC 1.05%; spring, MIC 1.90%; summer, MIC 1.90%; fall, MIC 3.0%) | [23] |
| Summer (February): bicyclogermacrene (14.80%), α-phellandrene (11.0%), and α-pinene (10.10%) | |||||
| Fall (May): α-pinene (25.10%), β-pinene (22.30%), and bicyclogermacrene (9.10%) | |||||
| Winter (August): α-pinene (20.10%), β-pinene (18.50%), and bicyclogermanrene (10.60%) | |||||
| N. megapotamica | Campo grande, MS | Stem bark | Profile I: Elemicin (41.70%), (E)-asarone (19.70%), α-pinene (8.50%), (Z)-β-ocimene (4.0%) | Antileishmanial (L. amazonensis, amastigotes, IC50 19.0 µg/mL), cytotoxic, fibroblast cells (NIH/3T3, IC50 162.30 µg/mL) sarcoma cells (J774A.1, IC50 221.60 µg/mL) | [28] |
| N. megapotamica | Campo grande, MS | Stem bark | Profile II: α-asarone (42.4%), α-cadinol (14.4%), τ-cadinol (8.10%), and δ-Cadinene (5.8%) | Antileishmanial (Leishmania infantum, amastigotes, IC50 12.50 µg/mL; L. amazonenses, amastigotes, IC50 21.30 µg/mL). cytotoxic, cells fibroblast cells (NIH/3T3, IC50 252.60 µg/mL); sarcoma cells (J774.A1, IC50 415.60 µg/mL) | [28] |
| N. puberula | Santarém, PA | Leaf | Apiole (22.20%), β-caryophyllene (15.10%) and β-pinene (13.30%) | Antibacterial (Escherichia coli, MIC 19.50 μL/mL; Bacillus cereus, MIC 625.0 μL/mL; Staphylococcus aureus, MIC 625.0 μL/mL; Staphylococcus epidermidis, MIC 625.0 μL/mL, microbroth dilution method), cytotoxic (MCF-7 mammary adenocarcinoma, IC50 64.5 μg/mL) | [29] |
| O. acutifolia | São Francisco de Assis, RS | Leaf | Caryophyllene oxide (56.90%), calarene epoxide (11.74%), τ-elemene (8.17%), | Anesthetic effect (silver catfish, Rhamdia quelen) at 300–900 μL/L (13–18 min). | [49] |
| O. bicolor | Curitiba, PR | Leaf | δ-Cadinene (7.39%), β-sesquiphellandrene (6.67%), β-elemene (5.41%), and α-cadinol (5.23%) | Antioxidant (DPPH method, EC50 > 500 μg/mL); antibacterial, microdilution method (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacter aerogenes, Klebsiella pneumoniae, Staphylococcus epidermidis and Salmonella typhimurium, MIC > 1000 μg/mL), toxicological (Artemia salina, LC50 40.10 μg/mL) | [34] |
| O. bracteosa | Santa Rita, PB | Stem bark | δ-Cadinene (12.40%), ledene (11.10%), globulol (10.1%), and aromadendrene (4.2%) | Molluscicidal (Biomphalaria glabrata, LC90 8.30 µg/mL) | [35] |
| O. caniculata | Caxiuanã National Forest, Melgaço, PA | Leaf | β-selinene (20.30%), β-caryophyllene (18.90%), 7-epi-α-selinene (14.30%), and bicyclogermacrene (10.40%) | Antibacterial, microdilution method (Escherichia coli, MIC 19.50 µg/mL; Staphylococcus epidermidis, MIC 312.50 µg/mL; Staphylococcus aureus, MIC 625.0 µg/mL; Bacillus cereus, MIC 312.50 µg/mL), cytotoxic (MCF-7 mammary adenocarcinoma, IC50 67.70 μg/mL) | [36] |
| O. caudata | Caxiuanã National Forest, Melgaço, PA | Leaf | Bicyclogermacrene (29.60%), germacrene D (19.90%), α-pinene (9.80%), and β-pinene (9.70%) | Antibacterial, microdilution method (Escherichia coli, MIC 19.50 µg/mL; Staphylococcus epidermidis, MIC 625.0 µg/mL; Staphylococcus aureus, MIC 625.0 µg/mL, Bacillus cereus, MIC 312.50 µg/mL), cytotoxic (MCF-7 mammary adenocarcinoma, IC50 64.0 μg/mL) | [36] |
| O. cujumary | Caxiuanã National Forest, Melgaço, PA | Leaf | β-caryophyllene (22.20%), caryophyllene oxide (12.40%), 2-tridecanone (7.30%), and δ-cadinene (6.60%) | Antibacterial, microdilution method (Escherichia coli, MIC 19.50 µg/mL; Staphylococcus epidermidis, MIC 625.0 µg/mL; Staphylococcus aureus, MIC 625.0 µg/mL, Bacillus cereus, MIC 625.0 µg/mL), cytotoxic (MCF-7 mammary adenocarcinoma, IC50 63.90 μg/mL) | [36] |
| O. duckei | Santa Rita, PB | Leaf, Steam bark, Fruits, and roots | Profile I: β-caryophyllene (60.54%), α-humulene (4.63%), δ-selinene (4.40%), and δ-cadinene (1.69%) | Cardiovascular (Wistar rats model) EO at 1.0, 5.0, 10.0 and 15.0 mg/kg. - Induced hypotension Leaves: (7.0, 15.0, 21.0 and 37.0%, respectively) Stem Bark: (8.0, 25.0, 38.0, 27.0%, respectively) Fruits: (6.0, 8.0, 18.0 and 26.0%, respectively) Roots: (4.0, 20.0, 33.0, 25.0%, respectively) - bradycardia leaves: (3.0, 9.0, 18.0 and 53.0%, respectively) Stem Bark: (5.0, 22.0, 53.0, 49.0%, respectively) Fruits: (3.0, 3.0, 12.0 and 35.0%, respectively) Roots: (3.0, 30.0, 57.0 and 35.0%, respectively) | [38] |
| Stem Bark: β-eudesmol (27.51%), α-pinene (9.02%), limonene (6.65%), and borneol (6.18%) | |||||
| Fruits: limonene (30.12%), β-pinene (12.25%), α-pinene (9.89%), and myrcene (7.86%); | |||||
| Roots: elemol (24.31%), β-elemene (16.69%), β-eudesmol (13.44%), and borneol (3.69%) | |||||
| O. elegans | Restinga de Jurubatiba National Park, Carapebus, RJ | Leaf | Sesquirosefuran (92.2%) | Antiparasitic, Rhipicephalus (Boophilus) microplus (larval packet test [LPT], LC50 59.68 mg/mL [24 h] and 25.59 mg/mL [48 h]; adult immersion test [AIT], LC50 4.96 mg/mL and LC90 17.37 mg/mL; larval repellency test [RT], LC50 0.04 mg/mL and LC90 1.24 mg/mL) | [51] |
| O. gardneri | Igarassu, PE | Leaf | Germacrene D (26.96%), bicyclogermacrene (20.73%), and viridiflorol (5.52%) | Acaricidal (Tetranychus urticae, 1.50 to 2.50 µL/cm2 of EO, percentages of repellency from 17.32% to 68%) | [40] |
| O. gardneri | not reported | Leaf | β-caryophyllene (29.28%), α-pinene (15.40%), kaurene (18.35%), and β-pinene (8.93%) | Molluscicidal (Biomphalaria glabrata, LC90 16.50 mg/mL, LC50 9.70 mg/mL, and LC10 2.80 mg/mL) | [41] |
| O. lancifolia | Santa Maria, RS | Leaf | Seasonal study (fall): caryophyllene oxide (40.6%), allo-himachalol (8.0%), bulnesol (6.9%), bicyclogermacrene (6.1%) | Antifungal (Fusarium moniliforme, mycelial growth inhibition in 67.50% at 1.0 µL/mL) | [50] |
| O. lancifolia | Santa Maria, RS | Leaf | Seasonal study (fall): β-chenopodiol (20.9%), (Z)-nerolidyl acetate (9.3%), and caryophyllene oxide (7%) | Antifungal (Fusarium moniliforme, mycelial growth inhibition in around 50.0% at 1.0 µL/mL) | [50] |
| O. lancifolia | Santa Maria, RS | Inflorescences | Seasonal study: caryophyllene oxide (34.90%), bicyclogermacrene (8.10%), and atractylone (4.90%) | Antifungal (Fusarium moniliforme, mycelial growth inhibition in around 60.0% at 1.0 µL/mL) | [50] |
| O. lancifolia | Santa Maria, RS | Fruit | Seasonal study: caryophyllene oxide (42.10%), bicyclogermacrene (9.90%), and (E)-β-ocimene (3.10%) | Antifungal (Fusarium moniliforme, mycelial growth inhibition in around 62.0% at 1.0 µL/mL) | [50] |
| O. nigrescens | Manaus, AM | Leaf | β-caryophyllene (37.90%), β-pinene (6.90%), α-pinene (6.60%), linalool (5.50%), and α-copaene (6.20%) | Platelet aggregation activity (anti-aggregant factor with 10.80%) | [44] |
| O. notata | Carapebus, RJ | Leaf | β-caryophyllene (22.90%), germacrene A (22.70%), and α-pinene (8.70%) | Toxicological (Artemia salina, LC50 2.37 μg/mL) | [42] |
| O. odorifera | Machado, MG | Leaf | Profile I: safrole (36.30%), γ-cadinene (6.60%), camphor (6.50%), and α-copaene (6.0%) | Antileishmanial (Leishmania amazonensis, amastigotes, IC50 4,67 μg/mL), cytotoxic (mice BALB/c peritonal macrophages (CC50 49.52 μg/mL) | [55] |
| O. odorifera | Marcelino Ramos, RS | Leaf | Profile II: camphor (43.0%), safrole (42.0%), camphene (6.0%), limonene (3.0%) | Insecticidal and repellent (maize weevil Sitophilus zeamais, LD50 14.10 μL or 0.09 μL/cm2) | [53] |
| O. odorifera | Marcelino Ramos, RS | Leaf | Profile II: safrole (40.23%), camphor (34.35%), and limonene (7.42%) | Antibacterial, disc diffusion method: Gram-negative (Acinetobacter sp, Aeromonas sp, Citrobacter freundii, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Salmonella choleraesuis, Serratia marcescens, Shigella flexneri, Yersinia enterocolitica) and Gram-positive (Enterococcus faecalis, Micrococcus luteus, Sarcina sp, Staphylococcus epidermidis, Streptococcus mutans, Staphylococcus aureus), no MIC values reported; antioxidant, DPPH (IC50 46.03 mg/mL) | [54] |
| O. splendens | Manaus, AM | Leaf | β-caryophyllene (51.0%), caryophyllene oxide (9.90%), α-humulene (6.20%) | Platelet aggregation activity (anti-aggregant factor with 11.74%) | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, J.K.A.M.; Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes. Biomolecules 2020, 10, 869. https://doi.org/10.3390/biom10060869
Xavier JKAM, Alves NSF, Setzer WN, da Silva JKR. Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes. Biomolecules. 2020; 10(6):869. https://doi.org/10.3390/biom10060869
Chicago/Turabian StyleXavier, Júlia Karla A. M., Nayara Sabrina F. Alves, William N. Setzer, and Joyce Kelly R. da Silva. 2020. "Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes" Biomolecules 10, no. 6: 869. https://doi.org/10.3390/biom10060869
APA StyleXavier, J. K. A. M., Alves, N. S. F., Setzer, W. N., & da Silva, J. K. R. (2020). Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes. Biomolecules, 10(6), 869. https://doi.org/10.3390/biom10060869