8-Hydroxyquinoline-2-Carboxylic Acid as Possible Molybdophore: A Multi-Technique Approach to Define Its Chemical Speciation, Coordination and Sequestering Ability in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus and Procedure for Potentiometric Measurements
2.3. Apparatus and Procedure for Spectrophotometric Measurements
2.4. Apparatus and Procedure for Voltammetric Measurements
2.5. Apparatus and Procedure for ESI-MS Measurements
2.6. Procedure for Quantum Mechanical Calculations
2.7. Thermodynamic Calculations
3. Results and Discussion
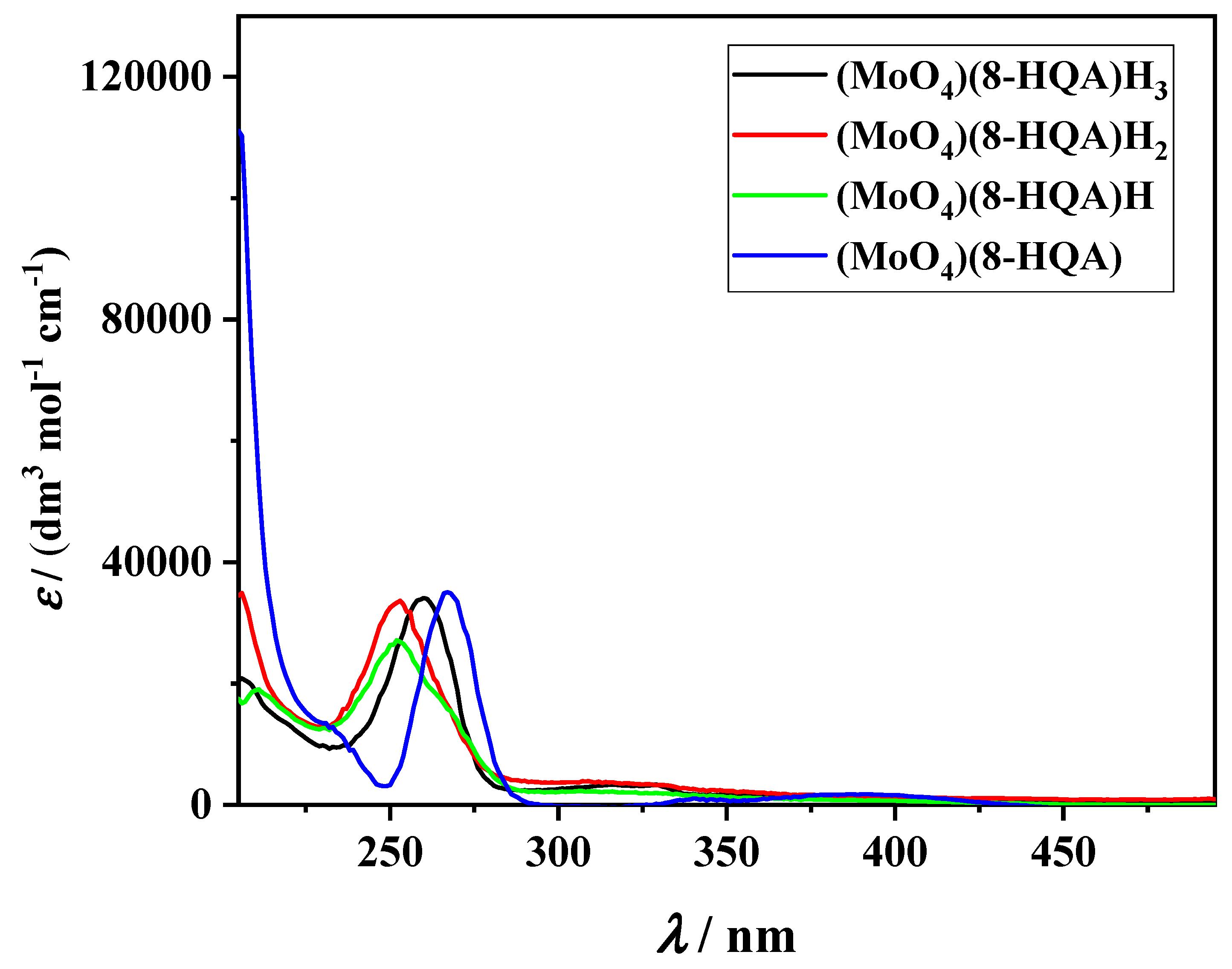
3.1. Determination of the Speciation Model. Nature and Stability of (MoO4)p(8-HQA)qHr(2p+2q−r)− Complexes: Potentiometric and Spectrophotometric Investigation
3.1.1. Acid–Base Properties of MoO42− and 8-HQA
3.1.2. Stability Constants of (MoO4)p(8-HQA)qHr(2p+2q−r)− Species
3.2. Rebuttal of the Speciation Model. Towards Poly-8-HQA Complexes: Voltammetric Investigation
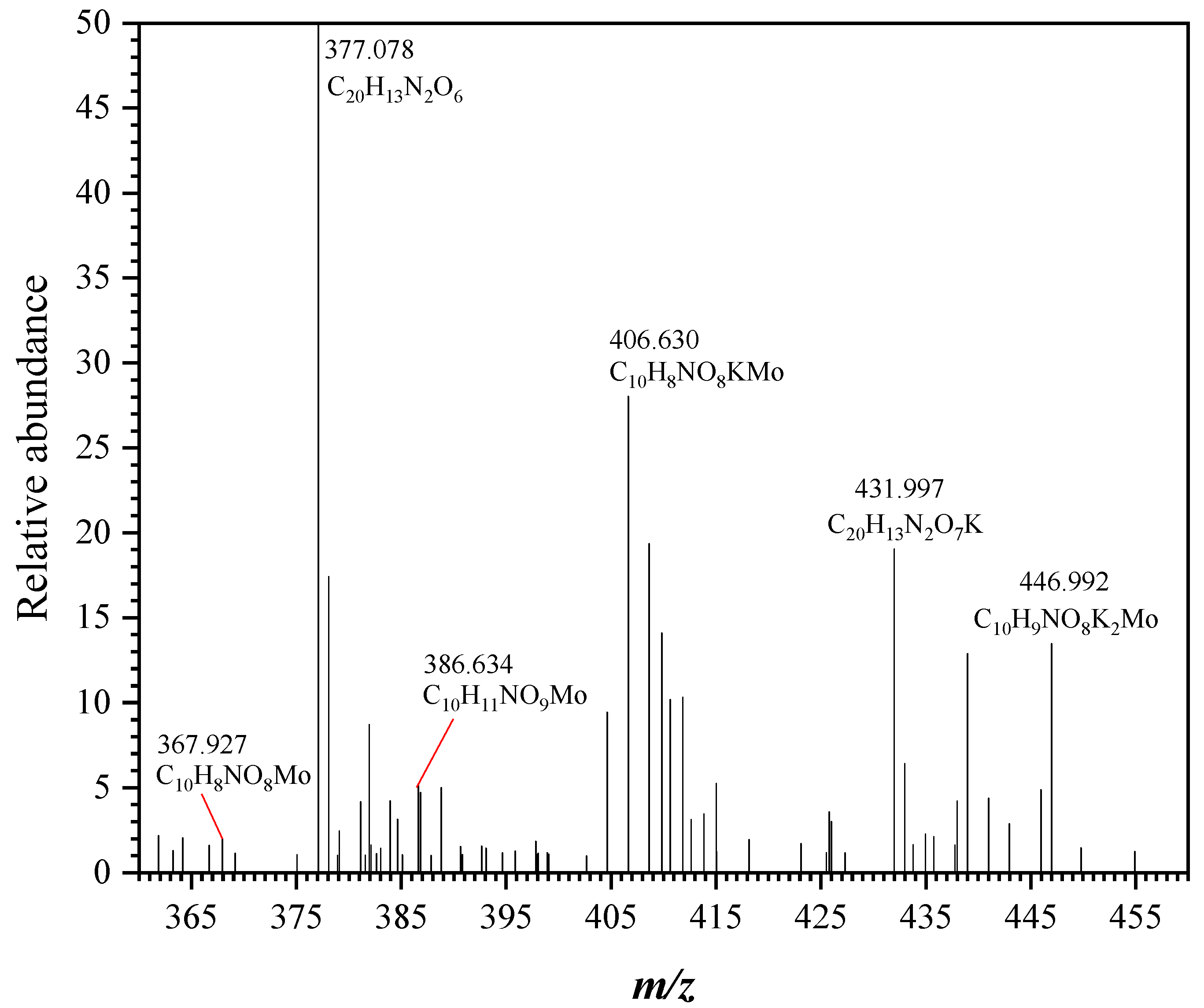
3.3. Identification of (MoO4)p(8-HQA)qHr Species and Coordination of Mo(VI): ESI-MS Investigation
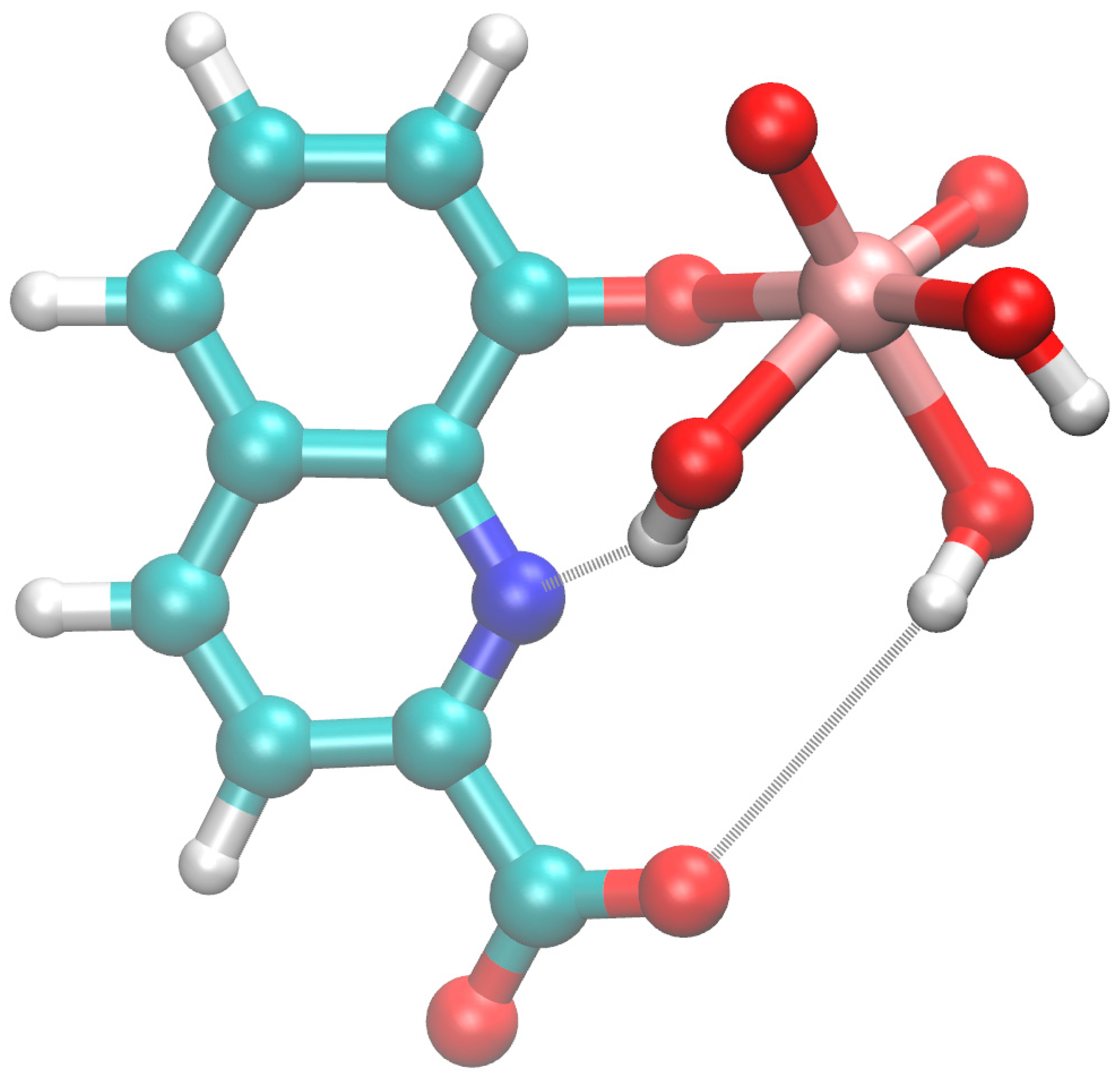
3.4. Possible Binding Modes: Quantum Mechanical Calculations
3.5. 8-HQA as a Possible Molybdophore: Sequestering Ability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pesek, J.; Svoboda, J.; Sattler, M.; Bartram, S.; Boland, W. Biosynthesis of 8-hydroxyquinoline-2-carboxylic acid, an iron chelator from the gut of the lepidopteran Spodoptera littoralis. Org. Biomol. Chem. 2014, 13, 178–184. [Google Scholar] [CrossRef]
- Gama, S.; Frontauria, M.; Ueberschaar, N.; Brancato, G.; Milea, D.; Sammartano, S.; Plass, W. Thermodynamic study on 8-hydroxyquinoline-2-carboxylic acid as a chelating agent for iron found in the gut of Noctuid larvae. New J. Chem. 2018, 42, 8062–8073. [Google Scholar] [CrossRef]
- Walczak, K.; Langner, E.; Szalast, K.; Makuch-Kocka, A.; Pożarowski, P.; Plech, T. A Tryptophan Metabolite, 8-Hydroxyquinaldic Acid, Exerts Antiproliferative and Anti-Migratory Effects on Colorectal Cancer Cells. Molecules 2020, 25, 1655. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.K.; Kline, S.J. Multidentate Chelators Based on the 8-Hydroxyquinoline Unit. European Patent Application EP0308757B1, 30 June 1993. [Google Scholar]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug. Des. Devel. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.n.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. Med. Chem. Commun. 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Capodagli, G.C.; Sedhom, W.G.; Jackson, M.; Ahrendt, K.A.; Pegan, S.D. A Noncompetitive Inhibitor for Mycobacterium tuberculosis’s Class IIa Fructose 1,6-Bisphosphate Aldolase. Biochemistry 2014, 53, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigel, A.; Sigel, H. Molybdenum and Tungsten: Their Roles in Biological Processes; Marcel Dekker Inc.: Basel, Switzerland, 2002; Volume 39, p. 856. [Google Scholar]
- Anke, M.K. Molybdenum. In Elements and Their Compounds in the Environment, 2nd ed.; Merian, E., Anke, M., Ihnat, M., Stoeppler, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 1007–1037. [Google Scholar]
- Garrett, R.M.; Johnson, J.L.; Graf, T.N.; Feigenbaum, A.; Rajagopalan, K.V. Human sulfite oxidase R160Q: Identification of the mutation in a sulfite oxidase-deficient patient and expression and characterization of the mutant enzyme. Proc. Natl. Acad. Sci. USA 1998, 95, 6394. [Google Scholar] [CrossRef] [Green Version]
- Terao, M.; Kurosaki, M.; Demontis, S.; Zanotta, S.; Garattini, E. Isolation and characterization of the human aldehyde oxidase gene: Conservation of intron/exon boundaries with the xanthine oxidoreductase gene indicates a common origin. Biochem. J. 1998, 332, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R. Structure and function of xanthine oxidoreductase: Where are we now? Free Rad. Biol. Med. 2002, 33, 774–797. [Google Scholar] [CrossRef]
- Wahl, B.; Reichmann, D.; Niks, D.; Krompholz, N.; Havemeyer, A.; Clement, B.; Messerschmidt, T.; Rothkegel, M.; Biester, H.; Hille, R.; et al. Biochemical and spectroscopic characterization of the human mitochondrial amidoxime reducing components hmARC-1 and hmARC-2 suggests the existence of a new molybdenum enzyme family in eukaryotes. J. Biol. Chem. 2010, 285, 37847–37859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotny, J.A. Molybdenum Nutriture in Humans. J. Evid. Bas. Compl. Altern. Med. 2011, 16, 164–168. [Google Scholar] [CrossRef]
- Schwarz, G. Molybdenum cofactor and human disease. Curr. Opin. Chem. Biol. 2016, 31, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Atwal, P.S.; Scaglia, F. Molybdenum cofactor deficiency. Mol. Genet. Metab. 2016, 117, 1–4. [Google Scholar] [CrossRef]
- Bourke, C.A. Molybdenum Deficiency Produces Motor Nervous Effects That Are Consistent with Amyotrophic Lateral Sclerosis. Front. Neurol. 2016, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Cigala, R.M.; Crea, F.; De Stefano, C.; Lando, G.; Milea, D.; Sammartano, S. Electrochemical Study on the Stability of Phytate Complexes with Cu2+, Pb2+, Zn2+, and Ni2+: A Comparison of Different Techniques. J. Chem. Eng. Data 2010, 55, 4757–4767. [Google Scholar] [CrossRef]
- De Stefano, C.; Lando, G.; Milea, D.; Pettignano, A.; Sammartano, S. Formation and Stability of Cadmium(II)/Phytate Complexes by Different Electrochemical Techniques. Critical Analysis of Results. J. Solut. Chem. 2010, 39, 179–195. [Google Scholar] [CrossRef]
- Foti, C.; Lando, G.; Millero, F.J.; Sammartano, S. Experimental study and modeling of inorganic Cd2+ speciation in natural waters. Environ. Chem. 2011, 8, 320–331. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mennucci, B.; Cammi, R.; Tomasi, J. Excited states and solvatochromic shifts within a nonequilibrium solvation approach: A new formulation of the integral equation formalism method at the self-consistent field, configuration interaction, and multiconfiguration self-consistent field level. J. Chem. Phys. 1998, 109, 2798–2807. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, C.; Mineo, P.; Rigano, C.; Sammartano, S. Ionic Strength Dependence of Formation Constants. XVII. The Calculation of Equilibrium Concentrations and Formation Constants. Ann. Chim. (Rome) 1993, 83, 243–277. [Google Scholar]
- Gans, P. Hyperquad. Available online: http://www.hyperquad.co.uk/ (accessed on 29 May 2020).
- Crea, F.; De Stefano, C.; Foti, C.; Milea, D.; Sammartano, S. Chelating agents for the sequestration of mercury(II) and monomethyl mercury (II). Curr. Med. Chem. 2014, 21, 3819–3836. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; De Stefano, C.; Irto, A.; Milea, D.; Pettignano, A.; Sammartano, S. Modeling the acid-base properties of molybdate(VI) in different ionic media, ionic strengths and temperatures, by EDH, SIT and Pitzer equations. J. Mol. Liq. 2017, 229, 15–26. [Google Scholar] [CrossRef]
- Lingane, J.J. Interpretation of the Polarographic Waves of Complex Metal Ions. Chem. Rev. 1941, 29, 1–35. [Google Scholar] [CrossRef]
- DeFord, D.D.; Hume, D.N. The Determination of Consecutive Formation Constants of Complex Ions from Polarographic Data. J. Am. Chem. Soc. 1951, 73, 5321–5322. [Google Scholar] [CrossRef]
- Crow, D.R. Polarography of Metal Complexes; Academic Press: London, UK, 1969. [Google Scholar]
- Krishnan, C.V.; Garnett, M.; Hsiao, B.; Chu, B. Electrochemical measurements of isopolyoxomolybdates: 1. pH dependent behavior of sodium molybdate. Int. J. Electrochem. Sci. 2007, 2, 29–51. [Google Scholar]
- Yoder, C.H.; Christie, E.L.; Morford, J.L. 95Mo NMR study of the effect of structure on complexation of molybdate with alpha and beta hydroxy carboxylic acid ligands. Polyhedron 2016, 114, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Duhme, A.K.; Hider, R.C.; Naldrett, M.J.; Pau, R.N. The stability of the molybdenum-azotochelin complex and its effect on siderophore production in Azotobacter vinelandii. J. Biol. Inorg. Chem. 1998, 3, 520–526. [Google Scholar] [CrossRef]
- Farkas, E.; Csoka, H.; Gama, S.; Santos, M.A. Dihydroxamate based siderophore model, piperazine-1,4-bis-(N-methyl-acetohydroxamic acid (PIPDMAHA), as a chelating agent of molybdenum(VI). Talanta 2002, 57, 935–943. [Google Scholar] [CrossRef]
- Santos, M.A.; Gama, S.; Pessoa, J.C.; Oliveira, M.C.; Toth, I.; Farkas, E. Complexation of molybdenum(VI) with bis(3-hydroxy-4-pyridinone)amino acid derivatives. Eur. J. Inorg. Chem. 2007, 2007, 1728–1737. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Clares, M.P.; Garcia-Espana, E. Addressing selectivity criteria in binding equilibria. Coord. Chem. Rev. 2012, 256, 13–27. [Google Scholar] [CrossRef]







| p:q:r | log βpqr 1 |
|---|---|
| 1:0:1 | 3.97 ± 0.02 |
| 1:0:2 | 7.40 ± 0.02 |
| 0:1:1 | 9.55 ± 0.01 |
| 0:1:2 | 13.49 ± 0.02 |
| p:q:r | log βpqr 1 | ||
|---|---|---|---|
| ISE-H+ | UV/Vis | Proposed | |
| 1:1:3 | 24.73 ± 0.03 | 24.73 ± 0.02 | 24.73 ± 0.04 |
| 1:1:2 | 21.48 ± 0.02 | 21.26 ± 0.02 | 21.37 ± 0.03 |
| 1:1:1 | 17.00 ± 0.02 | 16.99 ± 0.03 | 17.00 ± 0.04 |
| 1:1:0 | 6.12 ± 0.03 | 5.92 ± 0.03 | 6.02 ± 0.05 |
| MoO42− | |||||||||
| pH | 2.5 | 3.5 | 4.5 | 5.5 | 6.5 | 7.4 | 8.1 | 9.5 | 10.5 |
| pL0.5 | 6.29 | 7.18 | 7.46 | 7.44 | 7.44 | 7.44 | 7.42 | 7.18 | 6.58 |
| Fe3+ | |||||||||
| pH | 3.0 | 4.0 | 5.0 | 6.0 | 7.4 | 8.1 | 9.0 | 10.0 | |
| pL0.5 | 6.29 | 7.77 | 8.33 | 8.41 | 8.42 | 8.31 | 8.31 | 6.24 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, K.; Brancato, G.; Cacciola, F.; Crea, F.; Cataldo, S.; De Stefano, C.; Gama, S.; Lando, G.; Milea, D.; Mondello, L.; et al. 8-Hydroxyquinoline-2-Carboxylic Acid as Possible Molybdophore: A Multi-Technique Approach to Define Its Chemical Speciation, Coordination and Sequestering Ability in Aqueous Solution. Biomolecules 2020, 10, 930. https://doi.org/10.3390/biom10060930
Arena K, Brancato G, Cacciola F, Crea F, Cataldo S, De Stefano C, Gama S, Lando G, Milea D, Mondello L, et al. 8-Hydroxyquinoline-2-Carboxylic Acid as Possible Molybdophore: A Multi-Technique Approach to Define Its Chemical Speciation, Coordination and Sequestering Ability in Aqueous Solution. Biomolecules. 2020; 10(6):930. https://doi.org/10.3390/biom10060930
Chicago/Turabian StyleArena, Katia, Giuseppe Brancato, Francesco Cacciola, Francesco Crea, Salvatore Cataldo, Concetta De Stefano, Sofia Gama, Gabriele Lando, Demetrio Milea, Luigi Mondello, and et al. 2020. "8-Hydroxyquinoline-2-Carboxylic Acid as Possible Molybdophore: A Multi-Technique Approach to Define Its Chemical Speciation, Coordination and Sequestering Ability in Aqueous Solution" Biomolecules 10, no. 6: 930. https://doi.org/10.3390/biom10060930
APA StyleArena, K., Brancato, G., Cacciola, F., Crea, F., Cataldo, S., De Stefano, C., Gama, S., Lando, G., Milea, D., Mondello, L., Pettignano, A., Plass, W., & Sammartano, S. (2020). 8-Hydroxyquinoline-2-Carboxylic Acid as Possible Molybdophore: A Multi-Technique Approach to Define Its Chemical Speciation, Coordination and Sequestering Ability in Aqueous Solution. Biomolecules, 10(6), 930. https://doi.org/10.3390/biom10060930












