Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Human Osteoarthritic Chondrocytes and Cell Expansion
2.2. Inflammatory Model of 3D Microtissues for Small Molecules Testing
2.3. RNA Extraction for Sequencing and Gene Expression Analysis
2.4. Library Preparation for Next-Generation Sequencing and Ingenuity Pathway Analysis
2.5. Gene Expression Analysis
2.6. Matrix Metalloproteinase (MMP) Activity in Supernatants of Treated and Control Vehicle Samples
2.7. Immunoassay for Pro-Inflammatory Cytokine Quantification
2.8. Nuclear and Cytoplasmic Protein Extraction of Human OA Chondrocytes
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Whole-Genome RNA Sequencing and Gene Expression for the Differentially Expressed Genes
Gene Expression Analysis
3.2. Production of Cytokines in the Groups Treated with the Small Molecules Versus Control
3.3. MMP Activity of the Groups Treated with the Small Molecules Versus Control Vehicle
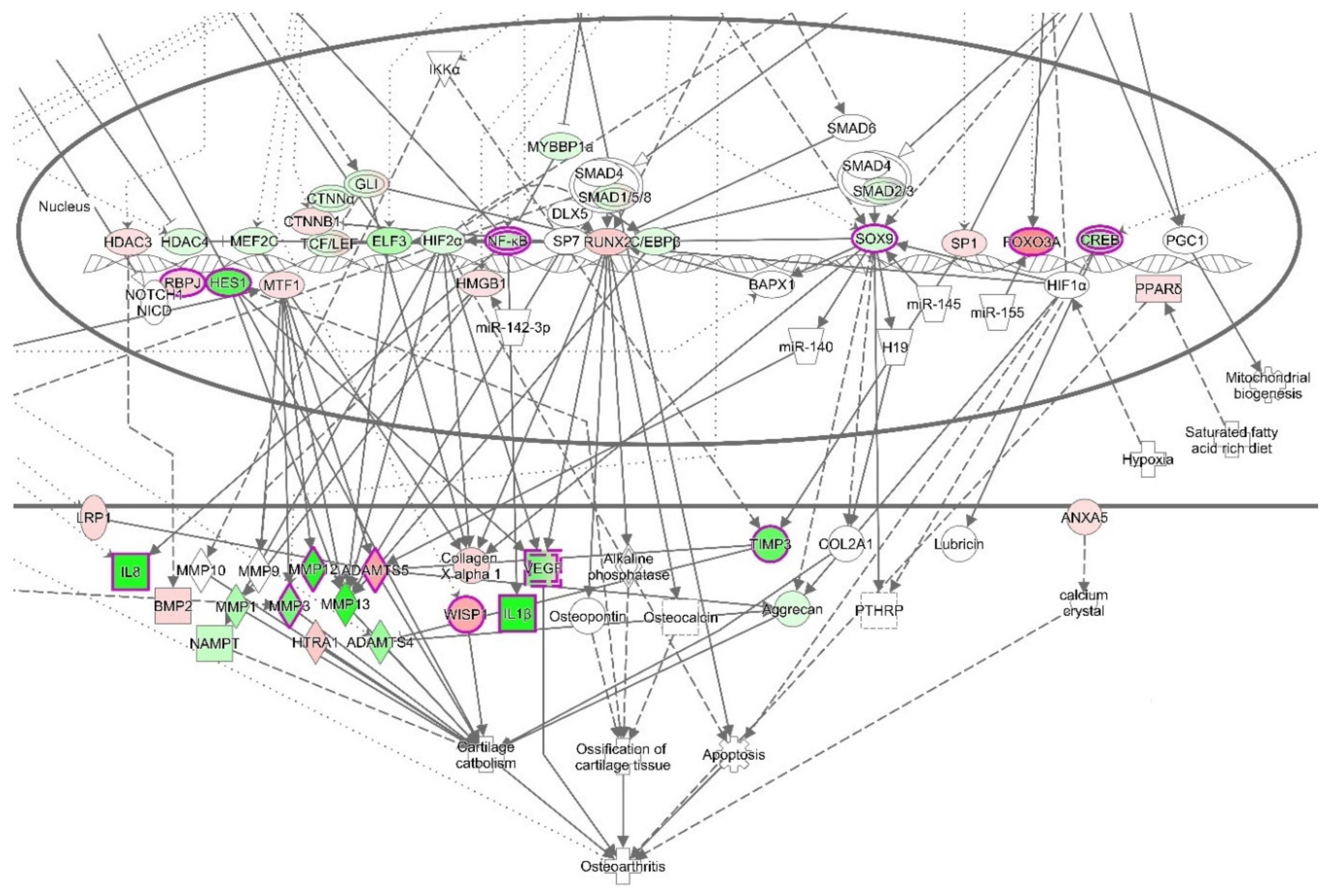
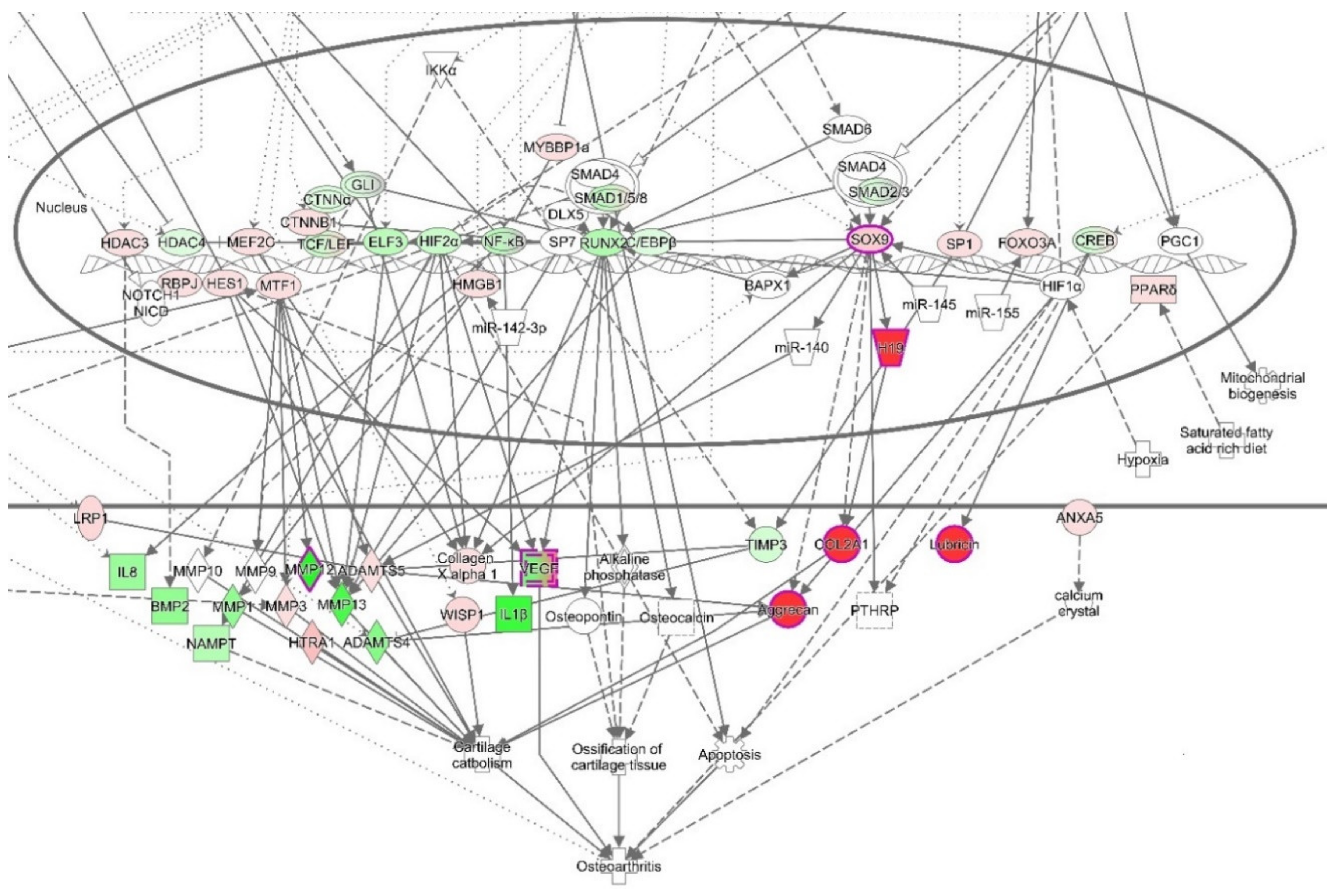
3.4. Ingenuity Pathways Analysis (IPA)
3.4.1. IPA’s Downstream Effect Analysis
3.4.2. IPA’s Upstream Regulator Analysis
3.4.3. The Canonical Pathways
3.5. RT2 Profiler PCR Array for the NF-κB Signaling Pathway
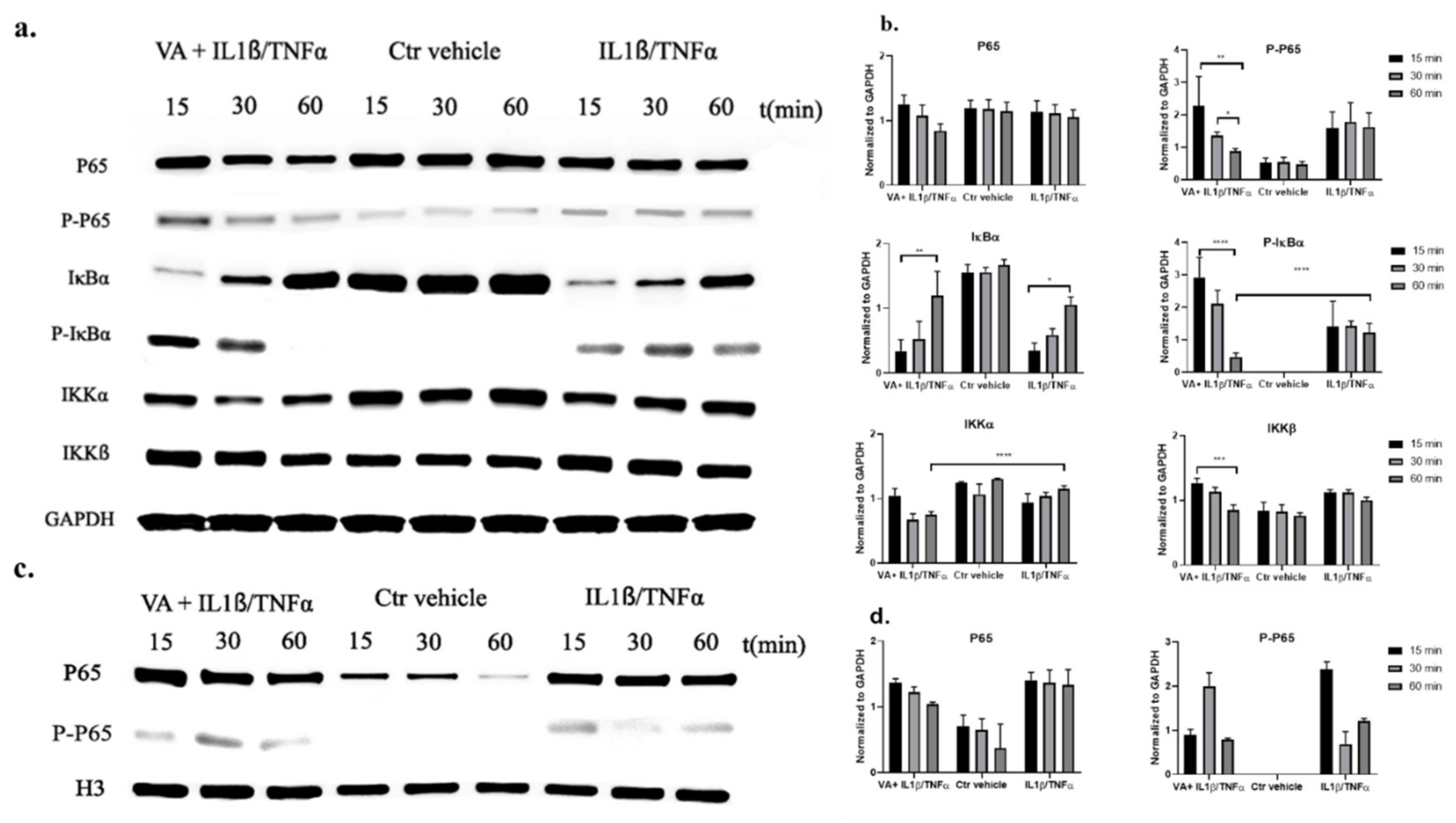
3.6. Western Blot Analysis for the NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Shen, J.; Jin, H.; Im, H.-J.; Sandy, J.; Chen, D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann. N. Y. Acad. Sci. 2011, 1240, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.R.; Agrícola, R.; Price, A.J.; Vincent, T.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Melchiorri, C.; Meliconi, R.; Frizziero, L.; Silvestri, T.; Pulsatelli, L.; Mazzetti, I.; Borzi, R.M.; Uguccioni, M.; Facchini, A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arheaw 1998, 41, 2165–2174. [Google Scholar] [CrossRef]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L.; Facchini, A. Signaling Pathways in Cartilage Repair. Int. J. Mol. Sci. 2014, 15, 8667–8698. [Google Scholar] [CrossRef]
- Kim, H.A.; Cho, M.-L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.-Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006, 54, 2152–2163. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Chondrocyte innate immune MyD88-dependent signaling drives pro-catabolic effects of the endogenous TLR2/TLR4 ligands LMW-HA and HMGB1. Arthritis Rheum. 2010, 62, 2004–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, E.; Otero, M.; Marcu, K.B.; Goldring, M.B. Pathophysiology of osteoarthritis: Canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open 2015, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Arnandis, I.; Guillén, M.I.; Gomar, F.; Pelletier, J.-P.; Martel-Pelletier, J.; Alcaraz, M.J. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1β in osteoarthritic synoviocytes. Arthritis Res. Ther. 2010, 12, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nefla, M.; Holzinger, D.; Berenbaum, F.; Jacques, C. The danger from within: Alarmins in arthritis. Nat. Rev. Rheumatol. 2016, 12, 669–683. [Google Scholar] [CrossRef]
- Aulin, C.; Lassacher, T.; Palmblad, K.; Harris, H.E. Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthr. Cartil. 2020. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.; Kopp, E.B. NF-κB AND REL PROTEINS: Evolutionarily Conserved Mediators of Immune Responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Hayden, M.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Tak, P.-P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Boil. 2009, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Favata, M.F.; Horiuchi, K.Y.; Manos, E.J.; Daulerio, A.J.; Stradley, D.A.; Feeser, W.S.; Van Dyk, D.E.; Pitts, W.; Earl, R.A.; Hobbs, F.; et al. Identification of a Novel Inhibitor of Mitogen-activated Protein Kinase Kinase. J. Boil. Chem. 1998, 273, 18623–18632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef] [Green Version]
- Pande, V.; Ramos, M.J. NF-κB in Human Disease: Current Inhibitors and Prospects for De Novo Structure Based Design of Inhibitors. Curr. Med. Chem. 2005, 12, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.R.; Pattoli, M.A.; Gregor, K.R.; Brassil, P.J.; MacMaster, J.F.; McIntyre, K.W.; Yang, X.; Iotzova, V.S.; Clarke, W.; Strnad, J.; et al. BMS-345541 Is a Highly Selective Inhibitor of IκB Kinase That Binds at an Allosteric Site of the Enzyme and Blocks NF-κB-dependent Transcription in Mice. J. Boil. Chem. 2002, 278, 1450–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Cao, C.; Zhang, Y.; Liu, G.; Ren, W.; Ye, Y.; Sun, T. PI3K/Akt inhibitor partly decreases TNF-α-induced activation of fibroblast-like synoviocytes in osteoarthritis. J. Orthop. Surg. Res. 2019, 14, 425. [Google Scholar] [CrossRef] [Green Version]
- Eräsalo, H.; Laavola, M.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Moilanen, E. PI3K Inhibitors LY294002 and IC87114 Reduce Inflammation in Carrageenan-Induced Paw Oedema and Down-Regulate Inflammatory Gene Expression in Activated Macrophages. Basic Clin. Pharmacol. Toxicol. 2014, 116, 53–61. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta Bioenerg. 2010, 1799, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef]
- Wieland, H.A.; Michaelis, M.; Kirschbaum, B.J.; Rudolphi, K.A. Osteoarthritis—An untreatable disease? Nat. Rev. Drug Discov. 2005, 4, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. Tumour necrosis factor α stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Kamper, S.J.; Wiggers, J.; O’Brien, K.M.; Lee, H.; Wolfenden, L.; Yoong, S.L.; Robson, E.K.; McAuley, J.H.; Hartvigsen, J.; et al. Musculoskeletal conditions may increase the risk of chronic disease: A systematic review and meta-analysis of cohort studies. BMC Med. 2018, 16, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.O.; Reda, M.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O., III; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Mobasheri, A. Natural Products for Promoting Joint Health and Managing Osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72–81. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, X.; Cheng, J.; Zhang, X.; Zhao, F.; Shi, W.; Ren, B.; Yu, H.; Yang, P.; Li, Z.; et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat. Commun. 2019, 10, 1914–1928. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.A.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A Stem Cell-Based Approach to Cartilage Repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Yano, F.; Hojo, H.; Ohba, S.; Fukai, A.; Hosaka, Y.; Ikeda, T.; Saito, T.; Hirata, M.; Chikuda, H.; Takato, T.; et al. A novel disease-modifying osteoarthritis drug candidate targeting Runx1. Ann. Rheum. Dis. 2012, 72, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Liu, W.; He, Y.; Huang, J.; Duan, L.; Xiong, J.; Liu, L.; Wang, D. Recent advances in kartogenin for cartilage regeneration. J. Drug Target. 2018, 27, 28–32. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, X.H.; Qin, C.; Tao, L.; Liu, X.; Shi, Z.; Zhang, C.L.; Tan, C.Y.; Chen, Y.Z.; Jiang, Y. Drug Discovery Prospect from Untapped Species: Indications from Approved Natural Product Drugs. PLoS ONE 2012, 7, e039782. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural Products as Sources of New Drugs over the Period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A. The Future of Osteoarthritis Therapeutics: Emerging Biological Therapy. Curr. Rheumatol. Rep. 2013, 15, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kismali, G.; Gupta, S.C. Natural Products for the Prevention and Treatment of Chronic Inflammatory Diseases: Integrating Traditional Medicine into Modern Chronic Diseases Care. Evid. Based Complement. Altern. Med. 2018, 2018, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussaieff, A.; Shohami, E.; Kashman, Y.; Fride, E.; Schmitz, M.L.; Renner, F.; Fiebich, B.L.; Munoz, E.; Ben-Neriah, Y.; Mechoulam, R. Incensole Acetate, a Novel Anti-Inflammatory Compound Isolated fromBoswelliaResin, Inhibits Nuclear Factor-κB Activation. Mol. Pharmacol. 2007, 72, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Tsai, K.-S.; Chan, D.-C.; Lan, K.C.; Chen, C.F.; Yang, R.-S.; Liu, S.-H. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J. Orthop. Res. 2013, 32, 573–580. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Zhou, S.; Luo, F.; Xu, W.; Wang, Q.; Tan, Q.; Chen, L.; Wang, J.; Chen, H.; et al. Anemonin attenuates osteoarthritis progression through inhibiting the activation of IL-1β/NF-κB pathway. J. Cell. Mol. Med. 2017, 21, 3231–3243. [Google Scholar] [CrossRef]
- Yu, S.-M.; Cho, H.; Kim, G.H.; Chung, K.-W.; Seo, S.-Y.; Kim, S.-J. Berberine induces dedifferentiation by actin cytoskeleton reorganization via phosphoinositide 3-kinase/Akt and p38 kinase pathways in rabbit articular chondrocytes. Exp. Boil. Med. 2016, 241, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shou, K.; Gong, C.; Yang, H.; Yang, Y.; Bao, T. Anti-Inflammatory Effect of Geniposide on Osteoarthritis by Suppressing the Activation of p38 MAPK Signaling Pathway. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Mei, Q.; Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015, 126, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. 2012, 20, 1647–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.M.; Qin, L.; Garnero, P.; Genant, H.K.; Zhang, G.; Dai, K.; Yao, X.; Gu, G.; Hao, Y.; Li, Z.; et al. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporos. Int. 2011, 23, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, L.-M.; Wang, K.-Z.; Yang, N.-P.; Yang, S.-H.; He, W.; Wang, Y.-S.; Wang, Z.-M.; Yang, P.; Liu, X.-Z.; et al. Herbal Fufang Xian Ling Gu Bao prevents corticosteroid-induced osteonecrosis of the femoral head-A first multicentre, randomised, double-blind, placebo-controlled clinical trial. J. Orthop. Transl. 2017, 12, 36–44. [Google Scholar] [CrossRef]
- Wang, F.; Shi, L.; Zhang, Y.; Wang, K.; Pei, F.; Zhu, H.; Shi, Z.; Tao, T.; Li, Z.; Zeng, P.; et al. A Traditional Herbal Formula Xianlinggubao for Pain Control and Function Improvement in Patients with Knee and Hand Osteoarthritis: A Multicenter, Randomized, Open-Label, Controlled Trial. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Yao, Z.-H.; Qin, Z.-F.; Cheng, H.; Wu, X.-M.; Dai, Y.; Wang, X.; Qin, L.; Ye, W.-C.; Yao, X.-S.; Qin, Z.-F. Simultaneous Quantification of Multiple Representative Components in the Xian-Ling-Gu-Bao Capsule by Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Tandem Mass Spectrometry. Molecules 2017, 22, 927. [Google Scholar] [CrossRef] [Green Version]
- Ziadlou, R.; Barbero, A.; Stoddart, M.J.; Wirth, M.; Li, Z.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S.; et al. Regulation of Inflammatory Response in Human Osteoarthritic Chondrocytes by Novel Herbal Small Molecules. Int. J. Mol. Sci. 2019, 20, 5745. [Google Scholar] [CrossRef] [Green Version]
- Müller, S.; Acevedo, L.; Wang, X.; Karim, M.Z.; Matta, A.; Mehrkens, A.; Schaeren, S.; Feliciano, S.; Jakob, M.; Martin, I.; et al. Notochordal cell conditioned medium (NCCM) regenerates end-stage human osteoarthritic articular chondrocytes and promotes a healthy phenotype. Arthritis Res. 2016, 18, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman-Blas, J.A.; Jimenez, S.A. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Ming, J.; Deng, M.; Li, Y.; Li, B.; Li, J.; Ma, Y.; Chen, Z.; Liu, S. Berberine-mediated up-regulation of surfactant protein D facilitates cartilage repair by modulating immune responses via the inhibition of TLR4/NF-ĸB signaling. Pharmacol. Res. 2020, 155, 104690–104701. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4/MyD88/NF-κB signal pathway. Drug Dev. Res. 2019, 80, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, C.; Tang, Q.; Zheng, W.; Feng, Z.; Yu, X.; Guo, X. Paeonol Inhibits IL-1β-Induced Inflammation via PI3K/Akt/NF-βB Pathways: In Vivo and Vitro Studies. Inflammation 2017, 40, 1698–1706. [Google Scholar] [CrossRef]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef]
- Jiang, Y.; Sang, W.; Wang, C.; Lu, H.; Zhang, T.; Wang, Z.; Liu, Y.; Xue, B.; Xue, S.; Cai, Z.; et al. Oxymatrine exerts protective effects on osteoarthritis via modulating chondrocyte homoeostasis and suppressing osteoclastogenesis. J. Cell. Mol. Med. 2018, 22, 3941–3954. [Google Scholar] [CrossRef]
- Ying, X.; Chen, X.; Cheng, S.; Shen, Y.; Peng, L.; Xu, H.Z. Piperine inhibits IL-β induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int. Immunopharmacol. 2013, 17, 293–299. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Z.; Bian, Y.; Su, W.; Li, X.; Li, S.; Wu, J.; Shi, L.; Song, Y.; Zheng, G.; et al. Scutellarin Attenuates the IL-1β-Induced Inflammation in Mouse Chondrocytes and Prevents Osteoarthritic Progression. Front. Pharmacol. 2020, 11, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Xi, Y.; Mao, Z.; Chu, X.; Zhang, R.; Ma, X.; Ni, B.; Cheng, H.; You, H. Vanillic acid attenuates cartilage degeneration by regulating the MAPK and PI3K/AKT/NF-κB pathways. Eur. J. Pharmacol. 2019, 859, 172481–172490. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-βB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Li, J.; Sundberg, E.; Aveberger, A.-C.; Palmblad, K.; Yang, H.; Tracey, K.J.; Andersson, U.; Harris, H.E. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003, 48, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kawahara, K.-I.; Yone, K.; Hashiguchi, T.; Yamakuchi, M.; Goto, M.; Inoue, K.; Yamada, S.; Ijiri, K.; Matsunaga, S.; et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003, 48, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Sundberg, E.; Ulfgren, A.-K.; Palmblad, K.; Li, J.; Wang, H.; Ulloa, L.; Yang, H.; Yan, X.-J.; Furie, R.; et al. High mobility group box chromosomal protein 1: A novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002, 46, 2598–2603. [Google Scholar] [CrossRef]
- Andersson, U.; Erlandsson-Harris, H. HMGB1 is a potent trigger of arthritis. J. Intern. Med. 2004, 255, 344–350. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [Green Version]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.F. Function of the chondrocyte PI-3 kinase-Akt signaling pathway is stimulus dependent. Osteoarthr. Cartil. 2015, 23, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Vlahos, C.J.; Matter, W.F.; Hui, K.Y.; Brown, R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Boil. Chem. 1994, 269, 5241–5248. [Google Scholar]
- Kong, D.; Yamori, T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci. 2007, 98, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Selectivity and therapeutic inhibition of kinases: To be or not to be? Nat. Immunol. 2009, 10, 356–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokosz, L.L.; Beasley, J.R.; Carroll, C.D.; Lin, T.; Zhao, J.; Appell, K.C.; Webb, M.L. Kinase inhibitors as drugs for chronic inflammatory and immunological diseases: Progress and challenges. Expert Opin. Ther. Targets 2008, 12, 883–903. [Google Scholar] [CrossRef] [PubMed]
- Barouch-Bentov, R.; Sauer, K. Mechanisms of drug resistance in kinases. Expert Opin. Investig. Drugs 2011, 20, 153–208. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Barreto, G.; Sandelin, J.; Salem, A.; Nordström, D.C.; Waris, E. Toll-like receptors and their soluble forms differ in the knee and thumb basal osteoarthritic joints. Acta Orthop. 2017, 88, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Carmody, R.J.; Chen, Y.H. Nuclear factor-kappaB: Activation and regulation during toll-like receptor signaling. Cell. Mol. Immunol. 2007, 4, 31–41. [Google Scholar]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3–mediated interferon-β induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Mori, K.; Hoshino, K.; Takeuchi, O.; Takeda, K.; Akira, S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-β Promoter in the Toll-Like Receptor Signaling. J. Immunol. 2002, 169, 6668–6672. [Google Scholar] [CrossRef] [Green Version]
- Loiarro, M.; Ruggiero, V.; Sette, C. Targeting TLR/IL-1R Signalling in Human Diseases. Mediat. Inflamm. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.A.; Palsson-McDermott, E.M.; Bowie, A.G.; Jefferies, C.A.; Mansell, A.; Brady, G.; Brint, E.; Dunne, A.; Gray, P.; Harte, M.T.; et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001, 413, 78–83. [Google Scholar] [CrossRef]
- Bonelli, M.; Dalwigk, K.; Platzer, A.; Calvo, I.O.; Hayer, S.; Niederreiter, B.; Holinka, J.; Sevelda, F.; Pap, T.; Steiner, G.; et al. IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes: JAKinibs suppress the interferon response in RA-FLSs. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, V.F.-S.; Tsui, R.; Caldwell, A.; Hoffmann, A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2010, 21, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, L.A.; Nwosu, L.; Wilson, D.; Hill, R.; Spendlove, I.; Bennett, A.; Scammell, B.E.; Walsh, D.A. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aveleira, C.; Castilho, Á.; Baptista, F.; Simões, N.; Fernandes, C.; Leal, E.C.; Ambrósio, A.F. High glucose and interleukin-1β downregulate interleukin-1 type I receptor (IL-1RI) in retinal endothelial cells by enhancing its degradation by a lysosome-dependent mechanism. Cytokine 2010, 49, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Syddall, C.M.; Reynard, L.N.; Young, D.A.; Loughlin, J. The Identification of Trans-acting Factors That Regulate the Expression of GDF5 via the Osteoarthritis Susceptibility SNP rs143383. PLoS Genet. 2013, 9, e1003557. [Google Scholar] [CrossRef] [Green Version]
- Hedbom, E.; Antonsson, P.; Hjerpe, A.; Aeschlimann, D.; Paulsson, M.; Rosa-Pimentel, E.; Sommarin, Y.; Wendel, M.; Oldberg, A.; Heinegard, D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. 1992, 267, 6132–6136. [Google Scholar]
- Xing, X.; Li, Z.; Yu, Z.; Cheng, G.; Li, D.; Li, Z. Effects of connective tissue growth factor (CTGF/CCN2) on condylar chondrocyte proliferation, migration, maturation, differentiation and signalling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 1447–1453. [Google Scholar] [CrossRef]
- Fujisawa, T.; Hattori, T.; Ono, M.; Uehara, J.; Kubota, S.; Kuboki, T.; Takigawa, M. CCN family 2/connective tissue growth factor (CCN2/CTGF) stimulates proliferation and differentiation of auricular chondrocytes. Osteoarthr. Cartil. 2008, 16, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, Y.; Guo, Y.; Ma, R.-F.; Fu, M.; Niu, J.-Z.; Gao, S.; Zhang, D. Herba Epimedii: An Ancient Chinese Herbal Medicine in the Prevention and Treatment of Osteoporosis. Curr. Pharm. Des. 2015, 22, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Xu, H.; Zhao, Y.-J.; Tang, D.; Xu, G.-H.; Holz, J.; Wang, J.; Cheng, S.-D.; Shi, Q.; Wang, Y.-J. Icariin Augments Bone Formation and Reverses the Phenotypes of Osteoprotegerin-Deficient Mice through the Activation of Wnt/β-Catenin-BMP Signaling. Evid. Based Complement. Altern. Med. 2013, 2013, 652317. [Google Scholar] [CrossRef] [Green Version]
- Shou, D.; Zhang, Y.; Shen, L.; Zheng, R.; Huang, X.; Mao, Z.; Yu, Z.; Wang, N.; Zhu, Y. Flavonoids of HerbaEpimediiEnhances Bone Repair in a Rabbit Model of Chronic Osteomyelitis During Post-infection Treatment and Stimulates Osteoblast Proliferation inVitro. Phytotherapy Res. 2016, 31, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Zhang, Y.; Shen, L.; Wang, N.; Xiong, X.; Zhang, L.; Cai, X.; Shou, D. Absorption and utilisation of epimedin C and icariin from Epimedii herba, and the regulatory mechanism via the BMP2/ Runx2 signalling pathway. Biomed. Pharmacother. 2019, 118, 109345–109353. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, F.; He, Q.; Wang, J.; Shiu, H.T.; Shu, Y.; Tsang, W.P.; Liang, S.; Zhao, K.; Wan, C. Flavonoid Compound Icariin Activates Hypoxia Inducible Factor-1α in Chondrocytes and Promotes Articular Cartilage Repair. PLoS ONE 2016, 11, e0148372. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Liu, Y.; Deng, X.; Yu, C.; Dai, N.; Yuan, X.; Chen, L.; Yu, S.; Si, W.; Wang, X.; et al. An inhibitor of cathepsin K, icariin suppresses cartilage and bone degradation in mice of collagen-induced arthritis. Phytomedicine 2013, 20, 975–979. [Google Scholar] [CrossRef]
- Liu, M.-H.; Sun, J.-S.; Tsai, S.-W.; Sheu, S.-Y.; Chen, M.-H. Icariin protects murine chondrocytes from lipopolysaccharide-induced inflammatory responses and extracellular matrix degradation. Nutr. Res. 2010, 30, 57–65. [Google Scholar] [CrossRef]
- Chen, J.; Long, F. mTOR signaling in skeletal development and disease. Bone Res. 2018, 6, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.; Rufino, A.; Judas, F.; Tenreiro, C.; Lopes, M.; Mendes, A.F. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: Modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthr. Cartil. 2011, 19, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, Y.; Zhao, M.; Jia, H.; Li, B.; Xing, D. Tormentic acid inhibits IL-1β-induced chondrocyte apoptosis by activating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2018, 17, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, T.; Xia, C.; Shi, L.; Wang, S.; Zheng, X.; Hu, T.; Zhang, B. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J. Cell. Mol. Med. 2013, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Hayashi, S.; Fujishiro, T.; Kanzaki, N.; Hashimoto, S.; Sakata, S.; Chinzei, N.; Nishiyama, T.; Kuroda, R.; Kurosaka, M. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J. Orthop. Res. 2013, 32, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Wu, Y.-T.; Hsueh, T.Y.; Lin, L.-C.; Tsai, T.-H. Pharmacokinetics and oral bioavailability of epimedin C after oral administration of epimedin C and Herba Epimedii extract in rats. Biomed. Chromatogr. 2013, 28, 630–636. [Google Scholar] [CrossRef]
- Jakob, M.; Démarteau, O.; Schäfer, D.; Hintermann, B.; Dick, W.; Heberer, M.; Martin, I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem. 2001, 81, 368–377. [Google Scholar] [CrossRef]








| Gene | Probe Type | Assay ID |
|---|---|---|
| CXCL12 | 5′ FAM-3′ NFQ | Hs03676656_mH |
| CCL11 | 5′ FAM-3′ NFQ | Hs00237013_m1 |
| IL23A | 5′ FAM-3′ NFQ | Hs00372324_m1 |
| MMP12 | 5′ FAM-3′ NFQ | Hs00159178_m1 |
| ADAMTS16 | 5′ FAM-3′ NFQ | Hs00373526_m1 |
| IL-6 | 5′ FAM-3′ NFQ | Hs00174131_m1 |
| COMP | 5′ FAM-3′ NFQ | Hs00164359_m1 |
| GDF-5 | 5′ FAM-3′ NFQ | Hs00167060_m1 |
| CCN2 | 5′ FAM-3′ NFQ | Hs00170014_m1 |
| 18s fast | 5′ FAM-3′ NFQ | Hs99999901_s1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules 2020, 10, 932. https://doi.org/10.3390/biom10060932
Ziadlou R, Barbero A, Martin I, Wang X, Qin L, Alini M, Grad S. Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules. 2020; 10(6):932. https://doi.org/10.3390/biom10060932
Chicago/Turabian StyleZiadlou, Reihane, Andrea Barbero, Ivan Martin, Xinluan Wang, Ling Qin, Mauro Alini, and Sibylle Grad. 2020. "Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes" Biomolecules 10, no. 6: 932. https://doi.org/10.3390/biom10060932





