Pre-Clinical Evaluation of Biological Bone Substitute Materials for Application in Highly Loaded Skeletal Sites
Abstract
1. Introduction
2. The Adaptive Load-Bearing Capacity of Bone
3. Biomechanical Considerations for the Design of BSMs
Biological BSMs
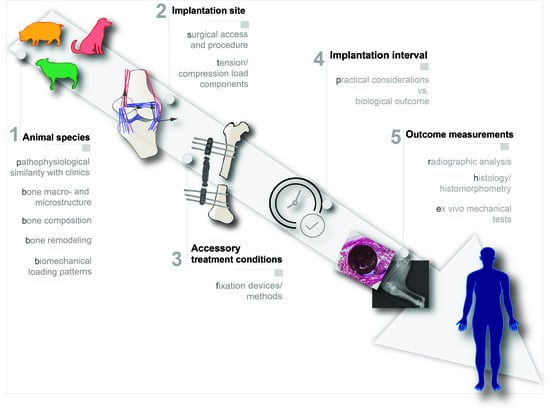
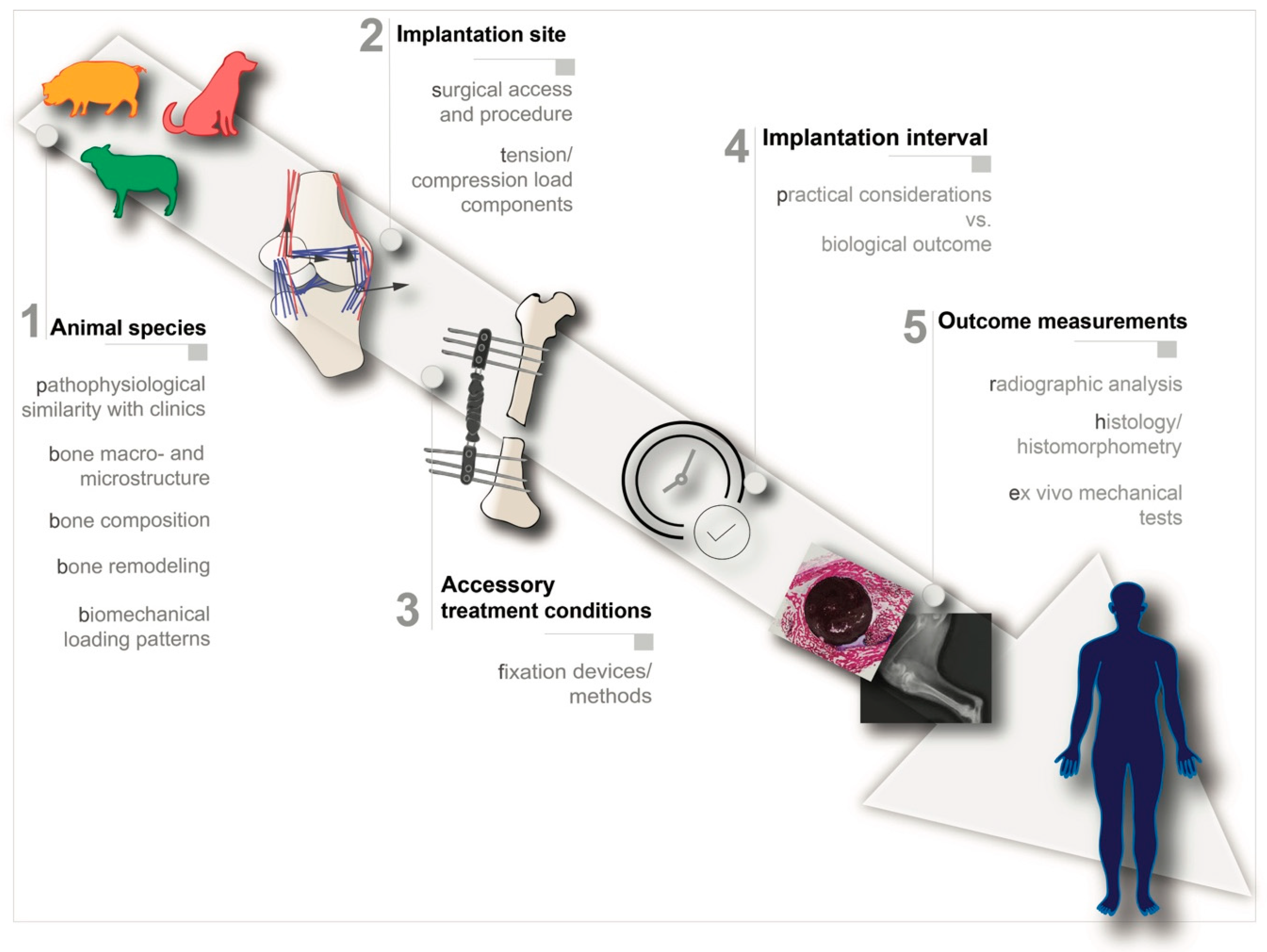
4. Animal Models for Critical Load-Bearing Bone Defects
- 1.
- Selection of the animal species: the selected animal species should resemble the human physiological and pathophysiological response as closely as possible;
- 2.
- Selection of implantation site: the selected implantation site should match the clinical setting both anatomically, biomechanically and surgically;
- 3.
- Accessory treatment conditions: the need for additional treatment conditions such as fixation devices should be carefully analyzed and mimic the real clinical intervention as much as possible;
- 4.
- Implantation period: the implantation period should be clinically relevant;
- 5.
- Outcome measurements: the experimental design should include concrete outcome measurement evaluations.
4.1. Selection of the Animal Species
4.1.1. Anatomic Analogy and Bone Macro- and Microstructure
4.1.2. Weight/Loading Patterns
4.2. Selection of Implantation Site
4.3. Acessory Treatment Conditions
4.4. Implantation Period
4.5. Outcome Measurements
5. Progress in the BSM Field—Clinical Translatability
6. Closing Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! Orthop. Trauma 2017, 31 (Suppl. 5), S20–S22. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The Critical Size Defect as an Experimental Model for Craniomandibulofacial Nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Sprio, S.; Tampieri, A.; Celotti, G.; Landi, E. Development of hydroxyapatite/calcium silicate composites addressed to the design of load-bearing bone scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.; Girardi, F.P.; Lane, J.M. The Biology of Bone Grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.; Kanakaris, N.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, W.; Schlickewei, C. The Use of Bone Substitutes in the Treatment of Bone Defects–the Clinical View and History. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Bongio, M.; Beucken, J.J.V.D.; Leeuwenburgh, S.; Jansen, J.A. Preclinical evaluation of injectable bone substitute materials. J. Tissue Eng. Regen. Med. 2012, 9, 191–209. [Google Scholar] [CrossRef]
- A El-Rashidy, A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Reichert, J.C.; Cipitria, A.; Epari, D.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A Tissue Engineering Solution for Segmental Defect Regeneration in Load-Bearing Long Bones. Sci. Transl. Med. 2012, 4, 141ra93. [Google Scholar] [CrossRef]
- Beniash, E. Biominerals—hierarchical nanocomposites: The example of bone. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 3, 47–69. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Reilly, D.T.; Burstein, A.H. The elastic and ultimate properties of compact bone tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef]
- Giesen, E.; Ding, M.; Dalstra, M.; Van Eijden, T. Mechanical properties of cancellous bone in the human mandibular condyle are anisotropic. J. Biomech. 2001, 34, 799–803. [Google Scholar] [CrossRef]
- Keaveny, T.M. Cancellous bone. In Handbook of Biomaterial Properties; Springer Science and Business Media LLC: Berlin, Germany, 1998; pp. 15–23. [Google Scholar]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Currey, J. Cortical Bone. In Handbook of Biomaterial Properties; Black, J., Ed.; Springer: Boston, MA, USA, 1998; pp. 3–14. [Google Scholar]
- Goldstein, S. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Lanyon, L.E.; Bourn, S. The influence of mechanical function on the development and remodeling of the tibia. An experimental study in sheep. J. Bone Jt. Surg. Am. 1979, 61, 263–273. [Google Scholar] [CrossRef]
- Lanyon, L. The success and failure of the adaptive response to functional load-bearing in averting bone fracture. Bone 1992, 13, S17–S21. [Google Scholar] [CrossRef]
- Lanyon, L.; Baggott, D. Mechanical function as an influence on the structure and form of bone. J. Bone Jt. Surg. Br. Vol. 1976, 58, 436–443. [Google Scholar] [CrossRef]
- Turner, C. Three rules for bone adaptation to mechanical stimuli. Bone 1998, 23, 399–407. [Google Scholar] [CrossRef]
- Pauwels, F. The Functional Adaptation of Bone through Growth in Length. In Biomechanics of the Locomotor Apparatus; Springer Science and Business Media LLC: Berlin, Germany, 1980; pp. 310–328. [Google Scholar]
- Klein-Nulend, J.; Bacabac, R.G.; Bakker, A.D. Mechanical loading and how it affects bone cells: The role of the osteocyte cytoskeleton in maintaining our skeleton. Eur. Cells Mater 2012, 24, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Goulet, R.; Goldstein, S.; Ciarelli, M.; Kuhn, J.; Brown, M.; Feldkamp, L. The relationship between the structural and orthogonal compressive properties of trabecular bone. J. Biomech. 1994, 27, 375–389. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone—role of the lacunocanalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. The Classic: On the Significance of the Architecture of the Spongy Substance for the Question of Bone Growth: A preliminary publication. Clin. Orthop. Relat. Res. 2011, 469, 3077–3078. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. The classic: On the inner architecture of bones and its importance for bone growth. Clin. Orthop. Relat. Res. 1870, 468, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef]
- Hutchinson, D.; Shipman, P.; Walker, A.; Bichell, D. The Human Skeleton. Pat Shipman, Alan Walker, and David Bichell. Harvard University Press, Cambridge, 1985. x + 343 pp., figures, tables, glossary, biblio., credits, index. $27.50 (cloth). Am. Antiq. 1987, 52, 438. [Google Scholar] [CrossRef]
- Bankoff, A.D.P. Biomechanical Characteristics of the Bone. Hum. Musculoskeletal Biomech. 2012, 61–87. [Google Scholar]
- Reed, W.J.; Mueller, R.W. Spiral fracture of the humerus in a ball thrower. Am. J. Emerg. Med. 1998, 16, 306–308. [Google Scholar] [CrossRef]
- Van der Stok, J.; Van Lieshout, E.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the Netherlands–A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Galovich, L. Álvarez; Pérez-Higueras, A.; Altonaga, J.R.; Orden, J.M.G.; Barba, M.L.M.; Morillo, M.T.C. Biomechanical, histological and histomorphometric analyses of calcium phosphate cement compared to PMMA for vertebral augmentation in a validated animal model. Eur. Spine J. 2011, 20, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Kushchayev, S.V. Percutaneous Vertebral Body Augmentations: The State of Art. Neuroimaging Clin. N. Am. 2019, 29, 495–513. [Google Scholar] [CrossRef]
- Grados, F.; Depriester, C.; Cayrolle, G.; Hardy, N.; Deramond, H.; Fardellone, P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology 2000, 39, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Rong, Z.; Wang, C.; Liu, X.; Zhang, F.; Zhang, Z.; Xu, J.-Z.; Dai, F. Clinical evaluation of a bone cement-injectable cannulated pedicle screw augmented with polymethylmethacrylate: 128 osteoporotic patients with 42 months of follow-up. Clinics 2019, 74, e346. [Google Scholar] [CrossRef] [PubMed]
- Babis, G.C.; Soucacos, P.N. Bone scaffolds: The role of mechanical stability and instrumentation. Injury 2005, 36, S38–S44. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kikuchi, N.; Hollister, S. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J. Biomech. 2004, 37, 623–636. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Hollister, S.; Maddox, R.; Taboas, J. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Fan, J.; Bi, L.; Wu, T.; Cao, L.; Wang, D.; Nan, K.; Chen, J.; Jin, D.; Jiang, S.; Pei, G. A combined chitosan/nano-size hydroxyapatite system for the controlled release of icariin. J. Mater. Sci. Mater. Electron. 2011, 23, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Lickorish, D.; Guan, L.; Davies, J. A three-phase, fully resorbable, polyester/calcium phosphate scaffold for bone tissue engineering: Evolution of scaffold design. Biomaterials 2007, 28, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Roohani, I.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite–PCL composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/Nano-Hydroxyapatite Composite Microsphere Scaffolds for Bone Tissue Engineering. Biomacromolecules 2008, 9, 1818–1825. [Google Scholar] [CrossRef]
- Puértolas, J.; Vadillo, J.; Sanchez-Salcedo, S.; Nieto, A.; Gómez-Barrena, E.; Vallet-Regí, M. Compression behaviour of biphasic calcium phosphate and biphasic calcium phosphate–agarose scaffolds for bone regeneration. Acta Biomater. 2011, 7, 841–847. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar] [CrossRef]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-Induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Zhu, C.-F.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of Biomechanics in Tissue Engineering of Bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef]
- Gauthier, O.; Weiss, P.; Bosco, J.; Daculsi, G.; Aguado, E.; Bouler, J.-M. Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calcium-phosphate bone substitutes. J. Biomed. Mater. Res. 1999, 47, 28–35. [Google Scholar] [CrossRef]
- Bacáková, L.; Filová, E.; Rypácek, F.; Švorčík, V.; Starý, V. Cell adhesion on artificial materials for tissue engineering. Physiol. Res. 2004, 53 (Suppl. 1), S35–S45. [Google Scholar] [PubMed]
- Hench, L.L.; Polak, J. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Giannoudis, P.V.; Einhorn, T.; Hankenson, K.D.; E Friedlaender, G.; Li, R.; Schemitsch, E.H. Growth Factors: Beyond Bone Morphogenetic Proteins. J. Orthop. Trauma 2010, 24, 543–546. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.T. BMPs: Options, Indications, and Effectiveness. J. Orthop. Trauma 2010, 24, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Winn, S.R.; Hu, Y.; Sfeir, C.; O Hollinger, J. Gene therapy approaches for modulating bone regeneration. Adv. Drug Deliv. Rev. 2000, 42, 121–138. [Google Scholar] [CrossRef]
- Hasan, A.; Byambaa, B.; Morshed, M.; Cheikh, M.I.; Shakoor, R.A.; Mustafy, T.; Marei, H. Advances in osteobiologic materials for bone substitutes. J. Tissue Eng. Regen. Med. 2018, 12, 1448–1468. [Google Scholar] [CrossRef] [PubMed]
- Oreffo, R.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- De Peppo, G.M.; Marcos-Campos, I.; Kahler, D.J.; Alsalman, D.; Shang, L.; Vunjak-Novakovic, G.; Marolt, D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 8680–8685. [Google Scholar] [CrossRef]
- Clarke, S.A.; Choi, S.Y.; McKechnie, M.; Burke, G.; Dunne, N.; Walker, G.M.; Cunningham, E.; Buchanan, F. Osteogenic cell response to 3-D hydroxyapatite scaffolds developed via replication of natural marine sponges. J. Mater. Sci. Mater. Electron. 2015, 27, 22. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Yang, S.-R.; Brey, E.M.; Chu, I.-M.; Cheng, M.-H. Hydrogel Delivery of Mesenchymal Stem Cell–Expressing Bone Morphogenetic Protein-2 Enhances Bone Defect Repair. Plast. Reconstr. Surg. Glob. Open 2016, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Nečas, A.; Proks, P.; Urbanová, L.; Srnec, R.; Stehlík, L.; Crha, M.; Rauser, P.; Planka, L.; Janovec, J.; Dvořák, M.; et al. Healing of Large Segmental Bone Defect after Implantation of Autogenous Cancellous Bone Graft in Comparison to Hydroxyapatite and 0.5% Collagen Scaffold Combined with Mesenchymal Stem Cells. Acta Veter. Brno. 2010, 79, 607–612. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Zhang, K.; Zhao, R.; Ye, X.; Chen, X.; Xiao, Z.; Yang, X.; Wang, J.; Zhang, K.; Fan, Y.; et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials 2017, 147, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, W.; Cui, L.; Cao, Y. Tissue-Engineered Bone Repair of Goat Femur Defects with Osteogenically Induced Bone Marrow Stromal Cells. Tissue Eng. 2006, 12, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Vagaská, B.; Bacáková, L.; Filová, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2009, 59, 309–322. [Google Scholar] [PubMed]
- Sciadini, M.F.; Johnson, K.D. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J. Orthop. Res. 2000, 18, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Oh, S.H.; Lee, S.L.; Kim, N.-H.; Choe, Y.H.; Yim, H.J.; Lee, J.-H. Bone regeneration by bone morphogenetic protein-2 from porous beads with leaf-stacked structure for critical-sized femur defect model in dogs. J. Biomater. Appl. 2020, 34, 1437–1448. [Google Scholar] [CrossRef]
- Manrique, E.; Chaparro, D.; Cebrián, J.L.; López-Durán, L. In vivo tricalcium phosphate, bone morphogenetic protein and autologous bone marrow biomechanical enhancement in vertebral fractures in a porcine model. Int. Orthop. 2014, 38, 1993–1999. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Liu, X.; Ge, B. Calcium Phosphate Cement with BMP-2-loaded Gelatin Microspheres Enhances Bone Healing in Osteoporosis: A Pilot Study. Clin. Orthop. Relat. Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Dallari, D.; Savarino, L.; Stagni, C.; Cenni, E.; Cenacchi, A.; Fornasari, P.M.; Albisinni, U.; Rimondi, E.; Baldini, N.; Giunti, A. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J. Bone Jt. Surg. Am. 2007, 89, 2413–2420. [Google Scholar] [CrossRef]
- Salazar, V.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- E Epstein, N. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4, S343–S352. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Lam, C.X.; Hutmacher, D.W.; Goh, J.C.; Wong, H.-K. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials 2009, 30, 5086–5093. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, M.G.; Overgaard, S.; Jespersen, S.M.; Ding, M. Comparison of synthetic bone graft ABM/P-15 and allograft on uninstrumented posterior lumbar spine fusion in sheep. J. Orthop. Surg. Res. 2019, 14, 2. [Google Scholar] [CrossRef]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; Ben David, S.; Gavrity, J.; Awad, H.A.; et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017, 9, eaal3128. [Google Scholar] [CrossRef]
- Balmayor, E.R.; Van Griensven, M. Gene Therapy for Bone Engineering. Front. Bioeng. Biotechnol. 2015, 3, 9–19. [Google Scholar] [CrossRef]
- Roohani, I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef]
- Kucko, N.; Schickert, S.D.L.; Marques, T.S.; Herber, R.-P.; Beuken, J.J.J.P.V.D.; Zuo, Y.; Leeuwenburgh, S.C.; Beucken, J.J.V.D. Tough and Osteocompatible Calcium Phosphate Cements Reinforced with Poly(vinyl alcohol) Fibers. ACS Biomater. Sci. Eng. 2019, 5, 2491–2505. [Google Scholar] [CrossRef]
- Maenz, S.; Brinkmann, O.; Kunisch, E.; Horbert, V.; Gunnella, F.; Bischoff, S.; Schubert, H.; Sachse, A.; Xin, L.; Günster, J.; et al. Enhanced bone formation in sheep vertebral bodies after minimally invasive treatment with a novel, PLGA fiber-reinforced brushite cement. Spine J. 2017, 17, 709–719. [Google Scholar] [CrossRef]
- Gunnella, F.; Kunisch, E.; Bungartz, M.; Maenz, S.; Horbert, V.; Xin, L.; Mika, J.; Borowski, J.; Bischoff, S.; Schubert, H.; et al. Low-dose BMP-2 is sufficient to enhance the bone formation induced by an injectable, PLGA fiber-reinforced, brushite-forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2017, 17, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, S.; Yunus, W.M.Z.W.; Ab Rahman, M.Z.; Ibrahim, N.A. Effect of organoclay on mechanical and thermal properties of polycaprolactone/chitosan/montmorillonite nanocomposites. J. Reinf. Plast. Compos. 2011, 30, 1045–1054. [Google Scholar] [CrossRef]
- Anitha, A.; Joseph, J.; Menon, D.; Nair, S.V.; Nair, M.B. Electrospun Yarn Reinforced NanoHA Composite Matrix as a Potential Bone Substitute for Enhanced Regeneration of Segmental Defects. Tissue Eng. Part A 2017, 23, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kawakami, K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomater. 2007, 3, 495–501. [Google Scholar] [CrossRef]
- Pek, Y.; Gao, S.; Arshad, M.M.; Leck, K.-J.; Ying, J.Y. Porous collagen-apatite nanocomposite foams as bone regeneration scaffolds. Biomaterials 2008, 29, 4300–4305. [Google Scholar] [CrossRef]
- Aerssens, J. Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- I Pearce, A.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Viateau, V.; Logeart-Avramoglou, D.; Guillemin, G.; Petite, H. Animal Models for Bone Tissue Engineering Purposes. In Sourcebook of Models for Biomedical Research; Springer Science and Business Media LLC: Berlin, Germany, 2008; pp. 725–736. [Google Scholar]
- Sparks, D.S.; Saifzadeh, S.; Savi, F.M.; Dlaska, C.E.; Berner, A.; Henkel, J.; Reichert, J.C.; Wullschleger, M.; Ren, J.; Cipitria, A.; et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020, 15, 877–924. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.; Lai, Y. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Schimandle, J.H.; Boden, S.D. Spine Update the Use of Animal Models to Study Spinal Fusion. Spine 1994, 19, 1998–2006. [Google Scholar] [CrossRef]
- Gutierrez, K.; Dicks, N.; Glanzner, W.G.; Agellon, L.B.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.; Fini, M.; Giavaresi, G.; Giardino, R. Sheep model in orthopedic research: A literature review. Comp. Med. 2001, 51, 292–299. [Google Scholar]
- Allen, M.J.; Hankenson, K.D.; Goodrich, L.; Boivin, G.P.; Von Rechenberg, B. Ethical use of animal models in musculoskeletal research. J. Orthop. Res. 2017, 35, 740–751. [Google Scholar] [CrossRef]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.; Schuetz, M.; Duda, G.N.; Schell, H.; Van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef] [PubMed]
- Berner, A.; Woodruff, M.A.; Lam, C.; Arafat, M.T.; Saifzadeh, S.; Steck, R.; Ren, J.; Nerlich, M.; Ekaputra, A.; Gibson, I.; et al. Effects of scaffold architecture on cranial bone healing. Int. J. Oral Maxillofac. Surg. 2014, 43, 506–513. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Kessler, P.; Wiltfang, J.; Schlegel, K.A. Bone Regeneration in Osseous Defects Using a Resorbable Nanoparticular Hydroxyapatite. J. Oral Maxillofac. Surg. 2005, 63, 1626–1633. [Google Scholar] [CrossRef]
- Mosekilde, L.; Weisbrode, S.E.; Safron, J.A.; Stills, H.F.; Jankowsky, M.L.; Ebert, D.C.; Danielsen, C.C.; Sogaard, C.H.; Franks, A.F.; Stevens, M.L.; et al. Calcium-restricted ovariectomized sinclair S-1 minipigs: An animal model of osteopenia and trabecular plate perforation. Bone 1993, 14, 379–382. [Google Scholar] [CrossRef]
- Jungbluth, P.; Spitzhorn, L.-S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Meinig, R.P.; Buesing, C.M.; Helm, J.; Gogolewski, S. Regeneration of Diaphyseal Bone Defects Using Resorbable Poly(L/DL-Lactide) and Poly(D-Lactide) Membranes in the Yucatan Pig Model. J. Orthop. Trauma 1997, 11, 551–558. [Google Scholar] [CrossRef]
- Kuhn, J.; Goldstein, S.; Ciarelli, M.; Matthews, L. The limitations of canine trabecular bone as a model for human: A biomechanical study. J. Biomech. 1989, 22, 95–107. [Google Scholar] [CrossRef]
- Nafei, A.; Danielsen, C.C.; Linde, F.; Hvid, I. Properties of growing trabecular ovine bone. Part I: Mechanical and physical properties. J. Bone Jt. Surg. Br. 2000, 82, 910–920. [Google Scholar] [CrossRef]
- Ravaglioli, A.; Krajewski, A.; Celotti, G.; Piancastelli, A.; Bacchini, B.; Montanari, L.; Zama, G.; Piombi, L. Mineral evolution of bone. Biomaterials 1996, 17, 617–622. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef]
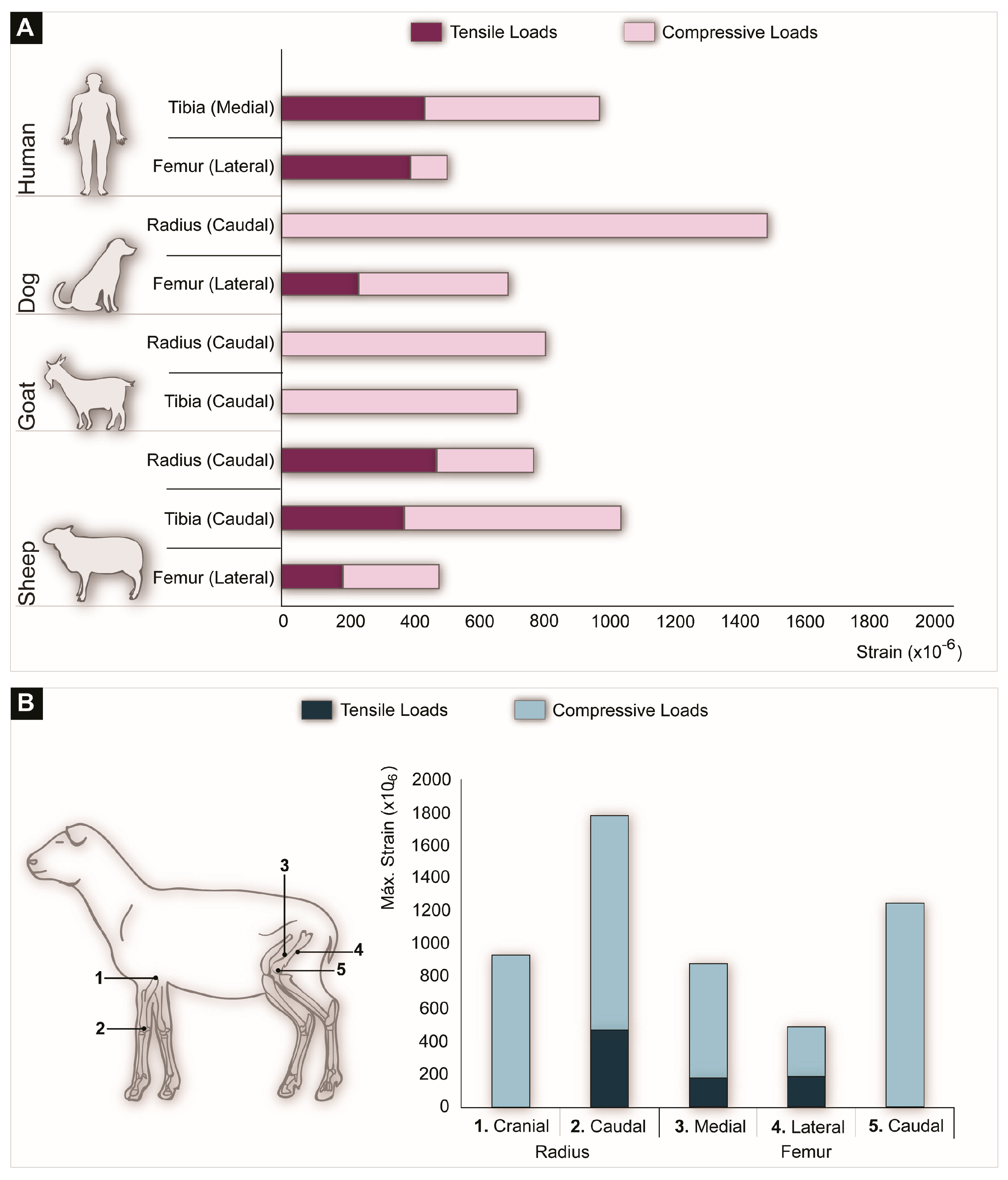
- Taylor, W.R.; Ehrig, R.M.; Heller, M.; Schell, H.; Seebeck, P.; Duda, G.N. Tibio-femoral joint contact forces in sheep. J. Biomech. 2006, 39, 791–798. [Google Scholar] [CrossRef]
- Goodship, A.E.; Lanyon, L.E.; McFie, H. Functional adaptation of bone to increased stress. An experimental study. J. Bone Jt. Surg. Am. 1979, 61, 539–546. [Google Scholar] [CrossRef]
- Lanyon, L.; Goodship, A.; Pye, C.; MacFie, J. Mechanically adaptive bone remodelling. J. Biomech. 1982, 15, 141–154. [Google Scholar] [CrossRef]
- Manley, P.A.; Schatzker, J.; Sumner-Smith, G. Evaluation of tension and compression forces in the canine femur in vivo. Arch. Orthop. Trauma Surg. 1982, 99, 213–216. [Google Scholar] [CrossRef]
- Rubin, C.T.; E Lanyon, L. Limb mechanics as a function of speed and gait: A study of functional strains in the radius and tibia of horse and dog. J. Exp. Boil. 1982, 101, 187–211. [Google Scholar]
- A Biewener, A.; Taylor, C.R. Bone strain: A determinant of gait and speed? J. Exp. Boil. 1986, 123, 383–400. [Google Scholar]
- Burr, D.; Milgrom, C.; Fyhrie, D.; Forwood, M.; Nyska, M.; Finestone, A.S.; Hoshaw, S.; Saiag, E.; Simkin, A. In vivo measurement of human tibial strains during vigorous activity. Bone 1996, 18, 405–410. [Google Scholar] [CrossRef]
- Lanyon, L.E.; Smith, R.N. Bone Strain in the Tibia during Normal Quadrupedal Locomotion. Acta Orthop. Scand. 1970, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Wagels, M.; Rowe, D.; Senewiratne, S.; Read, T.; Theile, D.R. Soft tissue reconstruction after compound tibial fracture: 235 cases over 12 years. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Boer, F.C.D.; Wippermann, B.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Haarman, H.J.T.M. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-I or autologous bone marrow. J. Orthop. Res. 2003, 21, 521–528. [Google Scholar] [CrossRef]
- Wieding, J.; Lindner, T.; Bergschmidt, P.; Bader, R. Biomechanical stability of novel mechanically adapted open-porous titanium scaffolds in metatarsal bone defects of sheep. Biomaterials 2015, 46, 35–47. [Google Scholar] [CrossRef]
- Gugala, Z.; Lindsey, R.W.; Gogolewski, S. New Approaches in the Treatment of Critical-Size Segmental Defects in Long Bones. Macromol. Symp. 2007, 253, 147–161. [Google Scholar] [CrossRef]
- ASTM. Standard guide for pre-clinical in vivo evaluation in critical size segmental bone defects. In ASTM F2721-09; ASTM, Ed.; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Dai, K.R.; Xu, X.L.; Tang, T.T.; Zhu, Z.A.; Yu, C.F.; Lou, J.R.; Zhang, X.L. Repairing of goat Tibial Bone Defects with BMP-2 Gene–Modified Tissue-Engineered Bone. Calcif. Tissue Int. 2005, 77, 55–61. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Basu, D. The repair of segmental bone defects with porous bioglass: An experimental study in goat. Res. Veter. Sci. 2009, 86, 162–173. [Google Scholar] [CrossRef]
- Kuttenberger, J.; Stübinger, S.; Waibel, A.; Werner, M.; Klasing, M.; Ivanenko, M.; Hering, P.; Von Rechenberg, B.; Sader, R.; Zeilhofer, H.-F. Computer-Guided CO2-Laser Osteotomy of the Sheep Tibia: Technical Prerequisites and First Results. Photomed. Laser Surg. 2008, 26, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Smit, T.H. The use of a quadruped as an in vivo model for the study of the spine–Biomechanical considerations. Eur. Spine J. 2002, 11, 137–144. [Google Scholar] [CrossRef]
- Turner, T.M.; Urban, R.M.; Singh, K.; Hall, D.; Renner, S.M.; Lim, T.-H.; Tomlinson, M.J.; An, H.S. Vertebroplasty comparing injectable calcium phosphate cement compared with polymethylmethacrylate in a unique canine vertebral body large defect model. Spine J. 2008, 8, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; Potes, J.; Queiroga, M.C.; Castro, J.L.; Pereira, A.M.F.; Rehman, S.; Dalgarno, K.; Ramos, A.; Vitale-Brovarone, C.; Reis, J. Percutaneous vertebroplasty: A new animal model. Spine J. 2016, 16, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, E.; Ahmed, A.K.; Lessing, N.; Pennington, Z.; Ishida, W.; Perdomo-Pantoja, A.; Lo, S.-F.; Howell, E.; Holmes, C.; Goodwin, C.R.; et al. Investigational growth factors utilized in animal models of spinal fusion: Systematic review. World J. Orthop. 2019, 10, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Schense, J.; Kronen, P.W.; Fouche, N.; Makara, M.; Kämpf, K.; Steffen, T.; Von Rechenberg, B. Feasibility Study of a Standardized Novel Animal Model for Cervical Vertebral Augmentation in Sheep Using a PTH Derivate Bioactive Material. Veter. Sci. 2014, 1, 96–120. [Google Scholar] [CrossRef]
- Zheng, G.B.; Yoon, B.H.; Lee, J.H. Comparison of the osteogenesis and fusion rates between activin A/BMP-2 chimera (AB204) and rhBMP-2 in a beagle’s posterolateral lumbar spine model. Spine J. 2017, 17, 1529–1536. [Google Scholar] [CrossRef]
- Takahashi, J.; Saito, N.; Ebara, S.; Kinoshita, T.; Itoh, H.; Okada, T.; Nozaki, K.; Takaoka, K. Anterior Thoracic Spinal Fusion in Dogs by Injection of Recombinant Human Bone Morphogenetic Protein-2 and a Synthetic Polymer. J. Spinal Disord. Tech. 2003, 16, 137–143. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, F.; Lineaweaver, W.C.; Zhang, J.; Jia, L.; Qi, J.; Wang, J.; Zhen, X. In Vivo Study of Hydroxyapatite-coated Hat Type Cervical Intervertebral Fusion Cage Combined With IGF-I and TGF-beta1 in the Goat Model. Clin. Spine Surg. 2016, 29, E267–E275. [Google Scholar] [CrossRef]
- Wilke, H.-J.; Kettler, A.; Claes, L. Are Sheep Spines a Valid Biomechanical Model for Human Spines? Spine 1997, 22, 2365–2374. [Google Scholar] [CrossRef]
- Wilke, H.-J.; Kettler, A.; Wenger, K.H.; Claes, L.E. Anatomy of the sheep spine and its comparison to the human spine. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 1997, 247, 542–555. [Google Scholar] [CrossRef]
- Turner, A. The sheep as a model for osteoporosis in humans. Veter. J. 2002, 163, 232–239. [Google Scholar] [CrossRef]
- Drespe, I.H.; Polzhofer, G.K.; Turner, A.S.; Grauer, J.N. Animal models for spinal fusion. Spine J. 2005, 5, S209–S216. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-G.; Hao, D.; He, B.-R.; Wu, Q.-N.; Liu, T.-J.; Wang, X.-D.; Guo, H.; Fang, X.-Y. Posterior atlantoaxial fixation: A review of all techniques. Spine J. 2015, 15, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Talbot, M.; Zdero, R.; Garneau, D.; Cole, P.A.; Schemitsch, E.H. Fixation of long bone segmental defects: A biomechanical study. Injury 2008, 39, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sisk, T.D. External fixation. Historic review, advantages, disadvantages, complications, and indications. Clin. Orthop. Relat. Res. 1983, 180, 15–22. [Google Scholar]
- Leunig, M.; Hertel, R. Thermal necrosis after tibial reaming for intramedullary nail fixation. A report of three cases. J. Bone Jt. Surg. Br. 1996, 78, 584–587. [Google Scholar] [CrossRef]
- Rahal, S.C.; Teixeira, C.; Volpi, R.; Taga, R.; Cestari, T.; Granjeiro, J.M.; Vulcano, L.; Corrêa, M. Tibial segmental bone defect treated with bone plate and cage filled with either xenogeneic composite or autologous cortical bone graft. Veter Comp. Orthop. Traumatol. 2007, 20, 269–276. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Corsi, A.; Francioso, E.; Di Comite, M.; Monetti, F.; Scaglione, S.; Favia, A.; Crovace, A.; Bianco, P.; Cancedda, R. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng. 2006, 12, 1261–1273. [Google Scholar] [CrossRef]
- Fountain, S.; Windolf, M.; Henkel, J.; Tavakoli, A.; Schuetz, M.; Hutmacher, D.W.; Epari, D.; Akbarzadeh, A.T. Monitoring Healing Progression and Characterizing the Mechanical Environment in Preclinical Models for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 47–57. [Google Scholar] [CrossRef]
- Liebschner, M.; Wettergreen, M.A. Optimization of bone scaffold engineering for load bearing applications. Top. Tissue Eng. 2003, 1, 1–39. [Google Scholar]
- Runyan, C.; Vu, A.T.; Rumburg, A.; Bove, K.; Racadio, J.; A Billmire, D.; Taylor, J.A. Repair of a Critical Porcine Tibial Defect by Means of Allograft Revitalization. Plast. Reconstr. Surg. 2015, 136, 461–473. [Google Scholar] [CrossRef]
- Sedlin, E.D.; Hirsch, C. Factors Affecting the Determination of the Physical Properties of Femoral Cortical Bone. Acta Orthop. Scand. 1966, 37, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Stefan, U.; Michael, B.; Schmoelz, W. Effects of three different preservation methods on the mechanical properties of human and bovine cortical bone. Bone 2010, 47, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Arbit, H.M.; Herrick, J.L.; Segovis, S.G.; Maran, A.; Yaszemski, M.J. Tissue engineered constructs: Perspectives on clinical translation. Ann. Biomed. Eng. 2015, 43, 796–804. [Google Scholar] [CrossRef]
- Hollister, S. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.; Murphy, W.L. Scaffold Translation: Barriers between Concept and Clinic. Tissue Eng. Part B Rev. 2011, 17, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-Engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Lopa, S.; Recordati, C.; Talò, G.; Turrisi, C.; Bottagisio, M.; Losa, M.; Scanziani, E.; Moretti, M. In Vivo Bone Formation within Engineered Hydroxyapatite Scaffolds in a Sheep Model. Calcif. Tissue Int. 2016, 99, 209–223. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Mechanical Characteristics 1 | |
|---|---|---|
| Cortical Bone | Cancellous Bone | |
| Compressive strength (MPa) | 70.0–200.0 | 0.1–30.0 |
| Tensile strength (MPa) | 90.0–170.0 | 10.0–20.0 |
| Flexural strength (MPa) | 135.0–193.0 | 10.0–20.0 |
| Ultimate strain at fracture (%) | 1.0–3.0 | 5.0–7.0 |
| Elastic modulus (GPa) | 3.0–30.0 | 0.1–5.0 |
| Porosity (%) | 5.0–30.0 | 50.0–95.0 |
| Animal | Bone | Segmental Bone Defect | BSM | Fixation Method | Time-Points (Weeks) | Outcome Measurements | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Species | Weight (Kgs) | Type | Dimensions (cm) | Method of Production | ||||||
| Pig | Yucatán mini-pigs (Sus scrofa) | 37.0 ± 3.6 | Tibia | Partial segmental defect | 1 | Oscillating bone saw | Collagen scaffold/microbubble-enhanced BMP6 plasmid | Internal fixation (custom-made six-hole LC-DCP plates) | 1, 2 and 3 | Protein expression analysis, µCT scan, histology and histomorphometry and ex-vivo mechanical test (i.e., torsional) | [79] |
| Mini-pigs (Sus scrofa domesticus) | N.I. | Femur | Total osseous mid-diaphyseal defect | 1.5 | Oscillating bone saw | Nanocomposite scaffold HaP/collagen/BMSCs | Internal fixation (LC-DCP plates (4.5 mm-thick) fixed with four cortical titanium locking screws (diameter: 4.5 mm) | 16 | Plain X-ray, µCT scan, histology and histomorphometry | [64] | |
| Dog | Mongrel dogs (Canis lupus familiaris) | 30.3 ± 8.6 | Radius | Total osteoperiosteal middiaphyseal defect | 2.5 | Oscillating bone saw | rhBMP2/collagen sponge carrier | External fixation | 24 | Plain X-ray, histology and histomorphometry and ex-vivo mechanical test (i.e., torsional) | [68] |
| Mongrel dogs (Canis lupus familiaris) | 4.5 ± 0.5 | Femur | Total osteoperiosteal middiaphyseal defect | 1.1 | Oscillating bone saw | PCL bread scaffolds, PCL bead scaffold/BMP2 | 2.0 mm Intramedullary pin and 2.7 mm universal locking plate | 4, 8 and 24 | Plain X-ray, serum chemistry, histology and histomorphometry and RT-qPCR | [69] | |
| Goat | N.I. | 19.6 ± 3.4 | Femur | Total osteoperiosteal mid-diaphyseal cortical defect | 2.5 | Oscillating bone saw | Coral cylinder/BMSCs | Internal fixation rod and interlocking nails | 16 and 32 | Plain X-ray, histology and histomorphometry and ex-vivo mechanical test (i.e., three-point bending) | [66] |
| Sheep | North-Holland and black-faced sheep (Ovis aries) | 54.2 ± 7.6 | Tibia | Total osseous total mid-diaphyseal defect | 3 | Oscillating bone saw | Granular porous HaP/rhOP-1, Granular porous HaP/autologous bone marrow aspirate | Intramedullary nail | 12 | Plain X-ray, histology and histomorphometry and ex-vivo mechanical test (i.e., torsional) | [118] |
| German blackheaded mutton sheep (Ovis aries) | 68.1 ± 8.4 | Metatarsus | Total osseous mid-diaphyseal defect | 2 | Oscillating bone saw | Titanium (Ti6Al4V) implants/collagen/β-TCP | Internal fixation (LCP 3.5 mm-thick, stainless steel, 8-holes) | 12 and 24 | Plain X-ray, µCT scan, BMD and ex-vivo mechanical test (i.e., torsional) | [119] | |
| Animal | Vertebral Defect | BSM | Time-Points (Weeks) | Outcome Measurements | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Species | Osteoporotic/Osteopenic Condition | Average Weight (kg) | Selected Vertebral Segments | Defect Size (Diameter × Depth) | Surgical Technique | ||||
| Pig | Piétrain (Sus scrofa domesticus) | No | N.I. | L3 | 10 × N.I. mm | N.I. | TCP, TCP/rhBMP7, TCP/autologous bone marrow aspirate | 4 | Plain X-ray, ex-vivo mechanical test (i.e., compression) | [70] |
| Goat | Domestic goat (Capra aegagrus hircus) | Yes | 17.0 ± 1.5 | L2 and L4 | 5 × 10 mm | Lateral retro-peritoneal exposure of spine | rhBMP2/GM/CPC, rhBMP2/CPC | 6 and 16 | µCT scan, DEX, histology and histomorphometry, ex-vivo mechanical test (i.e., push-out/compression) | [71] |
| Sheep | Merino sheep (Ovis aries) | Yes | 90.9 ± 10.7 | L1, L4, L5 | 5.0 × 14.0 mm | Fluoroscopy-guided minimally invasive ventrolateral approach | CPC/PLGA fibers, CPC/PLGA fibers/BMP2 | 12 and 36 | Plain X-ray, µCT scan, DXA, histology and histomorphometry, mechanical testing (i.e., compression) | [83,84] |
| Swiss alpine sheep (Ovis aries) | No | 72.6 ± 16.4 | C3–C5 | 2.8 × N.I. mm | Fluoroscopy-guided minimally invasive ventral approach | Fs/SrCo3, Fs/SrCo3/PTH. | 16 | Plain X-ray, µCT scan, histology and histomorphometry | [129] | |
| Animal | Spinal Fusion | BSM | Fixation Method | Time-Points (Weeks) | Outcome Measurements | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Species | Average Weight (kg) | Vertebral Segment | Method of Vertebrae Dislocation | Surgical Approach | |||||
| Dog | Beagle (Canis lupus familiaris) | 14.5 ± 0.5 | L1/L2 and L4/L5 | Vertebrae were decorticated by high speed burr | Posterolateral approach | BCP, BCP/rhBMP2 and BCP/AB204 | N.U. | 8 | Plain X-ray, µCT scan, manual palpation, histology and histomorphometry | [130] |
| Beagle (Canis lupus familiaris) | 10.5 ± 1.5 | T9/T10 | No dislocation, only curetting of the anterior longitudinal ligament and intervertebral disc | Anterolateral approach | RhBMP2/PLA-PEG | N.U. | 4, 8 and 12 months | Plain X-ray, µCT scan, manual palpation, histology and histomorphometry | [131] | |
| Goat | N.I. | N.I. | C3/C4 | Anterior discectomy | Right anterolateral approach | Hat shaped titanium cervical intervertebral fusion cage coated with HaP, IGF-I and TGF-β1 | N.U. | 1, 2, 4, 8, 12 | Plain X-ray, ex-vivo mechanical test (i.e., compression and bending), histology and histomorphometry | [132] |
| Sheep | Texas/Gotland breed sheep (Ovis aries) | 715 ± 15.5 | L2/L3 and L4/L5 | Vertebrae were decorticated by high speed burr | Posterior approach | i-Factor™ Flex (ABM+P-15) | N.U. | 18 | µCT scan, histology and histomorphometry | [78] |
| Mechanical Test | Schematic Representation | Advantages | Disadvantages | Observations | |
|---|---|---|---|---|---|
| Tensile test |  | Specimen is usually a round bar with a reduced middle region and a length-to-diameter ratio of 5:1. | Allows for relatively easy assessment of the strain of bone (by using strain gauges). | 1. Usually requires large specimens; 2. Some bending might be applied to the specimen, leading to measurement errors; 3. Requires the specimen to be machined; 4. Only one component of load is applied—incomplete evaluation of the mechanical properties. | 1. Easier to perform for cortical bone than cancellous since cancellous bone is difficult to clamp; 2. Tensile load is calculated by dividing the applied force divided by the cross-sectional area in the midsection of the specimen. |
| Compression test |  | Specimen is usually a cube or cylinder having a length-to-diameter ratio of 2:1. | 1. Usually requires small specimens; 2. Fabrication of the specimens is easier than for tensile tests. | 1. The presence of “end effects”1 often leads to errors; 2. Strain is very difficult to measure; 3. Only one component of load is applied—incomplete evaluation of the mechanical properties. | Reducing the size of the specimen increases the risk of “end-effects” 1. |
| Bending test |  | Can be performed in a 3- or 4-point bending set-up. | Both components of load are applied—tensile stresses are present on one side of the specimen and compressive stresses on the opposite side. | 1. Highly influenced by the size and shape of the specimen—defects throughout the specimen may lead to non-accurate results; 2. A 3-point bending produces several transverse shear stresses in the middle of the specimen while 4-point bending model applies almost pure bending stresses. | 1. Since bone is weaker in tension than compression, failure usually occurs on the tensile side of the bone; 2. Positioning of the specimen should be very precise, since each loading point has to be equal to obtain accurate results. |
| Torsion test |  | Specimen has a reduced central portion to ensure that the failure occurs in the middle part. | 1. Measures the biomechanical properties of bone under shear stress; 2. When the specimen is twisted, shear stresses vary from zero at the center of the specimen to the maximum value at the surface; 3. Both compression and tension are present. | 1. Requires the specimen to be machined; 2. Practical issues may occur (i.e., clamping the sample to the testing device). | Testing strongly influenced by the shape of the specimen. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lacerda Schickert, S.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Jansen, J.A. Pre-Clinical Evaluation of Biological Bone Substitute Materials for Application in Highly Loaded Skeletal Sites. Biomolecules 2020, 10, 883. https://doi.org/10.3390/biom10060883
de Lacerda Schickert S, van den Beucken JJJP, Leeuwenburgh SCG, Jansen JA. Pre-Clinical Evaluation of Biological Bone Substitute Materials for Application in Highly Loaded Skeletal Sites. Biomolecules. 2020; 10(6):883. https://doi.org/10.3390/biom10060883
Chicago/Turabian Stylede Lacerda Schickert, Sónia, Jeroen J.J.P. van den Beucken, Sander C.G. Leeuwenburgh, and John A. Jansen. 2020. "Pre-Clinical Evaluation of Biological Bone Substitute Materials for Application in Highly Loaded Skeletal Sites" Biomolecules 10, no. 6: 883. https://doi.org/10.3390/biom10060883
APA Stylede Lacerda Schickert, S., van den Beucken, J. J. J. P., Leeuwenburgh, S. C. G., & Jansen, J. A. (2020). Pre-Clinical Evaluation of Biological Bone Substitute Materials for Application in Highly Loaded Skeletal Sites. Biomolecules, 10(6), 883. https://doi.org/10.3390/biom10060883