Ten-Year Research Update Review: Antiviral Activities from Marine Organisms
Abstract
:1. Introduction
2. Marine Bacteria and Fungi
2.1. Marine Bacteria
2.2. Marine Fungi
3. Marine Microalgae
4. Seaweeds
5. Marine Plants
5.1. Seagrasses
5.2. Mangroves
6. Marine Invertebrates
6.1. Sponges
6.2. Mollusks
6.3. Cnidarians
6.4. Crustaceans
6.5. Echinoderms
6.6. Tunicates
6.7. Other Invertebrates
7. Marine Vertebrates
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneider, S.H. Encyclopedia of Climate and Weather; Oxford University Press: New York, NY, USA, 2011; Volume 1. [Google Scholar]
- Jaspars, M.; De Pascale, D.; Andersen, J.H.; Reyes, F.; Crawford, A.D.; Ianora, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef]
- Gerwick, W. Drugs from the Sea: The Search Continues. J. Pharm. Technol. 1987, 3, 136–141. [Google Scholar] [CrossRef]
- Lauritano, C.; Ianora, A. Grand Challenges in Marine Biotechnology: Overview of Recent EU-Funded Projects. In Grand Challenges in Marine Biotechnology; Springer: Cham, Switzerland, 2018; pp. 425–449. [Google Scholar]
- Poorvin, L.; Rinta-Kanto, J.M.; Hutchins, D.A.; Wilhelm, S.W. Viral release of iron and its bioavailability to marine plankton. Limnol. Oceanogr. 2004, 49, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Miller, K.M.; Di Cicco, E.; Schulze, A.D.; Kaukinen, K.H.; Ming, T.J.; Li, S.; Tabata, A.; Teffer, A.; Patterson, D.A.; et al. Endangered wild salmon infected by newly discovered viruses. Elife 2019, 8, e47615. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.L.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Wang, H.X.; Fang, E.F.; Lam, S.K.; Ngai, P.H.K.; et al. Antifungal and antiviral products of marine organisms. Appl. Microbiol. Biotechnol. 2014, 98, 3475–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plattet, P.; Alves, L.; Herren, M.; Aguilar, H.C. Measles virus fusion protein: Structure, function and inhibition. Viruses 2016, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Strand, M.; Carlsson, M.; Uvell, H.; Islam, K.; Edlund, K.; Cullman, I.; Altermark, B.; Mei, Y.F.; Elofsson, M.; Willassen, N.P.; et al. Isolation and characterization of anti-adenoviral secondary metabolites from marine actinobacteria. Mar. Drugs 2014, 12, 799–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.K.; Kaur, P.; Leong, S.T.; Tan, L.T.; Prinsep, M.R.; Chu, J.J.H. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs 2014, 12, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suthindhiran, K.; Sarath Babu, V.; Kannabiran, K.; Ishaq Ahmed, V.P.; Sahul Hameed, A.S. Anti-fish nodaviral activity of furan-2-yl acetate extracted from marine Streptomyces spp. Nat. Prod. Res. 2011, 25, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Al- Nahas Characterization of an exopolysaccharide-producing marine bacterium, isolate Pseudoalteromonas sp. AM. African J. Microbiol. Res. 2011, 5, 3823–3831.
- Silva, T.; Salomon, P.S.; Salomon, P.; Hamerski, L.; Walter, J.; B. Menezes, R.; Siqueira, J.E.; Santos, A.; Santos, J.A.M.; Ferme, N.; et al. Inhibitory effect of microalgae and cyanobacteria extracts on influenza virus replication and neuraminidase activity. PeerJ 2018, 26, e5716. [Google Scholar] [CrossRef] [Green Version]
- Raveh, A.; Delekta, P.C.; Dobry, C.J.; Peng, W.; Schultz, P.J.; Blakely, P.K.; Tai, A.W.; Matainaho, T.; Irani, D.N.; Sherman, D.H.; et al. Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PLoS ONE 2013, 8, e0082318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, C.; Lin, X.; Lu, X.; Wan, J.; Zhou, X.; Liao, S.; Tu, Z.; Xu, S.; Liu, Y. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. (Tokyo) 2015, 68, 121–125. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum mxh-x73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vazquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vilchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef] [Green Version]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Shushni, M.A.M.; Singh, R.; Mentel, R.; Lindequist, U. Balticolid: A new 12-membered macrolide with antiviral activity from an Ascomycetous fungus of marine origin. Mar. Drugs 2011, 9, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Nong, X.H.; Huang, Z.H.; Qi, S.H. Antifungal and Antiviral Cyclic Peptides from the Deep-Sea-Derived Fungus Simplicillium obclavatum EIODSF 020. J. Agric. Food Chem. 2017, 65, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 7, 1182–1186. [Google Scholar] [CrossRef]
- Wu, G.; Sun, X.; Yu, G.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Cladosins A-E, hybrid polyketides from a deep-sea-derived fungus, Cladosporium sphaerospermum. J. Nat. Prod. 2014, 77, 270–275. [Google Scholar] [CrossRef]
- Wang, J.F.; Lin, X.P.; Qin, C.; Liao, S.R.; Wan, J.T.; Zhang, T.Y.; Liu, J.; Fredimoses, M.; Chen, H.; Yang, B.; et al. Antimicrobial and antiviral sesquiterpenoids from sponge-associated fungus, Aspergillus sydowii ZSDS1-F6. J. Antibiot. 2014, 67, 581–583. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. New rubrolides from the marine-derived fungus Aspergillus terreus OUCMDZ-1925. J. Antibiot. 2014, 67, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Zhang, X.; Du, L.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014, 77, 424–428. [Google Scholar] [CrossRef]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Tan, Q.W.; Ouyang, M.A.; Shen, S.; Li, W. Bioactive metabolites from a marine-derived strain of the fungus Neosartorya fischeri. Nat. Prod. Res. 2012, 26, 1402–1407. [Google Scholar] [CrossRef]
- Shen, S.; Li, W.; Wang, J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum 0312F 1. Nat. Prod. Res. 2013, 27, 2286–2291. [Google Scholar] [CrossRef]
- Zhao, F.; Qin, Y.H.; Zheng, X.; Zhao, H.W.; Chai, D.Y.; Li, W.; Pu, M.X.; Zuo, X.S.; Qian, W.; Ni, P.; et al. Biogeography and Adaptive evolution of Streptomyces Strains from saline environments. Sci. Rep. 2016, 6, 32718. [Google Scholar] [CrossRef] [Green Version]
- Jose, P.A.; Jha, B. Intertidal marine sediment harbours Actinobacteria with promising bioactive and biosynthetic potential. Sci. Rep. 2017, 7, 10041. [Google Scholar] [CrossRef]
- Yang, C.; Qian, R.; Xu, Y.; Yi, J.; Gu, Y.; Liu, X.; Yu, H.; Jiao, B.; Lu, X.; Zhang, W. Marine Actinomycetes-derived Natural Products. Curr. Top. Med. Chem. 2019, 19, 2868–2918. [Google Scholar] [CrossRef] [PubMed]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Polish J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamjam, M.; Sivalingam, P.; Deng, Z.; Hong, K. Deep sea actinomycetes and their secondary metabolites. Front. Microbiol. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.u.; Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Tasneem, U. Emerging biopharmaceuticals from marine actinobacteria. Environ. Toxicol. Pharmacol. 2017, 49, 34–47. [Google Scholar] [CrossRef]
- Zlotnik, I.; Peacock, S.; Grant, D.P.; Batter-Hatton, D. The pathogenesis of western equine encephalitis virus (W.E.E.) in adult hamsters with special reference to the long and short term effects on the C.N.S. of the attenuated clone 15 variant. Br. J. Exp. Pathol. 1972, 53, 59–77. [Google Scholar]
- Steele, K.E.; Twenhafel, N.A. Review paper: Pathology of animal models of alphavirus encephalitis. Vet. Pathol. 2010, 47, 790–805. [Google Scholar] [CrossRef]
- Tong, J.; Trapido-Rosenthal, H.; Wang, J.; Wang, Y.; Li, Q.X.; Lu, Y. Antiviral activities and putative identification of compounds in microbial extracts from the Hawaiian coastal waters. Mar. Drugs 2012, 10, 521–538. [Google Scholar] [CrossRef]
- Buckwold, V.E.; Wei, J.; Wenzel-Mathers, M.; Russell, J. Synergistic in vitro interactions between alpha interferon and ribavirin against bovine viral diarrhea virus and yellow fever virus as surrogate models of hepatitis C virus replication. Antimicrob. Agents Chemother. 2003, 47, 2293–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckwold, V.E.; Beer, B.E.; Donis, R.O. Bovine viral diarrhea virus as a surrogate model of Hepatitis C virus for the evaluation of antiviral agents. Antiviral Res. 2003, 60, 1–15. [Google Scholar] [CrossRef]
- Santiago Bastos, J.C.; Konecny Kohn, L.; Fantinatti-Garboggini, F.; Aiello Padilla, M.; Furtado Flores, E.; da Silva, B.P.; de Menezes, C.B.A.; Weis Arns, C. Antiviral activity of Bacillus sp. isolated from the marine sponge petromica citrina against bovine viral diarrhea virus, a surrogate model of the hepatitis C virus. Viruses 2013, 5, 1219–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royo, J.L.; Maeso, I.; Irimia, M.; Gao, F.; Peter, I.S.; Lopes, C.S.; D’Aniello, S.; Casares, F.; Davidson, E.H.; Garcia-Fernandez, J.; et al. Transphyletic conservation of developmental regulatory state in animal evolution. Proc. Natl. Acad. Sci. USA 2011, 108, 14186–14191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermeyer, T.H.o.J. Anti-infective Natural Products from Cyanobacteria. Planta Med. 2015, 81, 1309–1325. [Google Scholar] [PubMed] [Green Version]
- Sharaf, M.; Amara, A.; Aboul-Enein, A.; Helmi, S.; Ballot, A.; Astani, A.; Schnitzler, P. Molecular authentication and characterization of the antiherpetic activity of the cyanobacterium Arthrospira fusiformis. Pharmazie 2010, 65, 132–136. [Google Scholar]
- Kiuru, P.; Valeria D’Auria, M.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Chlipala, G.E.; Tri, P.H.; Hung, N.V.; Krunic, A.; Shim, S.H.; Soejarto, D.D.; Orjala, J. Nhatrangins A and B, aplysiatoxin-related metabolites from the marine cyanobacterium Lyngbya majuscula from Vietnam. J. Nat. Prod. 2010, 73, 784–787. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.E.; Blackman, A.J.; Cheuk, C.E.; Mynderse, J.S.; Matsumoto, G.K.; Clardy, J.; Woodard, R.W.; Craig, J.C. Absolute Stereochemistries of the Aplysiatoxins and Oscillatoxin A. J. Org. Chem. 1984, 49, 2484–2489. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Raposo, M.F.; de Morais, R.M.; Bernardo de Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, R. Antiviral properties of cyanobacterium, Spirulina platensis—a Review. Int. J. Med. Pharm. Sci. 2013, 3, 1–10. [Google Scholar]
- Reichert, M.; Bergmann, S.M.; Hwang, J.; Buchholz, R.; Lindenberger, C. Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J. Fish Dis. 2017, 40, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [Green Version]
- Taishi, T.; Takechi, S.; Mori, S. First total synthesis of (±)-stachyflin. Tetrahedron Lett. 1998, 39, 4347–4350. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Nikzad, S.; Kadir, H.A.; Abubakar, S.; Zandi, K. Potential antiviral agents from marine fungi: An overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef]
- Rabenau, H.F.; Richter, M.; Doerr, H.W. Hand, foot and mouth disease: Seroprevalence of Coxsackie A16 and Enterovirus 71 in Germany. Med. Microbiol. Immunol. 2010, 199, 45–51. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, E.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2012, 24, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Martinez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.O.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Martinez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martin, J.; Diaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef] [Green Version]
- Riccio, G.; Lauritano, C. Microalgae with immunomodulatory activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, C.; Ianora, A. Marine organisms with anti-diabetes properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef]
- Lauritano, C.; Martín, J.; De La Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First identification of marine diatoms with anti-tuberculosis activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef] [Green Version]
- Brillatz, T.; Lauritano, C.; Jacmin, M.; Khamma, S.; Marcourt, L.; Righi, D.; Romano, G.; Esposito, F.; Ianora, A.; Queiroz, E.F.; et al. Zebrafish-based identification of the antiseizure nucleoside inosine from the marine diatom Skeletonema marinoi. PLoS ONE 2018, 13, e0196195. [Google Scholar] [CrossRef] [Green Version]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Biotechnological Applications of Bioactive Peptides From Marine Sources. Adv. Microb. Physiol. 2018, 73, 171–220. [Google Scholar] [CrossRef]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and chlorophyll-derived molecules from the diatom Cylindrotheca closterium with anti-inflammatory activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [Green Version]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.B.; Atsumi, K.; Kanazashi, M.; Shibayama, T.; Okamoto, K.; Kawahara, T.; Hayashi, T. In vitro and in vivo anti-herpes simplex virus activity of monogalactosyl diacylglyceride from Coccomyxa sp. KJ (IPOD FERM BP-22254), a green microalga. PLoS ONE 2019, 14, e0219305. [Google Scholar] [CrossRef] [Green Version]
- Gastineau, R.; Pouvreau, J.B.; Hellio, C.; Morançais, M.; Fleurence, J.; Gaudin, P.; Bourgougnon, N.; Mouget, J.L. Biological activities of purified marennine, the blue pigment responsible for the greening of oysters. J. Agric. Food Chem. 2012, 60, 3599–3605. [Google Scholar] [CrossRef]
- Gastineau, R.; Hardivillier, Y.; Leignel, V.; Tekaya, N.; Morançais, M.; Fleurence, J.; Davidovich, N.; Jacquette, B.; Gaudin, P.; Hellio, C.; et al. Greening effect on oysters and biological activities of the blue pigments produced by the diatom Haslea karadagensis (Naviculaceae). Aquaculture 2012, 368–369, 61–67. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antiviral Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and characterization of Griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: From discovery to clinical application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [Green Version]
- Mattos, B.B.; Romanos, M.T.V.; de Souza, L.M.; Sassaki, G.; Barreto-Bergter, E. Glycolipids from macroalgae: Potential biomolecules for marine biotechnology? Brazilian J. Pharmacogn. 2011, 12, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs 2016, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xie, E.; Zheng, K.; Fredimoses, M.; Yang, X.; Zhou, X.; Wang, Y.; Yang, B.; Lin, X.; Liu, J.; et al. Nutritional and chemical composition and antiviral activity of cultivated seaweed Sargassum naozhouense Tseng et Lu. Mar. Drugs 2013, 11, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.Y.; Kim, Y.M.; Park, S.J.; Rho, M.C.; Kim, S.J.; Lee, W.S. Influenza virus neuraminidase inhibitory activity of phlorotannins from the edible brown alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.; Wacyk, J.M.; Carrasco, J.; Cortez-San Martín, M.A. Red macroalgae Pyropia columbina and Gracilaria chilensis: Sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J. Appl. Phycol. 2016, 28, 1343–1351. [Google Scholar] [CrossRef]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorganic Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Declarador, R.S.; Serrano, A.E.; Corre, V.L. Ulvan extract acts as immunostimulant against white spot syndrome virus (WSSV) in juvenile black tiger shrimp Penaeus monodon. AACL Bioflux 2014, 7, 153–161. [Google Scholar]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Park, J.W.; Lee, N.H.; Na, H.K.; Surh, Y.J.; You, H.J.; Chung, M.H.; Hyun, J.W. Triphlorethol-A induces heme oxygenase-1 via activation of ERK and NF-E2 related factor 2 transcription factor. FEBS Lett. 2007, 581, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Stadler, K.; Masignani, V.; Eickmann, M.; Becker, S.; Abrignani, S.; Klenk, H.D.; Rappuoli, R. SARS— beginning to understand a new virus. Nat. Rev. Microbiol. 2003, 1, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, J.W.; Torrecillas, S.; Dimitroglou, A.; Rider, S.; Davies, S.J.; Izquierdo, M.S. Enhancing the natural defences and barrier protection of aquaculture species. Aquac. Res. 2010, 41, 345–355. [Google Scholar] [CrossRef]
- Zeng, K.; Xu, H.; Chen, K.; Zhu, J.; Zhou, Y.; Zhang, Q.; Mantian, M. Effects of taurine on glutamate uptake and degradation in Müller cells under diabetic conditions via antioxidant mechanism. Mol. Cell. Neurosci. 2010, 45, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, C.D.; Briske-Anderson, M.; Nielsen, F.H. An interaction between dietary silicon and arginine affects immune function indicated by con-a-induced dna synthesis of rat splenic T-lymphocytes. Biol. Trace Elem. Res. 2002, 87, 133–142. [Google Scholar] [PubMed]
- Morais, S.; Castanheira, F.; Martinez-Rubio, L.; Conceição, L.E.C.; Tocher, D.R. Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Δ4 activity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Rubio, L.; Morais, S.; Evensen, Ø.; Wadsworth, S.; Vecino, J.G.; Ruohonen, K.; Bell, J.G.; Tocher, D.R. Effect of functional feeds on fatty acid and eicosanoid metabolism in liver and head kidney of Atlantic salmon (Salmo salar L.) with experimentally induced Heart and Skeletal Muscle Inflammation. Fish Shellfish Immunol. 2013, 34, 1533–1545. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.C.; Xu, J.Y.; Liu, H.P. Cellular entry of white spot syndrome virus and antiviral immunity mediated by cellular receptors in crustaceans. Fish Shellfish Immunol. 2019, 93, 580–588. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ooi, E.V.; Ang, P.O. Antiviral activities of extracts from Hong Kong seaweeds. J. Zhejiang Univ. Sci. B 2008, 9, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef]
- Bilan, M.I.; Vinogradova, E.V.; Tsvetkova, E.A.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A sulfated glucuronofucan containing both fucofuranose and fucopyranose residues from the brown alga Chordaria flagelliformis. Carbohydr. Res. 2008, 343, 2605–2612. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef]
- McClure, M.O.; Whitby, D.; Patience, C.; Gooderham, N.J.; Bradshaw, A.; Cheingsong-Popov, R.; Weber, J.N.; Davies, D.S.; Cook, G.M.W.; Keynes, R.J.; et al. Dextrin sulphate and fucoidan are potent inhibitors of HIV infection in vitro. Antivir. Chem. Chemother. 1991, 2, 149–156. [Google Scholar] [CrossRef]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef]
- Olsen, J.L.; Rouzé, P.; Verhelst, B.; Lin, Y.C.; Bayer, T.; Collen, J.; Dattolo, E.; De Paoli, E.; Dittami, S.; Maumus, F.; et al. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 2016, 530, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Procaccini, G.; Ruocco, M.; Marín-Guirao, L.; Dattolo, E.; Brunet, C.; D’Esposito, D.; Lauritano, C.; Mazzuca, S.; Serra, I.A.; Bernardo, L.; et al. Depth-specific fluctuations of gene expression and protein abundance modulate the photophysiology in the seagrass Posidonia oceanica. Sci. Rep. 2017, 7, 42890. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmusson, L.M.; Lauritano, C.; Procaccini, G.; Gullström, M.; Buapet, P.; Björk, M. Respiratory oxygen consumption in the seagrass Zostera marina varies on a diel basis and is partly affected by light. Mar. Biol. 2017, 164, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravaglioli, C.; Lauritano, C.; Buia, M.C.; Balestri, E.; Capocchi, A.; Fontanini, D.; Pardi, G.; Tamburello, L.; Procaccini, G.; Bulleri, F. Nutrient Loading Fosters Seagrass Productivity under Ocean Acidification. Sci. Rep. 2017, 7, 13732. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ruocco, M.; Dattolo, E.; Buia, M.C.; Silva, J.; Santos, R.; Olivé, I.; Costa, M.M.; Procaccini, G. Response of key stress-related genes of the seagrass Posidonia oceanica in the vicinity of submarine volcanic vents. Biogeosciences 2015, 12, 4185–4194. [Google Scholar] [CrossRef] [Green Version]
- Olivé, I.; Silva, J.; Lauritano, C.; Costa, M.M.; Ruocco, M.; Procaccini, G.; Santos, R. Linking gene expression to productivity to unravel long-and short-term responses of seagrasses exposed to CO2 in volcanic vents. Sci. Rep. 2017, 7, 42287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkum, A.W.D.; Orth, R.J.; Duarte, C.M. Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; ISBN 140202942X. [Google Scholar]
- De La Torre-Castro, M.; Rönnbäck, P. Links between humans and seagrasses - An example from tropical East Africa. Ocean Coast. Manag. 2004, 47, 361–387. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Wang, L.; Jia, M.; Yin, D.; Tian, J. A review of remote sensing for mangrove forests: 1956–2018. Remote Sens. Environ. 2019, 231, 111223. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Bio. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Mohammed, M.M.D.; Hamdy, A.H.A.; El-Fiky, N.M.; Mettwally, W.S.A.; El-Beih, A.A.; Kobayashi, N. Anti-influenza A virus activity of a new dihydrochalcone diglycoside isolated from the Egyptian seagrass Thalassodendron ciliatum (Forsk.) den Hartog. Nat. Prod. Res. 2014, 28, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, A.H.A.; Mettwallya, W.S.A.; El Fotouh, M.A.; Rodriguez, B.; El-Dewany, A.I.; El-Toumy, S.A.A.; Hussein, A.A. Bioactive phenolic compounds from the Egyptian Red Sea seagrass Thalassodendron ciliatum. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2012, 67, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Abou El-Kassem, L.T. Thalassiolin D: A new flavone O-glucoside Sulphate from the seagrass Thalassia hemprichii. Nat. Prod. Res. 2017, 31, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Huang, X.Y.; Li, J.; Xin, G.R.; Guo, Y.W. Absolute configurations of integracins A, B, and 15′-dehydroxy-integracin B. Chirality 2012, 24, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, Z.; Shen, L.; Pedpradab, P.; Bruhn, T.; Wu, J.; Bringmann, G. Antiviral Limonoids Including Khayanolides from the Trang Mangrove Plant Xylocarpus moluccensis. J. Nat. Prod. 2015, 78, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.K.; Li, P.L.; Qiao, D.; Zhang, X.W.; Chu, M.J.; Qin, G.F.; Tang, X.L.; Li, G.Q. Cytotoxic and antiviral triterpenoids from the mangrove plant Sonneratia paracaseolaris. Molecules 2017, 22, 1319. [Google Scholar] [CrossRef] [Green Version]
- Krylova, N.V.; Leonova, G.N.; Maystrovskaya, O.S.; Popov, A.M.; Artyukov, A.A. Mechanisms of Antiviral Activity of the Polyphenol Complex from Seagrass of the Zosteraceae Family against Tick-Borne Encephalitis Virus. Bull. Exp. Biol. Med. 2018, 165, 61–63. [Google Scholar] [CrossRef]
- Hawas, U.W. A new 8-hydroxy flavone o-xyloside sulfate and antibacterial activity from the egyptian seagrass Thalassia hemprichii. Chem. Nat. Compd. 2014, 50, 629–632. [Google Scholar] [CrossRef]
- Regalado, E.L.; Rodríguez, M.; Menéndez, R.; Concepción, Á.A.; Nogueiras, C.; Laguna, A.; Rodríguez, A.A.; Williams, D.E.; Lorenzo-Luaces, P.; Valdés, O.; et al. Repair of UVB-damaged skin by the antioxidant sulphated flavone glycoside thalassiolin B isolated from the marine plant Thalassia testudinum Banks ex König. Mar. Biotechnol. 2009, 11, 74–80. [Google Scholar] [CrossRef]
- Popov, A.M.; Krivoshapko, O.N.; Klimovich, A.A.; Artyukov, A.A. Biological activity and mechanisms of therapeutic action of rosmarinic acid, luteolin and its sulphated derivatives. Biomeditsinskaya Khimiya 2016, 62, 22–30. [Google Scholar] [CrossRef]
- Tian, M.; Dai, H.; Li, X.; Wang, B. Chemical constituents of marine medicinal mangrove plant Sonneratia caseolaris. Chinese J. Oceanol. Limnol. 2009, 27, 288–296. [Google Scholar] [CrossRef]
- Sadhu, S.K.; Ahmed, F.; Ohtsuki, T.; Ishibashi, M. Flavonoids from Sonneratia caseolaris. J. Nat. Med. 2006, 60, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Wen, Y.; Li, X.W.; Zhao, Y.; Zhao, Z.; Hu, J.F. Chemical constituents from the fruits of Sonneratia caseolaris and Sonneratia ovata (Sonneratiaceae). Biochem. Syst. Ecol. 2009, 37, 1–5. [Google Scholar] [CrossRef]
- Ross, A.B.; Kamal-Eldin, A.; Åman, P. Dietary Alkylresorcinols: Absorption, Bioactivities, and Possible Use as Biomarkers of Whole-grain Wheat- and Rye-rich Foods. Nutr. Rev. 2004, 62, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.; Xu, Z.; Lee, K.-H.; Spitzer, T.; Kitrinos, P.; McDonald, O.; Jones, R.; Garvey, E. Fungal Metabolites as Potent Protein Kinase Inhibitors: Identification of a Novel Metabolite and Novel Activities of Known Metabolites. Lett. Drug Des. Discov. 2005, 100, 24–29. [Google Scholar] [CrossRef]
- Shibazaki, M.; Tanaka, K.; Nagai, K.; Watanabe, M.; Fujita, S.; Suzuki, K.; Okada, G.; Yamamoto, T. YM-92447 (spinosulfate A), a neuraminidase inhibitor produced by an unidentified pycnidial fungus. J. Antibiot. (Tokyo) 2004, 57, 812–815. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Huang, Y.; Tan, F.; He, X.; Boufford, D.E. Phylogenetic analysis of the Sonneratiaceae and its relationship to Lythraceae based on ITS sequences of nrDNA. J. Plant Res. 2000, 113, 253–258. [Google Scholar] [CrossRef]
- Ravangpai, W.; Sommit, D.; Teerawatananond, T.; Sinpranee, N.; Palaga, T.; Pengpreecha, S.; Muangsin, N.; Pudhom, K. Limonoids from seeds of Thai Xylocarpus moluccensis. Bioorganic Med. Chem. Lett. 2011, 21, 4485–4489. [Google Scholar] [CrossRef]
- Li, J.; Li, M.Y.; Feng, G.; Xiao, Q.; Sinkkonen, J.; Satyanandamurty, T.; Wu, J. Limonoids from the seeds of a Godavari mangrove, Xylocarpus moluccensis. Phytochemistry 2010, 71, 1917–1924. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009-2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other mechanisms of action. Mar. Drugs 2017, 15, 273. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and other mechanisms. Mar. Drugs 2020, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, A.R.; Mohanram, M.S.G.; Balasubramanian, B.; Kim, I.H.; Seedevi, P.; Mohan, K.; Kanagasabai, S.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Marine invertebrates’ proteins: A recent update on functional property. J. King Saud Univ. 2020, 32, 1496–1502. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, M.; Falkenberg, M.D.B. An overview of the marine natural products in clinical trials and on the market. J. Coast. Life Med. 2015, 3, 421–428. [Google Scholar]
- Yamashita, A.; Tamaki, M.; Kasai, H.; Tanaka, T.; Otoguro, T.; Ryo, A.; Maekawa, S.; Enomoto, N.; de Voogd, N.J.; Tanaka, J.; et al. Inhibitory effects of metachromin A on hepatitis B virus production via impairment of the viral promoter activity. Antiviral Res. 2017, 145, 136–145. [Google Scholar] [CrossRef]
- Yamashita, A.; Fujimoto, Y.; Tamaki, M.; Setiawan, A.; Tanaka, T.; Okuyama-Dobashi, K.; Kasai, H.; Watashi, K.; Wakita, T.; Toyama, M.; et al. Identification of antiviral agents targeting hepatitis B virus promoter from extracts of Indonesian marine organisms by a novel cell-based screening assay. Mar. Drugs 2015, 13, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Tani, H.; et al. Inhibition of hepatitis C virus NS3 helicase by manoalide. J. Nat. Prod. 2012, 75, 650–654. [Google Scholar] [CrossRef]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Tani, H.; et al. Psammaplin A inhibits hepatitis C virus NS3 helicase. J. Nat. Med. 2013, 67, 765–772. [Google Scholar] [CrossRef]
- Yu, H.B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.H.; Gan, J.H.; Hu, W.Z.; Lin, H.W. Aaptamine derivatives with antifungal and anti-HIV-1 activities from the South China sea sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef]
- Fan, G.; Li, Z.; Shen, S.; Zeng, Y.; Yang, Y.; Xu, M.; Bruhn, T.; Bruhn, H.; Morschhäuser, J.; Bringmann, G.; et al. Baculiferins A-O, O-sulfated pyrrole alkaloids with anti-HIV-1 activity, from the Chinese marine sponge Iotrochota baculifera. Bioorganic Med. Chem. 2010, 18, 5466–5474. [Google Scholar] [CrossRef]
- Tietjen, I.; Williams, D.E.; Read, S.; Kuang, X.T.; Mwimanzi, P.; Wilhelm, E.; Markle, T.; Kinloch, N.N.; Naphen, C.N.; Tenney, K.; et al. Inhibition of NF-κB-dependent HIV-1 replication by the marine natural product Bengamide A. Antiviral Res. 2018, 152, 94–103. [Google Scholar] [CrossRef]
- Lu, Z.; Van Wagoner, R.M.; Harper, M.K.; Baker, H.L.; Hooper, J.N.A.; Bewley, C.A.; Ireland, C.M. Mirabamides E-H, HIV-inhibitory depsipeptides from the sponge Stelletta clavosa. Bone 2012, 74, 185–193. [Google Scholar]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Physiol. Behav. 2015, 56, 4215–4219. [Google Scholar] [CrossRef] [Green Version]
- Palem, J.R.; Bedadala, G.R.; Sayed, K.A.E.; Hsia, S.V. Manzamine A as a novel inhibitor of Herpes simplex virus type-1 replication in cultured corneal cells. Planta Med. 2011, 77, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, T.D.R.; Quiroz, C.G.; Rigotto, C.; De Oliveira, S.Q.; De Almeida, M.T.R.; Bianco, É.M.; Moritz, M.I.G.; Carraro, J.L.; Palermo, J.A.; Cabrera, G.; et al. Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae). Mar. Drugs 2013, 11, 4176–4192. [Google Scholar] [CrossRef]
- González-Almela, E.; Sanz, M.A.; García-Moreno, M.; Northcote, P.; Pelletier, J.; Carrasco, L. Differential action of pateamine A on translation of genomic and subgenomic mRNAs from Sindbis virus. Virology 2015, 484, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Guo, J.; Liu, Y.; Lu, A.; Wang, Z.; Li, Y.; Yang, S.; Wang, Q. Marine natural product development: First discovery of Nortopsentin alkaloids as novel antiviral, anti-phytopathogenic-fungus, and insecticidal agents. J. Agric. Food Chem. 2018, 66, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, N.V.; Zagorodnya, S.D.; Moshtanska, V.; Dolashka, P.; Baranova, G.V.; Golovan, A.V.; Kurova, A.O. Antiviral activity of hemocyanin isolated from marine snail Rapana venosa. Antiviral Res. 2011, 90, 606–610. [Google Scholar] [CrossRef]
- Dolashka, P.; Nesterova, N.; Zagorodnya, S.; Aleksandar, D.; Baranova, G.; Golovan, A.; Voelter, W. Antiviral activity of Hemocyanin Rapana venosa and its isoforms against Epstein-Barr virus. Glob. J. Pharmacol. 2014, 8, 206–212. [Google Scholar]
- Zanjani, N.T.; Miranda-Saksena, M.; Valtchev, P.; Diefenbach, R.J.; Hueston, L.; Diefenbach, E.; Sairi, F.; Gomes, V.G.; Cunningham, A.L.; Dehghani, F. Abalone hemocyanin blocks the entry of herpes simplex virus 1 into cells: A potential new antiviral strategy. Antimicrob. Agents Chemother. 2016, 60, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Green, T.J.; Robinson, N.; Chataway, T.; Benkendorff, K.; O’Connor, W.; Speck, P. Evidence that the major hemolymph protein of the Pacific oyster, Crassostrea gigas, has antiviral activity against herpesviruses. Antiviral Res. 2014, 110, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.T.; Speck, P.; Doroudi, M.; Smith, B.; Benkendorff, K. Variation in the antiviral and antibacterial activity of abalone Haliotis laevigata, H. rubra and their hybrid in South Australia. Aquaculture 2011, 315, 242–249. [Google Scholar] [CrossRef]
- Novoa, B.; Romero, A.; Álvarez, Á.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral activity of Myticin C peptide from mussel: An ancient defense against herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [Green Version]
- Balseiro, P.; Falcó, A.; Romero, A.; Dios, S.; Martínez-López, A.; Figueras, A.; Estepa, A.; Novoa, B. Mytilus galloprovincialis myticin C: A chemotactic molecule with antiviral activity and immunoregulatory properties. PLoS ONE 2011, 6, e23140. [Google Scholar] [CrossRef] [Green Version]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; et al. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.K.; Yeh, T.T.; Duh, C.Y. Briacavatolides D-F, new briaranes from the Taiwanese octocoral Briareum excavatum. Mar. Drugs 2012, 10, 2103–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.T.; Wang, S.K.; Dai, C.F.; Duh, C.Y. Briacavatolides A-C, New briaranes from the Taiwanese octocoral Briareum excavatum. Mar. Drugs 2012, 10, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Chen, P.W.; Chen, H.P.; Wang, S.K.; Duh, C.Y. New cembranolides from the Dongsha Atoll soft coral Lobophytum durum. Mar. Drugs 2011, 9, 1307–1318. [Google Scholar] [CrossRef]
- Wang, S.K.; Hsieh, M.K.; Duh, C.Y. New diterpenoids from soft coral Sarcophyton ehrenbergi. Mar. Drugs 2013, 11, 4318–4327. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.Y.; Chuang, C.T.; Wang, S.K.; Wen, Z.H.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010, 73, 1184–1187. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, S.K.; Duh, C.Y. Polyhydroxylated steroids from the bamboo coral Isis hippuris. Mar. Drugs 2011, 9, 1829–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.Y.; Wang, S.K.; Duh, C.Y. Secocrassumol, a seco-cembranoid from the Dongsha Atoll soft coral lobophytum crassum. Mar. Drugs 2014, 12, 6028–6037. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.H.; Wang, Y.F.; Li, S.; Qian, P.Y.; Qi, S.H. Alkaloids and sesquiterpenes from the South China Sea gorgonian Echinogorgia pseudossapo. Mar. Drugs 2011, 9, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ibrahim, A.; Arafa, A.S. Anti-H5N1 virus metabolites from the Red Sea soft coral, Sinularia candidula. Tetrahedron Lett. 2013, 54, 2377–2381. [Google Scholar] [CrossRef]
- Gong, K.K.; Tang, X.L.; Zhang, G.; Cheng, C.L.; Zhang, X.W.; Li, P.N.; Li, G.Q. Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, F.; Shao, C.L.; Chen, M.; Zhang, M.Q.; Xu, K.X.; Meng, H.; Wang, C.Y. Antiviral C-25 epimers of 26-acetoxy steroids from the South China Sea gorgonian Echinogorgia rebekka. J. Nat. Prod. 2014, 77, 1488–1493. [Google Scholar] [CrossRef]
- Davis, R.; Zivanovic, S.; D’Souza, D.H.; Davidson, P.M. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol. 2012, 32, 57–62. [Google Scholar] [CrossRef]
- Du, Z.Q.; Wang, Y.; Ma, H.Y.; Shen, X.L.; Wang, K.; Du, J.; Yu, X.D.; Fang, W.H.; Li, X.C. A new crustin homologue (SpCrus6) involved in the antimicrobial and antiviral innate immunity in mud crab, Scylla paramamosain. Fish Shellfish Immunol. 2019, 84, 733–743. [Google Scholar] [CrossRef]
- Zhan, S.; Aweya, J.J.; Wang, F.; Yao, D.; Zhong, M.; Chen, J.; Li, S.; Zhang, Y. Litopenaeus vannamei attenuates white spot syndrome virus replication by specific antiviral peptides generated from hemocyanin. Dev. Comp. Immunol. 2019, 91, 50–61. [Google Scholar] [CrossRef]
- Liu, H.P.; Chen, R.Y.; Zhang, Q.X.; Wang, Q.Y.; Li, C.R.; Peng, H.; Cai, L.; Zheng, C.Q.; Wang, K.J. Characterization of two isoforms of antiliopolysacchride factors (Sp-ALFs) from the mud crab Scylla paramamosain. Fish Shellfish Immunol. 2012, 33, 1–10. [Google Scholar] [CrossRef]
- Du, Z.Q.; Ren, Q.; Huang, A.M.; Fang, W.H.; Zhou, J.F.; Gao, L.J.; Li, X.C. A novel peroxinectin involved in antiviral and antibacterial immunity of mud crab, Scylla paramamosain. Mol. Biol. Rep. 2013, 40, 6873–6881. [Google Scholar] [CrossRef]
- Peng, H.; Liu, H.P.; Chen, B.; Hao, H.; Wang, K.J. Optimized production of scygonadin in Pichia pastoris and analysis of its antimicrobial and antiviral activities. Protein Expr. Purif. 2012, 82, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, S.; Gao, J.; Zuo, H.; Yuan, J.; Weng, S.; He, J.; Xu, X. A single WAP domain (SWD)-containing protein with antiviral activity from Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 73, 167–174. [Google Scholar] [CrossRef]
- Ma, J.; Ruan, L.; Xu, X.; Gao, Z. Molecular characteristics of three thymosin-repeat proteins from Marsupenaeus japonicus and their responses to WSSV infection. Acta Oceanol. Sin. 2016, 35, 44–50. [Google Scholar] [CrossRef]
- Xin, Y.; Li, W.; Lu, L.; Zhou, L.; Victor, D.W.; Xuan, S. Antiviral effects of Stichopus japonicus acid mucopolysaccharide on hepatitis B virus transgenic mice. J. Ocean Univ. China 2016, 15, 719–725. [Google Scholar] [CrossRef]
- Wijanarko, A.; Lischer, K.; Hermansyah, H.; Pratami, D.K.; Sahlan, M. Antiviral activity of Acanthaster planci phospholipase A2 against human immunodeficiency virus. Vet. World 2018, 11, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Lum, K.Y.; Carroll, A.R.; Ekins, M.G.; Read, S.; Haq, Z.; Tietjen, I.; St John, J.; Davis, R.A. Capillasterin A, a novel pyrano[2–f]chromene from the Australian crinoid Capillaster multiradiatus. Mar. Drugs 2019, 17, 26. [Google Scholar] [CrossRef] [Green Version]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In vitro antiviral activities of enzymatic hydrolysates extracted from byproducts of the Atlantic holothurian Cucumaria frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- Pujol, C.A.; Sepúlveda, C.S.; Richmond, V.; Maier, M.S.; Damonte, E.B. Polyhydroxylated sulfated steroids derived from 5α-cholestanes as antiviral agents against Herpes simplex virus. Arch. Virol. 2016, 161, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Smitha, D.; Kumar, M.M.K.; Ramana, H.; Rao, D.V. Rubrolide R: A new furanone metabolite from the ascidian Synoicum of the Indian Ocean. Nat. Prod. Res. 2014, 28, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Harper, M.K.; Pond, C.D.; Barrows, L.R.; Ireland, C.M.; Van Wagoner, R.M. Thiazoline peptides and a tris-phenethyl urea from Didemnum molle with anti-HIV activity. J. Nat. Prod. 2012, 75, 1436–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, Y.; Chinen, T.; Yoshida, K.; Kudo, S.; Nagumo, Y.; Shiwa, Y.; Yamada, R.; Umihara, H.; Iwasaki, K.; Masumoto, H.; et al. Eudistomin C, an antitumor and antiviral natural product, targets 40S ribosome and inhibits protein translation. ChemBioChem 2016, 17, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, Z.; Li, G.; Liu, Y.; Xie, Y.; Wang, Q. First discovery of polycarpine, Polycarpaurines A and C, and their derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food Chem. 2016, 64, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Privatdegarilhe, M.; De Rudder, J. Effect of two arabinose nucleosides on the multiplication of Herpes virus and vaccine in cell culture. C. R. Hebd. Seances Acad. Sci. 1964, 259, 2725–2728. [Google Scholar] [PubMed]
- El-demerdash, A.; Tammam, M.A.; Atanasov, A.G.; Hooper, J.N.A.; Al-mourabit, A.; Kijjoa, A. Chemistry and biological activities of the marine sponges of the genera Mycale (Arenochalina), Bienna and Clathria. Mar. Drugs 2018, 16, 214. [Google Scholar] [CrossRef] [Green Version]
- Sakai, R.; Higa, T.; Jefford, C.W.; Bernardinelli, G. Manzamine A, a novel antitumor alkaloid from a sponge. J. Am. Chem. Soc. 1986, 108, 6404–6405. [Google Scholar] [CrossRef]
- Quinoa, E.; Adamczeski, M.; Crews, P.; Bakus, G.J. Bengamides, heterocyclic anthelmintics from a Jaspidae marine sponge. J. Org. Chem. 1986, 51, 4494–4497. [Google Scholar] [CrossRef]
- de Silva, E.D.; Scheuer, P.J. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis (Polejaeff). Tetrahedron Lett. 1980, 21, 1611–1614. [Google Scholar] [CrossRef]
- Arabshahi, L.; Schmitz, F.J. Brominated tyrosine metabolites from an unidentified sponge. J. Org. Chem. 1987, 52, 3584–3586. [Google Scholar] [CrossRef]
- Bianco, É.M.; De Oliveira, S.Q.; Rigotto, C.; Tonini, M.L.; Da Rosa Guimarães, T.; Bittencourt, F.; Gouvêa, L.P.; Aresi, C.; De Almeida, M.T.R.; Moritz, M.I.G.; et al. Anti-infective potential of marine invertebrates and seaweeds from the Brazilian coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, I.T.; Caon, T.; Lückemeyer, D.D.; Ramos, F.A.; Tello, E.; Arévalo-Ferro, C.; Schenke, E.P.; Duque, C.; Simões, C.M.O. Antiherpes screening of marine organisms from Colombian Caribbean Sea. Brazilian J. Pharmacogn. 2011, 21, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Suciati; Abdillah, I.; Uddin, M.I.; Fauzi, A.; Adianti, M.; Fuad, A. Antiviral activity of marine sponges Homaxinella tanitai and Microxina subtilis against hepatitis C virus. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 1642–1647. [Google Scholar]
- Ibrahim, H.A.H.; El-Naggar, H.A.; El-Damhougy, K.A.; Bashar, M.A.E.; Abou Senna, F.M. Callyspongia crassa and C. siphonella (Porifera, Callyspongiidae) as a potential source for medical bioactive substances, Aqaba Gulf, Red Sea, Egypt. J. Basic Appl. Zool. 2017, 78, 7. [Google Scholar] [CrossRef]
- Ka, E.; El-naggar, H.A.; Ibrahim, H.A.H.; Bashar, M.A.E.; Senna, F.M.A. Biological activities of some marine sponge extracts from Aqaba Gulf, Red Sea, Egypt. Int. J. Fish. Aquat. Stud. 2017, 5, 652–659. [Google Scholar]
- Dolashka, P.; Voelter, W. Antiviral activity of hemocyanins. Invertebr. Surviv. J. 2013, 10, 120–127. [Google Scholar]
- Dang, V.T.; Benkendorff, K.; Green, T.; Speck, P. Marine snails and slugs: A great place to look for antiviral drugs. J. Virol. 2015, 89, 8114–8118. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sairi, M.F.; Cunningham, T.; Gomes, V.; Dehghani, F.; Valtchev, P. Study on novel antibacterial and antiviral compounds from abalone as an important marine mollusc. J. Aquac. Mar. Biol. 2018, 7, 138–140. [Google Scholar]
- Dang, V.T.; Benkendorff, K.; Speck, P. In vitro antiviral activity against herpes simplex virus in the abalone Haliotis laevigata. J. Gen. Virol. 2011, 92, 627–637. [Google Scholar] [CrossRef]
- Zanjani, N.T.; Sairi, F.; Marshall, G.; Saksena, M.M.; Valtchev, P.; Gomes, V.G.; Cunningham, A.L.; Dehghani, F. Formulation of abalone hemocyanin with high antiviral activity and stability. Eur. J. Pharm. Sci. 2014, 53, 77–85. [Google Scholar] [CrossRef]
- Dang, V.T.; Speck, P.; Benkendorff, K. Influence of elevated temperatures on the immune response of abalone, Haliotis rubra. Fish Shellfish Immunol. 2012, 32, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef] [PubMed]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Van Ofwegen, L. Four new bioactive lobane diterpenes of the soft coral Lobophytum pauciflorum from Mindoro, Philippines. J. Nat. Prod. 1998, 61, 358–361. [Google Scholar] [CrossRef]
- Gopichand, Y.; Schmitz, F.J. Marine natural products: Fuscol, a new elemene-type diterpene alcohol from the gorgonian Eunicea fusca. Tetrahedron Lett. 1978, 19, 3641–3644. [Google Scholar] [CrossRef]
- Poet, E.S.; Ravi, B.N. Three new diterpenes from a soft coral Nephthea species. Aust. J. Chem. 1982, 35, 77–83. [Google Scholar] [CrossRef]
- Shin, J.; Fenical, W. Fuscosides A-D: Antiinflammatory diterpenoid glycosides of new structural classes from the Caribbean gorgonian Eunicea fusca. J. Org. Chem. 1991, 56, 3153–3158. [Google Scholar] [CrossRef]
- Chan, Y.S.; Ong, C.W.; Chuah, B.L.; Khoo, K.S.; Chye, F.Y.; Sit, N.W. Antimicrobial, antiviral and cytotoxic activities of selected marine organisms collected from the coastal areas of Malaysia. J. Mar. Sci. Technol. 2018, 26, 128–136. [Google Scholar]
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic and HIV-1 enzyme inhibitory activities of Red Sea marine organisms. BMC Complement. Altern. Med. 2014, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.Z.; Shi, L.J.; Zhao, Y.R.; Zhao, X.F.; Wang, J.X. β-Thymosins participate in antiviral immunity of red swamp crayfish (Procambarus clarkii). Dev. Comp. Immunol. 2015, 51, 213–225. [Google Scholar] [CrossRef]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N. Chitosan from marine crustaceans: Production, characterization and applications. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; InTech: Rijeka, Croatia, 2017; pp. 39–56. [Google Scholar]
- Kim, S.K.; Himaya, S.W.A. Triterpene glycosides from sea cucumbers and their biological activities. Adv. Food Nutr. Res. 2012, 65, 297–319. [Google Scholar]
- Kamyab, E.; Kellermann, M.Y.; Kunzmann, A.; Schupp, P.J. Chemical biodiversity and bioactivities of Saponins in Echinodermata with an emphasis on sea cucumbers (Holothuroidea). In YOUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 121–157. ISBN 9783030203894. [Google Scholar]
- Kornprobst, J.M.; Sallenave, C.; Barnathan, G. Sulfated compounds from marine organisms. Comp. Biochem. Physiol. 1998, 119B, 1–51. [Google Scholar]
- Comin, M.J.; Maier, M.S.; Roccatagliata, A.J.; Pujol, C.A.; Damonte, E.B. Evaluation of the antiviral activity of natural sulfated polyhydroxysteroids and their synthetic derivatives and analogs. Steroids 1999, 64, 335–340. [Google Scholar] [CrossRef]
- Maier, M.S.; Roccatagliata, A.J.; Kuriss, A.; Chludil, H.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the antarctic sea cucumber Staurocucumis liouvillei. J. Nat. Prod. 2001, 64, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Garrido Santos, G.A.; Murray, A.P.; Pujol, C.A.; Damonte, E.B.; Maier, M.S. Synthesis and antiviral activity of sulfated and acetylated derivatives of 2β,3α-dihydroxy-5α-cholestane. Steroids 2003, 68, 125–132. [Google Scholar] [CrossRef]
- Bahroodi, S.; Nematollahi, M.A.; Aghasadeghi, M.R.; Nazemi, M. In vitro evaluation of the antiviral activity and cytotoxicity efect of Holothuria leucospilota sea cucumber extracts from the Persian gulf. Infect. Epidemiol. Microbiol. 2018, 4, 153–157. [Google Scholar]
- Farshadpour, F.; Gharibi, S.; Taherzadeh, M.; Amirinejad, R.; Taherkhani, R.; Habibian, A.; Zandi, K. Antiviral activity of Holothuria sp. a sea cucumber against herpes simplex virus type 1 (HSV-1). Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 333–337. [Google Scholar] [PubMed]
- Fenical, W. Geranyl hydroquinone, a cancer-protective agent from the tunicate Aplidium species. Food Drugs Sea Proc. 1976, 4, 388–394. [Google Scholar]
- Menna, M. Antitumor potential of natural products from Mediterranean ascidians. Phytochem. Rev. 2009, 8, 461–472. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural products diversity of marine ascidians (Tunicates; Ascidiacea) and successful drugs in clinical development. Nat. Products Bioprospect. 2017, 7, 1–111. [Google Scholar] [CrossRef] [Green Version]
- Watters, D.J. Ascidian toxins with potential for drug development. Mar. Drugs 2018, 16, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, X.; Dong, B. Origins and bioactivities of natural compounds derived from marine ascidians and their symbionts. Mar. Drugs 2019, 17, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgiovine, C.; Bello, E.; Liguori, M.; Craparotta, I.; Mannarino, L.; Paracchini, L.; Beltrame, L.; Marchini, S.; Galmarini, C.M.; Mantovani, A.; et al. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br. J. Cancer 2017, 117, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Healy, P.C.; Quinn, R.J.; Tranter, C.J. Prunolides A, B, and C: Novel tetraphenolic bis-spiroketals from the Australian ascidian Synoicum prunum. J. Org. Chem. 1999, 64, 2680–2682. [Google Scholar] [CrossRef]
- Smith, C.J.; Hettich, R.L.; Jompa, J.; Tahir, A.; Buchanan, M.V.; Ireland, C.M. Cadiolides A and B, new metabolites from an ascidian of the genus Botryllus. J. Org. Chem. 1998, 63, 4147–4150. [Google Scholar] [CrossRef]
- Lake, R.J.; Blunt, J.W.; Munro, M.H.G. Eudistomins from the New Zealand ascidian Ritterella sigillinoides. Aust. J. Chem. 1989, 42, 1201–1206. [Google Scholar] [CrossRef]
- Su, J.; Jiang, L.; Wu, J.; Liu, Z.; Wu, Y. Anti-tumor and anti-virus activity of polysaccharides extracted from Sipunculus nudus (SNP) on Hepg2.2.15. Int. J. Biol. Macromol. 2016, 87, 597–602. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Rombout, J.H.W.M.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Collet, B. Innate immune responses of salmonid fish to viral infections. Dev. Comp. Immunol. 2014, 43, 160–173. [Google Scholar] [CrossRef]
- Wang, Z. APD: The Antimicrobial Peptide Database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliolo, L.; Silva, O.N.; Silva, P.A.; Costa, M.P.; Costa, C.R.; Nolasco, D.O.; Barbosa, J.A.R.G.; Silva, M.R.R.; Bemquerer, M.P.; Lima, L.M.P.; et al. Structural and Functional Characterization of a Multifunctional Alanine-Rich Peptide Analogue from Pleuronectes americanus. PLoS ONE 2012, 7, e47047. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; de Lima, L.M.P.; Migliolo, L.; Mendes, G.d.S.; de Jesus, M.G.; Franco, O.L.; Silva, P.A. Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus. Biopolymers 2017, 108, e22871. [Google Scholar] [CrossRef] [PubMed]
- Chappell, B.F.; Smith, K.G. Patterns of predation of native reef fish by invasive Indo-Pacific lionfish in the western Atlantic: Evidence of selectivity by a generalist predator. Glob. Ecol. Conserv. 2016, 8, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Sommeng, A.N.; Muhammad Yusuf Arya, R.; Ginting, M.J.; Pratami, D.K.; Hermansyah, H.; Sahlan, M.; Wijanarko, A. Antiretroviral activity of Pterois volitans (red lionfish) venom in the early development of human immunodeficiency virus/acquired immunodeficiency syndrome antiretroviral alternative source. Vet. World 2019, 12, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.C.; Doglio, A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-O.; Chakrabarti, B.K.; Guha-Niyogi, A.; Louder, M.K.; Mascola, J.R.; Ganesh, L.; Nabel, G.J. Lysis of Human Immunodeficiency Virus Type 1 by a Specific Secreted Human Phospholipase A2. J. Virol. 2007, 81, 1444–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Norman, G.A. Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs. JACC Basic Transl. Sci. 2016, 25, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Biopharmaceutical Research & Development: The Process Behind New Medicines. PhRMA. 2015. Available online: http://www.jtbaker.com/msds/englishhtml/a0338.htm (accessed on 28 June 2020).
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine natural products from microalgae: An -omics overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef] [Green Version]






| Compound/Extract | Organism | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Butenolides | Streptomyces sp. | Anti-adenoviral | Undetermined | [12] |
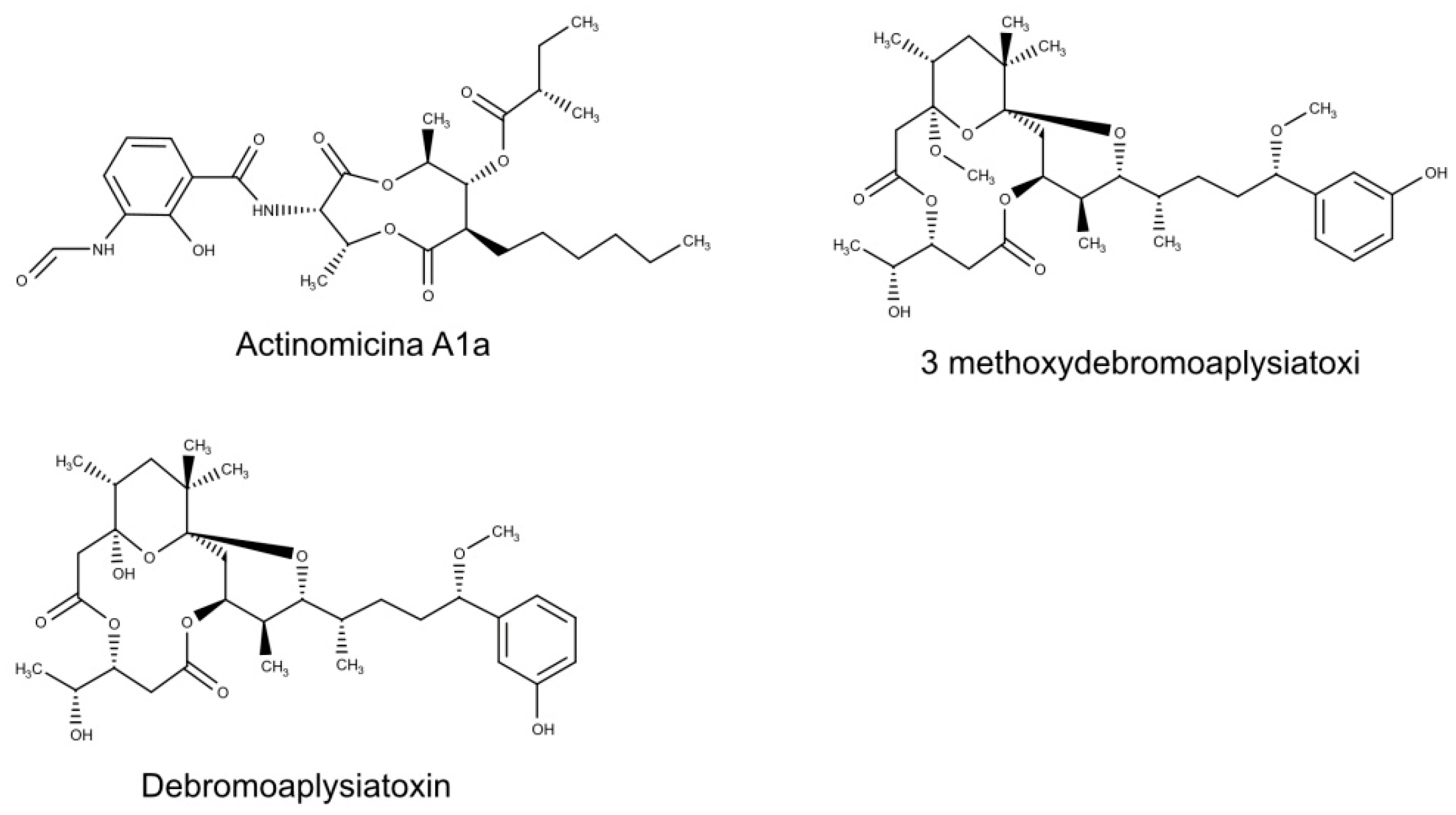
| Debromoaplysiatoxin; 3methoxydebromoaplysiatoxin | Trichodesmium erythraeum | CHIKV | Target replication cycle after viral entry | [13] |
| Furan-2-yl acetate | Streptomyces VITSDK1 spp. | FNV | Undetermined | [14] |
| EPS | Pseudoalteromonas sp. AM | HSV-1 | Undetermined | [15] |
| Chlorinated compounds | Leptolyngbya | Influenza A and B viruses | Inhibition neuraminidase activity and replication. | [16] |
| Antimycin A1a | Streptomyces kaviengensis | WEEV | inhibition of cellular mitochondrial electron transport chain | [17] |
| Compound/Extract | Organism | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Grisephenone A | Stachybotrys sp. | EV-71 | Not specified | [18] |
| Norlichexanthone-3,6,8-Trihydroxy-1-methylxanthone | Stachybotrys sp. | EV-71 | Not specified | [18] |
| Stachybogrisephenone B | Stachybotrys sp. | EV-71 | Not specified | [18] |
| Stachybotrins D | Stachybotrys chartarum MXH-X73 | HIV-1 | Not specified | [19,20] |
| Arisugacin A | Aspergillus terreus SCSGAF0162 | HSV-1 | Not specified | [21] |
| Aspergillipeptides D–E | Aspergillus sp. SCSIO 41501 | HSV-1 | Not specified | [22] |
| Aspernolide A | Aspergillus terreus SCSGAF0162 | HSV-1 | Not specified | [21] |
| Balticolid | Ascomycetous strain 222 | HSV-1 | Not specified | [23] |
| 11a-dehydroxyisoterreulactone A | Aspergillus terreus SCSGAF0162 | HSV-1 | Not specified | [21] |
| Isobutyrolactone II | Aspergillus terreus SCSGAF0162 | HSV-1 | Not specified | [21] |
| Simplicilliumtide J | Simplicillium obclavatum EIODSF 020 | HSV-1 | Not specified | [24] |
| Verlamelins A-B | Simplicillium obclavatum EIODSF 020 | HSV-1 | Not specified | [24] |
| Asperterrestide A | Aspergillus terreus SCSGAF0162 | Influenza A (H1N1 and H3N2) virus | Not specified | [25] |
| Cladosin C | Cladosporium sphaerospermum 2005-01-E3 | Influenza A (H1N1) virus | Not specified | [26] |
| Cordyol C | Aspergillus sydowii ZSDS1-F6 | Influenza A (H3N2) virus | Not specified | [27] |
| Diorcinol | Aspergillus sydowii ZSDS1-F6 | Influenza A (H3N2) virus | Not specified | [27] |
| (Z)-5-(Hydroxymenthyl)-2-(6′)-methylhept-2′-en-2′-yl)-phenol | Aspergillus sydowii ZSDS1-F6 | Influenza A (H3N2) virus | Not specified | [27] |
| Rubrolide S | Aspergillus terreus OUCMDZ-1925 | Influenza A (H1N1) virus | Not specified | [28] |
| Sorbicatechols A and B | Penicillium chrysogenum PJX-17 | Influenza A (H1N1) virus | Not specified | [29] |
| Alterporriol Q | Alternaria sp. ZJ-2008003 | PRRSV | Not specified | [30] |
| Tetrahydroaltersolanol C | Alternaria sp. ZJ-2008003 | PRRSV | Not specified | [30] |
| AGI-B4 | Neosartorya fischeri 1008F1 | TMV | Not specified | [31] |
| 3,4-dihydroxybenzoic acid | Neosartorya fischeri 1008F1 | TMV | Not specified | [31] |
| 2-(4-hydroxybenzyl) quinazolin-4(3H)-one | Penicillium oxalicum 0312F1 | TMV | Not specified | [32] |
| Methyl 4-hydroxyphenylacetate | Penicillium oxalicum 0312F1 | TMV | Not specified | [32] |
| Penipanoid C – 2-(4-hydroxybenzoyl) quinazolin-4(3H)-one | Penicillium oxalicum 0312F1 | TMV | Not specified | [32] |
| Compound/Extract | Organism | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| EPS | Porphyridium cruentum | HSV, VSV and Vaccinia virus | Reduction of virus-induced cytopathogenicity | [73] |
| MGDG | Coccomyxa sp. KJ | HSV | Structural changes in virus particles | [74] |
| Marennine-like pigment | Haslea karadagensis | HSV | Inhibition of plaque formation | [75,76] |
| Polysaccharide-rich fraction | DunaliellaSalina | HSV | Inhibition of plaque formation | [62] |
| Sulfated polysaccharide p-KG03 | Gyrodinium impudium | Influenza A virus (H1N1) and (H3N2) | Targeting virus particle attachment to cell surface receptors and internalization via virus–cell fusion | [77] |
| Compound/Extract | Organism | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Sulfate polysaccharides | Grateloupia filicina | AIV | Targeting virus particle attachment to cell | [81] |
| Sulfate polysaccharides | Ulva pertusa | AIV | Targeting virus particle attachment to cell | [81] |
| Sulfate polysaccharides | Sargassum qingdaoense | AIV | Targeting virus particle attachment to cell | [81] |
| Sulfated glucuronorhamnan | Monostroma nitidum | Enteroviruses | Targeting virus particle attachment to cell | [82] |
| Alginates and fucoidan | Sargassum naozhouense | HSV-1 | Targeting virus particle attachment to cell | [83] |
| Phlorofucofuroeckol | Ulva clathrata | Influenza A virus, H1N1, H3N2 and H9N2 | neuraminidases activity inhibition | [84] |
| EPA, fatty acids, omega w-3 | Gracilaria chilensis | ISA | Inhibition of viral replication | [85] |
| Ulvan | Ulva clathrata | NDV | Inhibited cell–cell fusion via a direct effect on the F0 protein | [86] |
| Fucoidan | Cladosiphon okamuranus | NDV | Inhibited cell–cell fusion | [86] |
| Diekol | Ulva clathrata | SARS-CoV | Inhibition of SARS-CoV 3CLpro | [87] |
| Ulvan | Ulva sp and Eteromorpha sp. | WSSV | Not reported | [88] |
| Compound/Extract | Organism | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Thalassiolin D—diosmetin 7-O-β-glucoside-2″-sulphate | Seagrass—Thalassia hemprichii | HCV | Inhibition of HCV NS3-NS4A protease | [126] |
| Dimeric Alkylresorcinols | Mangrove—Sonneratia hainanensis | HIV-1 | HIV-1 integrase inhibition | [127] |
| Asebotin, quercetin-3-O-β-d-xylopyranoside trans-caffeic acid | Seagrass—Thalassodendron ciliatum | HSV-1 | Inhibition of plaque formation | [125] |
| Khayanolides | Mangrove—Xylocarpus moluccensis | Influenza A virus (H1N1) | Cytopathic effect inhibition | [128] |
| 6′-O-rhamnosyl-(1‴→6″)-glucopyranosyl asebogenin—Thalassodendrone | Seagrass—Thalassodendron ciliatum | Influenza A virus | Reduce virus toxicity | [124] |
| Triterpenoids | Mangrove—Sonneratia paracaseolaris | Influenza A virus (H1N1) | Cytopathic effect inhibition | [129] |
| polyphenol complex | Seagrass—Zosteraceae | TBE virus | Reduction of virus titer | [130] |
| Compound/Extract | Species | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Metachromin A | Dactylospongia metachromia | HBV | Inhibition of HBV core promoter activity | [149] |
| Polybrominated diphenyl ethers | Dysidea sp. | HBV | Inhibition of HBV core promoter activity | [150] |
| Manoalide | Luffariella variabilis | HCV | Binding to a conserved helicase motif of the NS3 viral protein | [151] |
| Psammaplin A | Psammaplysilla sp., Poecillastra sp. and Jaspis sp. | HCV | Block of viral NS3 RNA helicase and ATPase activities | [152] |
| Aaptamine alkaloids | Aaptos aaptos | HIV-1 | Not specified | [153] |
| Baculiferins C, E–H and K–N | Iotrochota baculifera | HIV-1 | Interaction with Vif, APOBEC3G and gp41 proteins | [154] |
| Bengamide A | Jaspis cf. coriacea | HIV-1 | Interaction with LTR NF-κB response elements | [155] |
| Mirabamides E-H | Stelletta clavosa | HIV-1 | Not specified | [156] |
| Stellettapeptins A-B | Stelletta sp. | HIV-1 | Not specified | [157] |
| Manzamine A | Haliclona and Acanthostrongylophora genera | HSV-1 | Not specified | [158] |
| TSH fraction, halistanol sulfate and halistanol sulfate C | Petromica citrina | HSV-1 | Inhibition of viral attachment/penetration and reduction of ICP27 and gD levels | [159] |
| Pateamine A | Mycale sp. | SINV | Block of viral mRNA translation by targeting eIF4A complex | [160] |
| Nortopsentins | Spongosorites ruetzleri | TMV | Not specified | [161] |
| Compound/Extrasct | Species | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| RvH and the functional units RvH1-a/RvH2-e | Rapana venosa | EBV | Not specified | [162,163] |
| Three hemocyanin fractions | Haliotis rubra | HIV-1 | Binding to the viral surface through gD, gB, and gC glycoproteins | [164] |
| Cavortins | Crassotea gigas | HSV-1 | Not specified | [165] |
| Hemolymph | Haliotis laevigata | HSV-1 | Not specified | [166] |
| Myticin C, modified and nanoencapsulated | Mytilus galloprovincialis | OsHV-1, HSV-1/HSV-2 | Not specified | [167] |
| Hemolymph and Myticin C | Mytilus galloprovincialis | VHSV | Not specified | [168] |
| Compound/Extract | Species | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Norcembranoids and sesquiterpenoid | Sinularia kavarattiensis | CHIKV | Not specified | [169] |
| Briacavatolides C-F | Briareum excavatum | HCMV | Not specified | [170,171] |
| Durumolide J | Lobophytum durum | HCMV | Not specified | [172] |
| Ehrenbergol C and acetyl ehrenberoxide B | Sarcophyton ehrenbergi | HCMV | Not specified | [173] |
| Gyrosanols A and B | Sinularia gyrosa | HCMV | Not specified | [174] |
| Hipposterone N | Isis hippuris | HCMV | Not specified | [175] |
| Secocembranoid | Lobophytum crassum | HCMV | Not specified | [176] |
| Zoanthoxanthins | Echinogorgia pseudossapo | HSV-1 | Not specified | [177] |
| Polyhydroxylated sterol and ceramide derivatives | Sinularia candidula | Influenza A virus (H5N1) | Not specified | [178] |
| Polyhydroxylated steroids | Sarcophyton sp. | Influenza A virus (H1N1) | Not specified | [179] |
| Echrebsteroids A–C | Echinogorgia rebekka | RSV | Not specified | [180] |
| Compound/Extract | Species | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Chitosan | Several crustacean species | MS2/phi X174 phages and FCV-F9 | Not specified | [181] |
| Crustin, Sp-Crus6 | Scylla paramamosain | WSSV | Not specified | [182] |
| Hemocyanin, LvHcL48 | Litopenaeus vannamei | WSSV | Interaction to the viral envelope protein VP28 | [183] |
| Hemocyte proteins, Sp-ALFs | Scylla paramamosain | WSSV | Not specified | [184] |
| Peroxinectin analog, Sp-PX | Scylla paramamosain | WSSV | Not specified | [185] |
| Scygonadin | Scylla paramamosain | WSSV | Not specified | [186] |
| SWD, LvSWD3 | Litopenaeus vannamei | WSSV | Not specified | [187] |
| β-thymosin-repeat proteins | Marsupenaeus japonicus | WSSV | Not specified | [188] |
| Compound/Extract | Species | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Acidic mucopolysaccharide, SJAMP | Stichopus japonicus | HBV | Not specified | [189] |
| AP-PLA2 from crude venom | Acanthaster planci | HIV-1 | Not specified | [190] |
| Comaparvin | Capillaster multiradiatus | HIV-1 | Not specified | [191] |
| Seven hydrolysates | Cucumaria frondosa | HSV-1 | Not specified | [192] |
| Sulfated sterols | Echinoderms from cold waters | HSV-1, HSV-2 and PrV | Not specified | [193] |
| Compound/Extract | Species | Which Virus? | Mechanism of Action | Reference |
|---|---|---|---|---|
| Prunolide A and Cadiolide B | Synoicum prunum and Botryllus sp. | JEV | Not specified | [194] |
| Mollamide F, Molleurea A and Mollamide E | Didemnum molle | HIV-1 | Inhibition of viral replication and HIV-1 integrase | [195] |
| Eudistomin C | Ritterella sigillinoides | HSV-1 and IPV-1 | Interaction to the uS11-containing small ribosomal subunit | [196] |
| Polycarpaurines A and C | Polycarpa aurata | TMV | Not specified | [197] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccio, G.; Ruocco, N.; Mutalipassi, M.; Costantini, M.; Zupo, V.; Coppola, D.; de Pascale, D.; Lauritano, C. Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules 2020, 10, 1007. https://doi.org/10.3390/biom10071007
Riccio G, Ruocco N, Mutalipassi M, Costantini M, Zupo V, Coppola D, de Pascale D, Lauritano C. Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules. 2020; 10(7):1007. https://doi.org/10.3390/biom10071007
Chicago/Turabian StyleRiccio, Gennaro, Nadia Ruocco, Mirko Mutalipassi, Maria Costantini, Valerio Zupo, Daniela Coppola, Donatella de Pascale, and Chiara Lauritano. 2020. "Ten-Year Research Update Review: Antiviral Activities from Marine Organisms" Biomolecules 10, no. 7: 1007. https://doi.org/10.3390/biom10071007
APA StyleRiccio, G., Ruocco, N., Mutalipassi, M., Costantini, M., Zupo, V., Coppola, D., de Pascale, D., & Lauritano, C. (2020). Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules, 10(7), 1007. https://doi.org/10.3390/biom10071007












