A Novel Approach to Bacterial Expression and Purification of Myristoylated Forms of Neuronal Calcium Sensor Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antibodies
2.3. Genetic Constructs
2.4. Expression Protocol
2.5. Extraction of Recoverin, NCALD and NCS-1 as Soluble Proteins
2.6. Extraction from Inclusion Bodies as Insoluble Protein and Renaturation of NCS-1, GCAP1 and GCAP2
2.7. Purification of Myristoylated Recoverin, NCALD and NCS-1 by Hydrophobic Chromatography
2.8. Purification of Myristoylated GCAPs by Hydrophobic Chromatography
2.9. HPLC Detection of The Myristoylated Protein Forms
2.10. Mass Spectrometry Analysis
2.11. Fluorescence Measurements
2.12. Analytical Procedures
3. Results
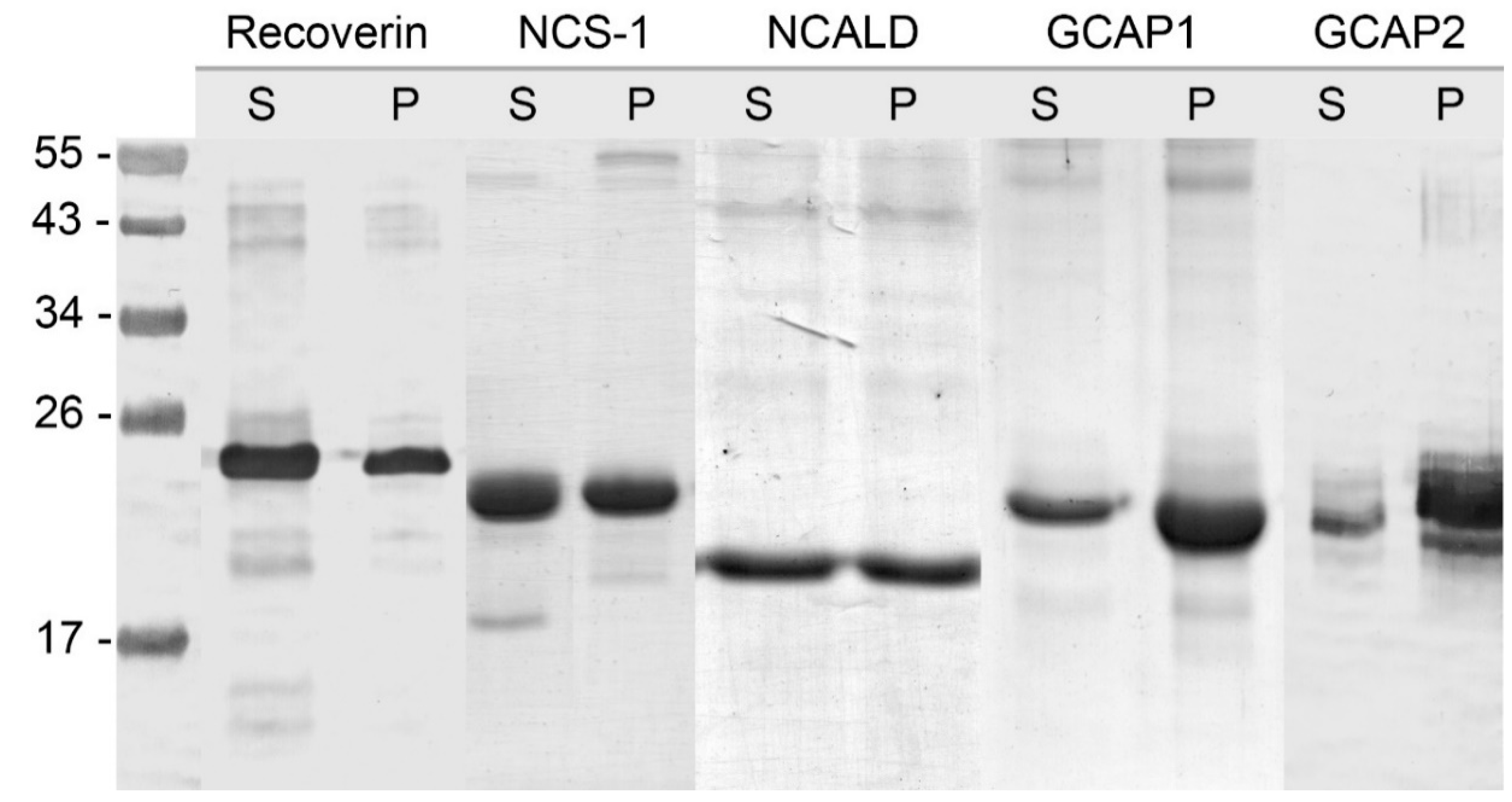
3.1. Analysis of Myristoylation and Solubility of NCS Proteins Expressed in Bacterial Cells
3.2. Optimization of Myristoylation of NCS-1 in Bacterial Cells
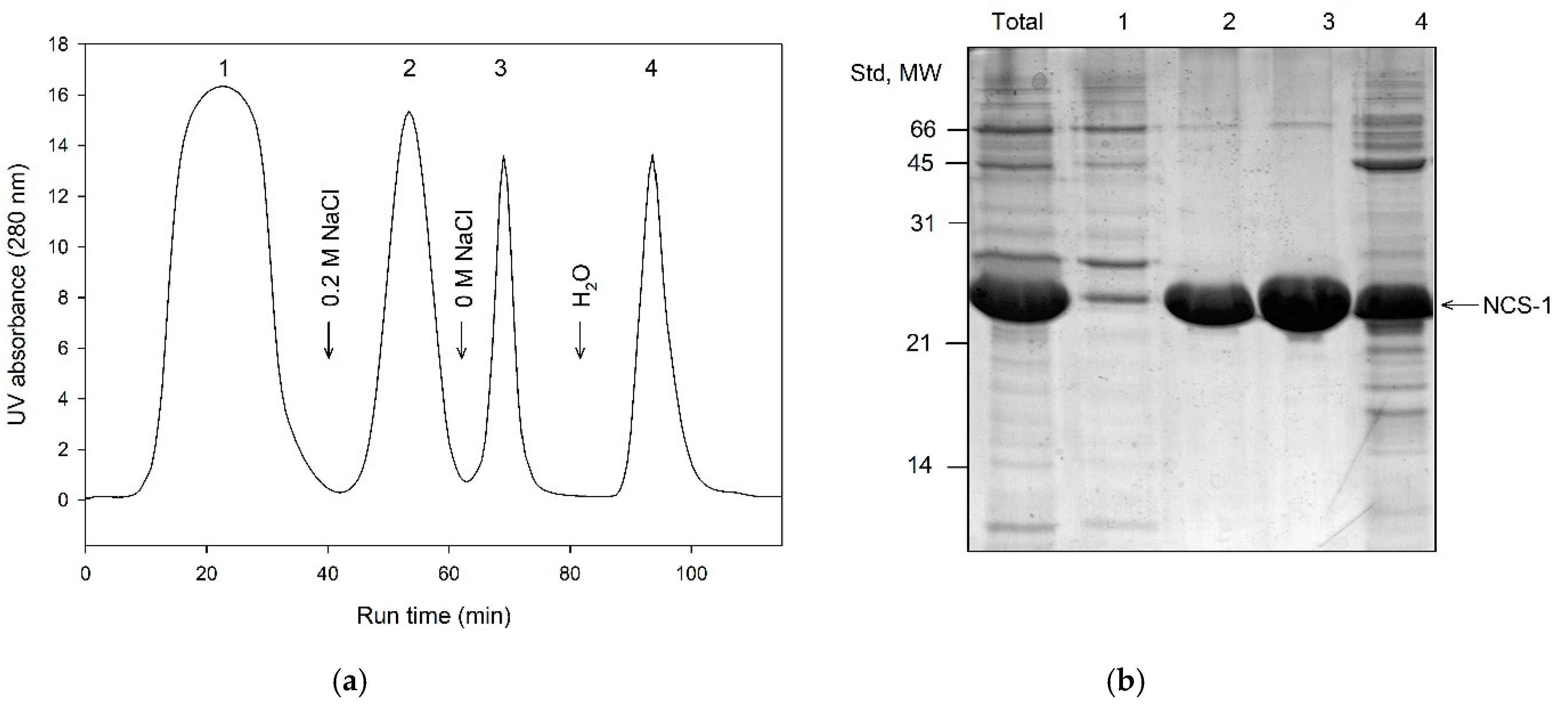
3.3. Primary Purification of Myristoylated Recoverin, NCALD and NCS-1: Phenyl Sepharose Chromatography
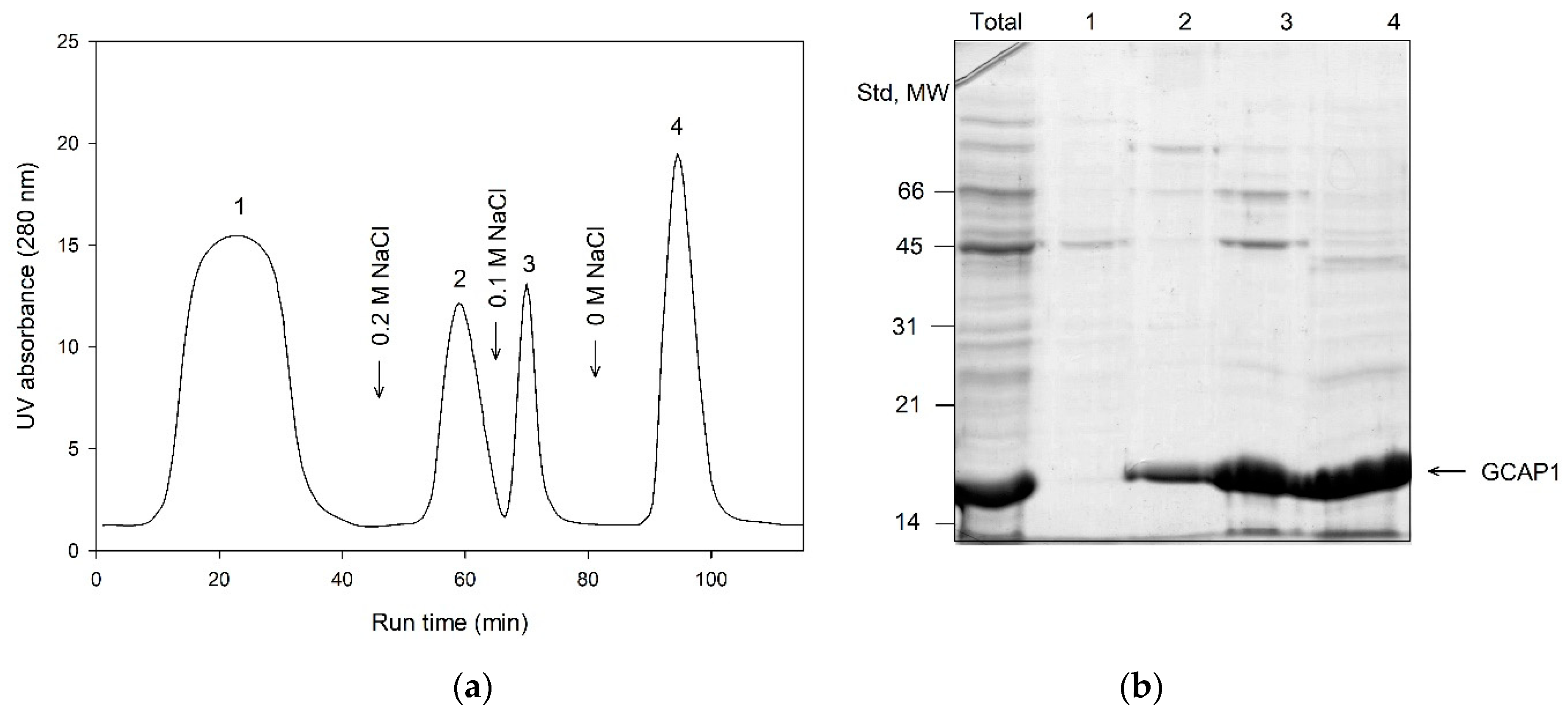
3.4. Primary Purification of Myristoylated GCAP1 and GCAP2: Toyopearl Butyl Chromatography
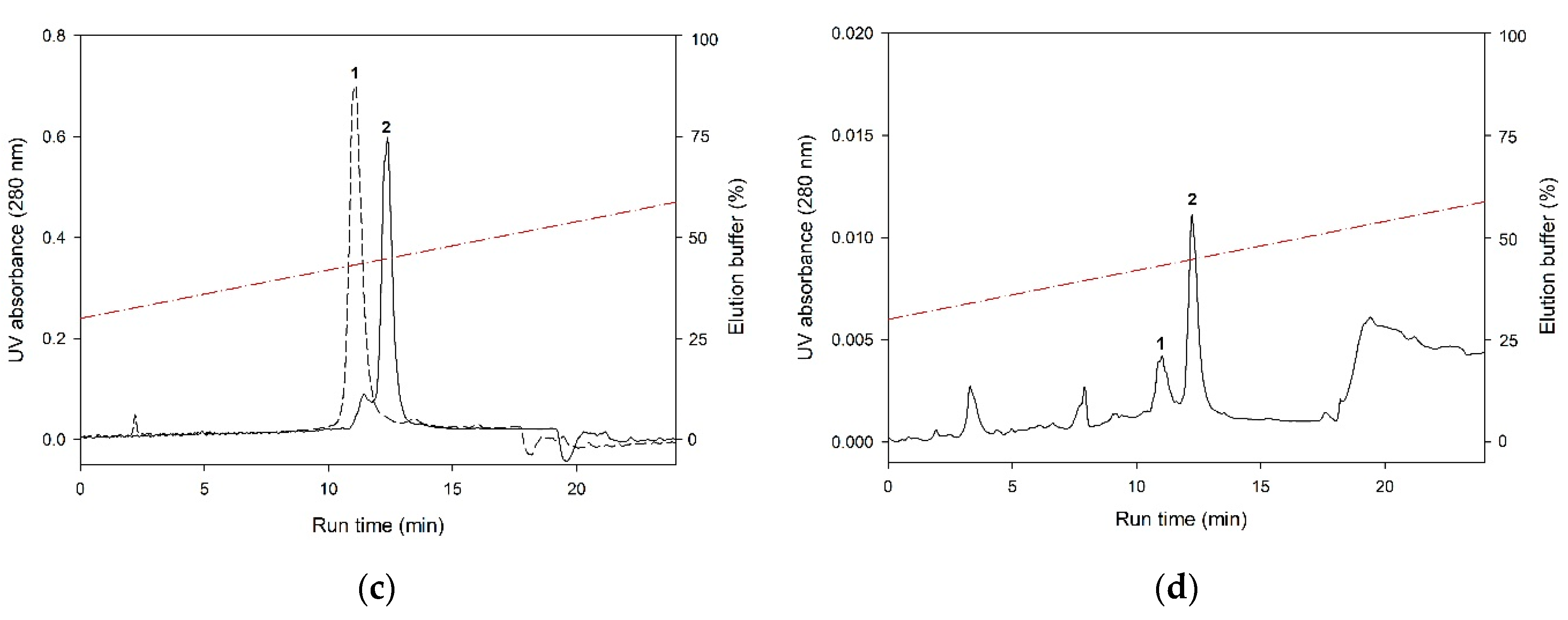
3.5. Final Separation of Myristoylated Forms of NCS Proteins: Butyl Sepharose Chromatography
3.6. Characterization of The Purified Myristoylated NCS Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NCS | neuronal calcium sensor; |
| NCS-1 | neuronal calcium sensor-1; |
| NMT1p | yeast N-myristoyltransferase; |
| HPLC | high performance liquid chromatography; |
| FPLC | fast protein liquid chromatography; |
| GCAP1 | guanylate activating protein 1; |
| GCAP2 | guanylate activating protein 2; |
| NCALD | neurocalcin δ; |
| DTT | 1,4-dithiothreitol; |
| EGTA | ethylene glycol-bis-N,N,N′,N′-tetraacetic acid; |
| Bis-ANS | 4,4’-Dianilino-1,1’-Binaphthyl-5,5’- disulfonic acid, dipotassium salt; |
| LC/ESI-MS | liquid chromatography electrospray ionization tandem mass spectrometric; |
| BCA | bicinchoninic acid assay; |
| TFA | trifluoroacetic acid; |
| ANS | 8-anilino-1-naphthalenesulfonic acid; |
| LB | lysogeny broth; |
| SDS PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis. |
References
- Burgoyne, R.D.; Helassa, N.; McCue, H.V.; Haynes, L.P. Calcium Sensors in Neuronal Function and Dysfunction. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, S. Neuronal calcium sensor-1: A multifunctional regulator of secretion. Biochem. Soc. Trans. 2003, 31, 828–832. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Nakao, S.; Wakabayashi, S. Emerging Roles of Neuronal Ca(2+) Sensor-1 in Cardiac and Neuronal Tissues: A Mini Review. Front. Mol. Neurosci. 2019, 12, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, J.; Neuhauss, S.C.F. The Binding Properties and Physiological Functions of Recoverin. Front. Mol. Neurosci. 2018, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.; Filipek, S.; Palczewski, K.; Sousa, M.C. Ca2+ -dependent regulation of phototransduction. Photochem. Photobiol. 2008, 84, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Braunewell, K.H.; Klein-Szanto, A.J. Visinin-like proteins (VSNLs): Interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+ -sensor proteins. Cell Tissue Res. 2009, 335, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Bähring, R. Kv channel-interacting proteins as neuronal and non-neuronal calcium sensors. Channels 2018, 12, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Dell’Orco, D.; Koch, K.W.; Kreutz, M.R.; Naranjo, J.R.; Schwaller, B. Editorial: Neuronal Calcium Sensors in Health and Disease. Front. Mol. Neurosci. 2019, 12, 278. [Google Scholar] [CrossRef]
- Bazhin, A.V.; Schadendorf, D.; Philippov, P.P.; Eichmüller, S.B. Recoverin as a cancer-retina antigen. Cancer Immunol. Immunother. CII 2007, 56, 110–116. [Google Scholar] [CrossRef]
- Boeckel, G.R.; Ehrlich, B.E. NCS-1 is a regulator of calcium signaling in health and disease. Biochim. Et Biophys. Acta. Mol. Cell Res. 2018, 1865, 1660–1667. [Google Scholar] [CrossRef]
- Groblewska, M.; Muszyński, P.; Wojtulewska-Supron, A.; Kulczyńska-Przybik, A.; Mroczko, B. The Role of Visinin-Like Protein-1 in the Pathophysiology of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2015, 47, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, S.E.; Nazipova, A.A.; Denesyuk, A.I.; Bakunts, A.G.; Zinchenko, D.V.; Lipkin, V.M.; Uversky, V.N.; Permyakov, E.A. Recoverin as a redox-sensitive protein. J. Proteome Res. 2007, 6, 1855–1863. [Google Scholar] [CrossRef]
- Liebl, M.P.; Kaya, A.M.; Tenzer, S.; Mittenzwei, R.; Koziollek-Drechsler, I.; Schild, H.; Moosmann, B.; Behl, C.; Clement, A.M. Dimerization of visinin-like protein 1 is regulated by oxidative stress and calcium and is a pathological hallmark of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2014, 72, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernii, E.Y.; Nazipova, A.A.; Nemashkalova, E.L.; Kazakov, A.S.; Gancharova, O.S.; Serebryakova, M.V.; Tikhomirova, N.K.; Baksheeva, V.E.; Vladimirov, V.I.; Zinchenko, D.V.; et al. Light-Induced Thiol Oxidation of Recoverin Affects Rhodopsin Desensitization. Front. Mol. Neurosci. 2018, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- De Raad, S.; Comte, M.; Nef, P.; Lenz, S.E.; Gundelfinger, E.D.; Cox, J.A. Distribution pattern of three neural calcium-binding proteins (NCS-1, VILIP and recoverin) in chicken, bovine and rat retina. Histochem. J. 1995, 27, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.-G.; Baumann, B.; Danos, P.; Diekmann, S.; Bogerts, B.; Gundelfinger, E.D.; Braunewell, K.H. Regional and cellular distribution of neural visinin-like protein immunoreactivities (VILIP-1 and VILIP-3) in human brain. J. Neurocytol. 1999, 28, 655–662. [Google Scholar] [CrossRef]
- Boekhoff, I.; Braunewell, K.H.; Andreini, I.; Breer, H.; Gundelfinger, E. The calcium-binding protein VILIP in olfactory neurons: Regulation of second messenger signaling. Eur. J. Cell Biol. 1997, 72, 151–158. [Google Scholar]
- Spilker, C.; Richter, K.; Smalla, K.H.; Manahan-Vaughan, D.; Gundelfinger, E.; Braunewell, K.H. The neuronal EF-hand calcium-binding protein visinin-like protein-3 is expressed in cerebellar Purkinje cells and shows a calcium-dependent membrane association. Neuroscience 2000, 96, 121–129. [Google Scholar] [CrossRef]
- Krishnan, A.; Venkataraman, V.; Fik-Rymarkiewicz, E.; Duda, T.; Sharma, R.K. Structural, biochemical, and functional characterization of the calcium sensor neurocalcin δ in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry 2004, 43, 2708–2723. [Google Scholar] [CrossRef]
- Nakano, A.; Terasawa, M.; Watanabe, M.; Okazaki, K.; Inoue, S.; Kato, M.; Nimura, Y.; Usuda, N.; Morita, T.; Hidaka, H. Distinct regional localization of neurocalcin, a Ca2+-binding protein, in the bovine adrenal gland. J. Endocrinol. 1993, 138, 283–NP. [Google Scholar] [CrossRef]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimmer, J.S.; et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef] [PubMed]
- McFerran, B.W.; Graham, M.E.; Burgoyne, R.D. Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J. Biol. Chem. 1998, 273, 22768–22772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.Y.; Jeromin, A.; Mikoshiba, K.; Wakabayashi, S. Neuronal calcium sensor 1 promotes immature heart function and hypertrophy by enhancing Ca2+ signals. Circ. Res. 2011, 109, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martone, M.E.; Edelmann, V.M.; Ellisman, M.H.; Nef, P. Cellular and subcellular distribution of the calcium-binding protein NCS-1 in the central nervous system of the rat. Cell Tissue Res. 1999, 295, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O.; Undie, A.S.; Kabbani, N.; Levenson, R.; Goldman-Rakic, P.S.; Lidow, M.S. Up-regulation of neuronal calcium sensor 1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc. Natl. Acad. Sci. USA 2003, 100, 313–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierke, P.; Zhao, C.; Brackmann, M.; Linke, B.; Heinemann, U.; Braunewell, K.H. Expression analysis of members of the neuronal calcium sensor protein family: Combining bioinformatics and Western blot analysis. Biochem. Biophys. Res. Commun. 2004, 323, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Zernii, E.Y.; Komolov, K.E.; Permyakov, S.E.; Kolpakova, T.; Dell’orco, D.; Poetzsch, A.; Knyazeva, E.L.; Grigoriev, I.I.; Permyakov, E.A.; Senin, I.I.; et al. Involvement of the recoverin C-terminal segment in recognition of the target enzyme rhodopsin kinase. Biochem. J. 2011, 435, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Baksheeva, V.E.; Nazipova, A.A.; Zinchenko, D.V.; Serebryakova, M.V.; Senin, I.I.; Permyakov, S.E.; Philippov, P.P.; Li, Y.; Zamyatnin, A.A.; Zernii, E.Y.; et al. Ca2+ -myristoyl switch in neuronal calcium sensor-1: A role of C-terminal segment. CNS Neurol. Disord. Drug Targets 2015, 14, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Zernii, E.Y.; Grigoriev, I.I.; Nazipova, A.A.; Scholten, A.; Kolpakova, T.V.; Zinchenko, D.V.; Kazakov, A.S.; Senin, I.I.; Permyakov, S.E.; Dell’Orco, D.; et al. Regulatory function of the C-terminal segment of guanylate cyclase-activating protein 2. Biochim. Et Biophys. Acta 2015, 1854, 1325–1337. [Google Scholar] [CrossRef]
- Burgoyne, R.D. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 2007, 8, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Permyakov, S.E.; Cherskaya, A.M.; Wasserman, L.A.; Khokhlova, T.I.; Senin, I.I.; Zargarov, A.A.; Zinchenko, D.V.; Zernii, E.Y.; Lipkin, V.M.; Philippov, P.P.; et al. Recoverin is a zinc-binding protein. J. Proteome Res. 2003, 2, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Roman, A.Y.; Baksheeva, V.E.; Nazipova, A.A.; Shevelyova, M.P.; Vladimirov, V.I.; Buyanova, M.F.; Zinchenko, D.V.; Zamyatnin, A.A., Jr.; Devred, F.; et al. Functional Status of Neuronal Calcium Sensor-1 Is Modulated by Zinc Binding. Front. Mol. Neurosci. 2018, 11, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, D.W.; Burgoyne, R.D. Role of myristoylation in the intracellular targeting of neuronal calcium sensor (NCS) proteins. Biochem. Soc. Trans. 2003, 31, 963–965. [Google Scholar] [CrossRef]
- Martin, D.D.; Beauchamp, E.; Berthiaume, L.G. Post-translational myristoylation: Fat matters in cellular life and death. Biochimie 2011, 93, 18–31. [Google Scholar] [CrossRef]
- Ames, J.B.; Ishima, R.; Tanaka, T.; Gordon, J.I.; Stryer, L.; Ikura, M. Molecular mechanics of calcium-myristoyl switches. Nature 1997, 389, 198–202. [Google Scholar] [CrossRef]
- Dizhoor, A.M.; Chen, C.K.; Olshevskaya, E.; Sinelnikova, V.V.; Phillipov, P.; Hurley, J.B. Role of the acylated amino terminus of recoverin in Ca (2+)-dependent membrane interaction. Science 1993, 259, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Calvez, P.; Schmidt, T.F.; Cantin, L.; Klinker, K.; Salesse, C. Phosphatidylserine allows observation of the calcium–myristoyl switch of recoverin and its preferential binding. J. Am. Chem. Soc. 2016, 138, 13533–13540. [Google Scholar] [CrossRef] [PubMed]
- Valentine, K.G.; Mesleh, M.F.; Opella, S.J.; Ikura, M.; Ames, J.B. Structure, topology, and dynamics of myristoylated recoverin bound to phospholipid bilayers. Biochemistry 2003, 42, 6333–6340. [Google Scholar] [CrossRef]
- Permyakov, S.E.; Zernii, E.Y.; Knyazeva, E.L.; Denesyuk, A.I.; Nazipova, A.A.; Kolpakova, T.V.; Zinchenko, D.V.; Philippov, P.P.; Permyakov, E.A.; Senin, I.I. Oxidation mimicking substitution of conservative cysteine in recoverin suppresses its membrane association. Amino Acids 2012, 42, 1435–1442. [Google Scholar] [CrossRef]
- Vijay-Kumar, S.; Kumar, V.D. Crystal structure of recombinant bovine neurocalcin. Nat. Struct. Biol. 1999, 6, 80–88. [Google Scholar] [CrossRef]
- Spilker, C.; Dresbach, T.; Braunewell, K.H. Reversible translocation and activity-dependent localization of the calcium–myristoyl switch protein VILIP-1 to different membrane compartments in living hippocampal neurons. J. Neurosci. 2002, 22, 7331–7339. [Google Scholar] [CrossRef] [Green Version]
- Spilker, C.; Braunewell, K.H. Calcium–myristoyl switch, subcellular localization, and calcium-dependent translocation of the neuronal calcium sensor protein VILIP-3, and comparison with VILIP-1 in hippocampal neurons. Mol. Cell. Neurosci. 2003, 24, 766–778. [Google Scholar] [CrossRef]
- McFerran, B.W.; Weiss, J.L.; Burgoyne, R.D. Neuronal Ca2+ Sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca2+-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca2+ signal transduction. J. Biol. Chem. 1999, 274, 30258–30265. [Google Scholar] [PubMed] [Green Version]
- O’Callaghan, D.W.; Burgoyne, R.D. Identification of residues that determine the absence of a Ca2+/myristoyl switch in neuronal calcium sensor-1. J. Biol. Chem. 2004, 279, 14347–14354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strahl, T.; Huttner, I.G.; Lusin, J.D.; Osawa, M.; King, D.; Thorner, J.; Ames, J.B. Structural insights into activation of phosphatidylinositol 4-kinase (Pik1) by yeast frequenin (Frq1). J. Biol. Chem. 2007, 282, 30949–30959. [Google Scholar] [CrossRef] [Green Version]
- Baksheeva, V.E.; Nemashkalova, E.L.; Firsov, A.M.; Zalevsky, A.O.; Vladimirov, V.I.; Tikhomirova, N.K.; Philippov, P.P.; Zamyatnin, A.A., Jr.; Zinchenko, D.V.; Antonenko, Y.N.; et al. Membrane Binding of Neuronal Calcium Sensor-1: Highly Specific Interaction with Phosphatidylinositol-3-Phosphate. Biomolecules 2020, 10, 164. [Google Scholar] [CrossRef] [Green Version]
- Peshenko, I.V.; Olshevskaya, E.V.; Lim, S.; Ames, J.B.; Dizhoor, A.M. Calcium-myristoyl Tug is a new mechanism for intramolecular tuning of calcium sensitivity and target enzyme interaction for guanylyl cyclase-activating protein 1: Dynamic connection between N-fatty acyl group and EF-hand controls calcium sensitivity. J. Biol. Chem. 2012, 287, 13972–13984. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.W.; Dell’orco, D. A calcium-relay mechanism in vertebrate phototransduction. ACS Chem. Neurosci. 2013, 4, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Calvert, P.D.; Klenchin, V.A.; Bownds, M.D. Rhodopsin Kinase Inhibition by Recoverin Function of recoverin myristoylation. J. Biol. Chem. 1995, 270, 24127–24129. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Peshenko, I.; Dizhoor, A.; Ames, J.B. Effects of Ca2+, Mg2+, and myristoylation on guanylyl cyclase activating protein 1 structure and stability. Biochemistry 2009, 48, 850–862. [Google Scholar] [CrossRef] [Green Version]
- Muralidhar, D.; Jobby, M.K.; Krishnan, K.; Annapurna, V.; Chary, K.V.; Jeromin, A.; Sharma, Y. Equilibrium Unfolding of Neuronal Calcium Sensor-1. N-terminal myristoylation influences unfolding and reduces protein stiffening in the presence of calcium. J. Biol. Chem. 2005, 280, 15569–15578. [Google Scholar] [CrossRef] [Green Version]
- Jeromin, A.; Muralidhar, D.; Parameswaran, M.N.; Roder, J.; Fairwell, T.; Scarlata, S.; Dowal, L.; Mustafi, S.M.; Chary, K.V.; Sharma, Y. N-terminal myristoylation regulates calcium-induced conformational changes in neuronal calcium sensor-1. J. Biol. Chem. 2004, 279, 27158–27167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, J.; Lim, S.; Ikura, M. Molecular structure and target recognition of neuronal calcium sensor proteins. Front. Mol. Neurosci. 2012, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocque, W.J.; McWherter, C.; Wood, D.; Gordon, J. A comparative analysis of the kinetic mechanism and peptide substrate specificity of human and Saccharomyces cerevisiae myristoyl-CoA: Protein N-myristoyltransferase. J. Biol. Chem. 1993, 268, 9964–9971. [Google Scholar]
- Hwang, J.Y.; Koch, K.W. Calcium-and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry 2002, 41, 13021–13028. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, P.; Penney, S.É.; Salesse, C. Single-step purification of myristoylated and nonmyristoylated recoverin and substrate dependence of myristoylation level. Anal. Biochem. 2006, 349, 25–32. [Google Scholar] [CrossRef]
- Li, C.; Pan, W.; Braunewell, K.H.; Ames, J.B. Structural analysis of Mg2+ and Ca2+ binding, myristoylation, and dimerization of the neuronal calcium sensor and visinin-like protein 1 (VILIP-1). J. Biol. Chem. 2011, 286, 6354–6366. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lim, S.; Braunewell, K.H.; Ames, J.B. Structure and Calcium Binding Properties of a Neuronal Calcium-Myristoyl Switch Protein, Visinin-Like Protein 3. PLoS ONE 2016, 11, e0165921. [Google Scholar] [CrossRef] [Green Version]
- Hoareau, E.; Belley, N.; Klinker, K.; Desbat, B.; Boisselier, É. Characterization of neurocalcin delta membrane binding by biophysical methods. Colloids Surf. B Biointerfaces 2019, 174, 291–299. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Koch, K.W. The myristoylation of the neuronal Ca2+-sensors guanylate cyclase-activating protein 1 and 2. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2002, 1600, 111–117. [Google Scholar] [CrossRef]
- Fisher, J.R.; Sharma, Y.; Iuliano, S.; Piccioti, R.A.; Krylov, D.; Hurley, J.; Roder, J.; Jeromin, A. Purification of myristoylated and nonmyristoylated neuronal calcium sensor-1 using single-step hydrophobic interaction chromatography. Protein Expr. Purif. 2000, 20, 66–72. [Google Scholar] [CrossRef] [PubMed]
- De Cotiis, D.A.; Woll, M.P.; Fox, T.E.; Hill, R.B.; Levenson, R.; Flanagan, J.M. Optimized expression and purification of myristoylated human neuronal calcium sensor 1 in E. coli. Protein Expr. Purif. 2008, 61, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandalaneni, S.; Karuppiah, V.; Saleem, M.; Haynes, L.P.; Burgoyne, R.D.; Mayans, O.; Derrick, J.P.; Lian, L.Y. Neuronal Calcium Sensor-1 Binds the D2 Dopamine Receptor and G-protein-coupled Receptor Kinase 1 (GRK1) Peptides Using Different Modes of Interactions. J. Biol. Chem. 2015, 290, 18744–18756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiergraber, O.H.; Senin, I.I.; Zernii, E.Y.; Churumova, V.A.; Kovaleva, N.A.; Nazipova, A.A.; Permyakov, S.E.; Permyakov, E.A.; Philippov, P.P.; Granzin, J.; et al. Tuning of a neuronal calcium sensor. J. Biol. Chem. 2006, 281, 37594–37602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senin, I.I.; Tikhomirova, N.K.; Churumova, V.A.; Grigoriev, I.I.; Kolpakova, T.A.; Zinchenko, D.V.; Philippov, P.P.; Zernii, E.Y. Amino acid sequences of two immune-dominant epitopes of recoverin are involved in Ca2+/recoverin-dependent inhibition of phosphorylation of rhodopsin. Biochem. Biokhimiia 2011, 76, 332–338. [Google Scholar] [CrossRef]
- Vladimirov, V.I.; Zernii, E.Y.; Baksheeva, V.E.; Wimberg, H.; Kazakov, A.S.; Tikhomirova, N.K.; Nemashkalova, E.L.; Mitkevich, V.A.; Zamyatnin, A.A., Jr.; Lipkin, V.M.; et al. Photoreceptor calcium sensor proteins in detergent-resistant membrane rafts are regulated via binding to caveolin-1. Cell Calcium 2018, 73, 55–69. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Tikhomirova, N.K.; Philippov, P.P.; Senin, I.I. Detection of annexin IV in bovine retinal rods. Biochem. Biokhimiia 2003, 68, 129–160. [Google Scholar] [CrossRef]
- Senin, I.I.; Zargarov, A.A.; Alekseev, A.M.; Gorodovikova, E.N.; Lipkin, V.M.; Philippov, P.P. N-myristoylation of recoverin enhances its efficiency as an inhibitor of rhodopsin kinase. FEBS Lett. 1995, 376, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Duronio, R.J.; Olins, P.O.; Gordon, J.I. Method for protein N-myristoylation. U.S. Patent 5,504,008, 2 April 1996. [Google Scholar]
- Burstein, E.A.; Emelyanenko, V.I. Log-normal description of fluorescence spectra of organic fluorophores. Photochem. Photobiol. 1996, 64, 316–320. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernii, E.Y.; Nazipova, A.A.; Gancharova, O.S.; Kazakov, A.S.; Serebryakova, M.V.; Zinchenko, D.V.; Tikhomirova, N.K.; Senin, I.I.; Philippov, P.P.; Permyakov, E.A.; et al. Light-induced disulfide dimerization of recoverin under ex vivo and in vivo conditions. Free Radic. Biol. Med. 2015, 83, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lange, C.; Helten, A.; Hoppner-Heitmann, D.; Duda, T.; Sharma, R.K.; Koch, K.W. Regulatory modes of rod outer segment membrane guanylate cyclase differ in catalytic efficiency and Ca(2+)-sensitivity. Eur. J. Biochem. 2003, 270, 3814–3821. [Google Scholar] [CrossRef] [PubMed]
- Ladant, D. Calcium and membrane binding properties of bovine neurocalcin expressed in Escherichia coli. J. Biol. Chem. 1995, 270, 3179–3185. [Google Scholar] [PubMed]
- Ames, B.; Ikura, M. Structure and membrane-targeting mechanism of retinal Ca 2+-binding proteins, recoverin and GCAP-2. In Photoreceptors and Calcium; Springer: Boston, MA, USA, 2020; pp. 333–348. [Google Scholar]
- Lim, S.; Dizhoor, A.; Ames, J. Structural diversity of neuronal calcium sensor proteins and insights for activation of retinal guanylyl cyclase by GCAP1. Front. Mol. Neurosci. 2014, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Aravind, P.; Chandra, K.; Reddy, P.P.; Jeromin, A.; Chary, K.V.; Sharma, Y. Regulatory and structural EF-hand motifs of neuronal calcium sensor-1: Mg 2+ modulates Ca 2+ binding, Ca 2+ -induced conformational changes, and equilibrium unfolding transitions. J. Mol. Biol. 2008, 376, 1100–1115. [Google Scholar] [CrossRef]
- Olshevskaya, E.V.; Hughes, R.E.; Hurley, J.B.; Dizhoor, A.M. Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J. Biol. Chem. 1997, 272, 14327–14333. [Google Scholar] [CrossRef] [Green Version]
- Dell’Orco, D.; Behnen, P.; Linse, S.; Koch, K.-W. Calcium binding, structural stability and guanylate cyclase activation in GCAP1 variants associated with human cone dystrophy. Cell. Mol. Life Sci. 2010, 67, 973–984. [Google Scholar] [CrossRef]
- Wang, B.; Boeckel, G.R.; Huynh, L.; Nguyen, L.; Cao, W.; De La Cruz, E.M.; Kaftan, E.J.; Ehrlich, B.E. Neuronal Calcium Sensor 1 Has Two Variants with Distinct Calcium Binding Characteristics. PLoS ONE 2016, 11, e0161414. [Google Scholar] [CrossRef]
- Zozulya, S.; Ladant, D.; Stryer, L. Expression and characterization of calcium-myristoyl switch proteins. In Methods in enzymology; Academic Press: New York, NY, USA, 1995; Volume 250, pp. 383–393. [Google Scholar]
- Burgoyne, R.D.; Weiss, J.L. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 2001, 353, 1–12. [Google Scholar] [CrossRef]
- Hughes, R.E.; Brzovic, P.S.; Klevit, R.E.; Hurley, J.B. Calcium-dependent solvation of the myristoyl group of recoverin. Biochemistry 1995, 34, 11410–11416. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, W.A.; Kobialka, M.; Kuropatwa, M.; Kurowska, E. Ca2+ differently affects hydrophobic properties of guanylyl cyclase-activating proteins (GCAPs) and recoverin. Acta Biochim. Pol. 2003, 50, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peshenko, I.V.; Dizhoor, A.M. Ca2+ and Mg2+ binding properties of GCAP-1. Evidence that Mg2+-bound form is the physiological activator of photoreceptor guanylyl cyclase. J. Biol. Chem. 2006, 281, 23830–23841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.A.; Durussel, I.; Comte, M.; Nef, S.; Nef, P.; Lenz, S.E.; Gundelfinger, E.D. Cation binding and conformational changes in VILIP and NCS-1, two neuron-specific calcium-binding proteins. J. Biol. Chem. 1994, 269, 32807–32813. [Google Scholar]
- Handley, M.T.; Lian, L.Y.; Haynes, L.P.; Burgoyne, R.D. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS ONE 2010, 5, e10534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidarsson, P.O.; Bjerrum-Bohr, I.J.; Jensen, G.A.; Pongs, O.; Finn, B.E.; Poulsen, F.M.; Kragelund, B.B. The C-terminal tail of human neuronal calcium sensor 1 regulates the conformational stability of the Ca2+-activated state. J. Mol. Biol. 2012, 417, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Stroh, S.; Eisenhaber, B.; Eisenhaber, F. N-terminal N-myristoylation of proteins: Refinement of the sequence motif and its taxon-specific differences. J. Mol. Biol. 2002, 317, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Zilberstein, D.; Agmon, V.; Schuldiner, S.; Padan, E. Escherichia coli intracellular pH, membrane potential, and cell growth. J. Bacteriol. 1984, 158, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, H.; Kobayashi, H. Bacterial responses to alkaline stress. Sci. Prog. 2003, 86, 271–282. [Google Scholar] [CrossRef]
- Towler, D.A.; Adams, S.P.; Eubanks, S.R.; Towery, D.S.; Jackson-Machelski, E.; Glaser, L.; Gordon, J.I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc. Natl. Acad. Sci. USA 1987, 84, 2708–2712. [Google Scholar] [CrossRef] [Green Version]
- Thinon, E.; Serwa, R.A.; Broncel, M.; Brannigan, J.A.; Brassat, U.; Wright, M.H.; Heal, W.P.; Wilkinson, A.J.; Mann, D.J.; Tate, E.W. Global profiling of co-and post-translationally N-myristoylated proteomes in human cells. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]


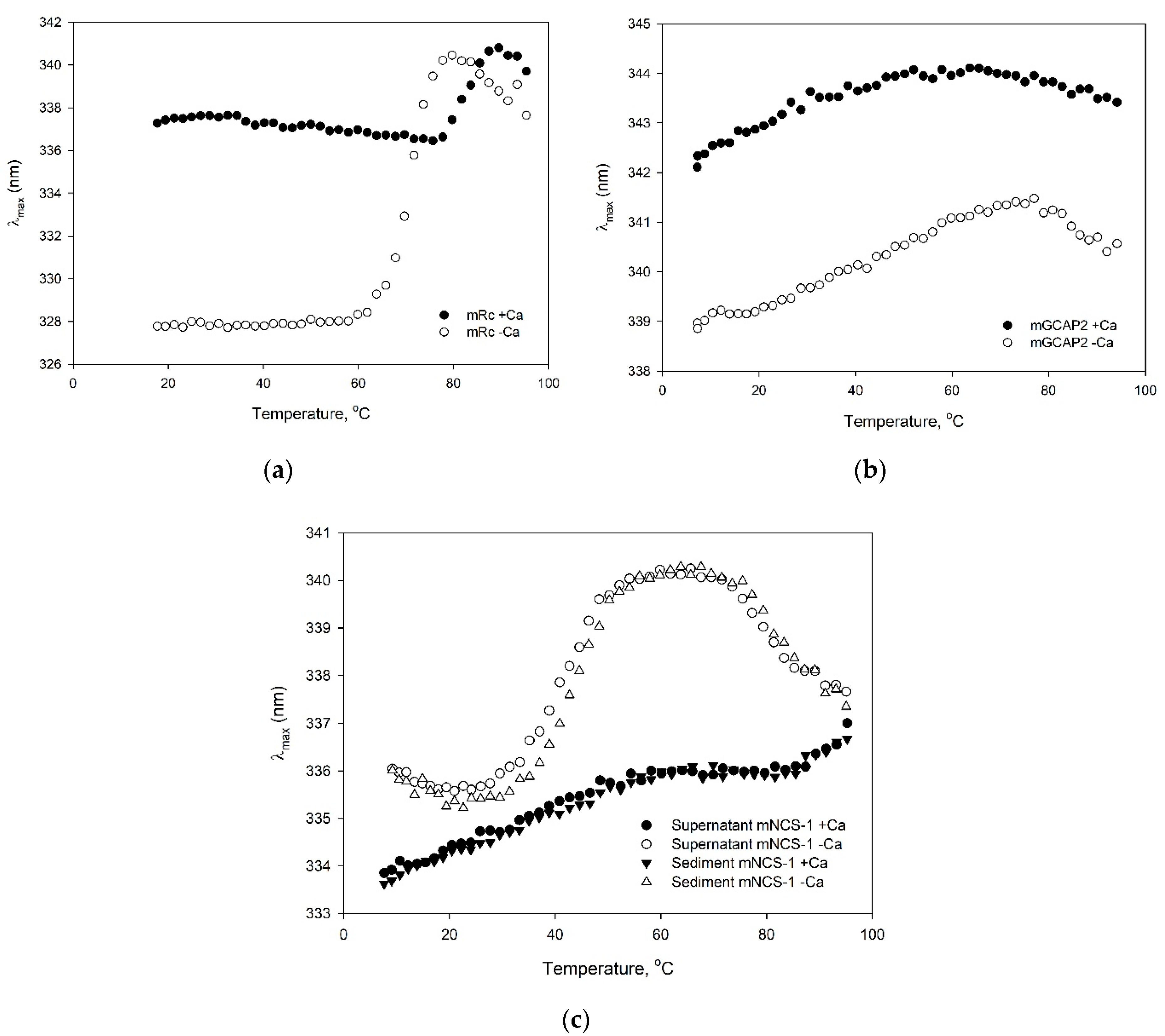

| Protein | Expression | Primary Purification | Final Separation | Molecular Weight, kDa ± SD * | Mid-Transition Temperature, °C ** | ||
|---|---|---|---|---|---|---|---|
| Supernatant | Pellet | ||||||
| Recoverin | Content, mg | 110 | 30 | 70 | 65 | 23411.0 ± 1.7 | 66.5 |
| Purity, % | 70 | 30 | 96 | 98 | |||
| Degree of myristoylation, % | 80–90 | 90 | 90–95 | 99 | |||
| NCALD | Content, mg | 90 | 25 | 60 | 50 | - | - |
| Purity, % | 65 | 35 | 97 | 99 | |||
| Degree of myristoylation, % | 90 | 90 | 97 | 99 | |||
| GCAP1 | Content, mg | 25 | 50 | 15 | 10 | - | - |
| Purity, % | 50 | 65 | 85 | 95 | |||
| Degree of myristoylation, % | 0 | 80 | 75–80 | 98 | |||
| GCAP2 | Content, mg | 15 | 35 | 12 | 10 | - | no cooperative transition |
| Purity, % | 0 | 60 | 85 | 94 | |||
| Degree of myristoylation, % | 0 | 80 | 75–80 | 96 | |||
| NCS-1 | Content, mg | 80 | 60 | 42 | 15 | 21957.0 ± 1.4 | 43.6 |
| Purity, % | 70 | 50 | 80 | 97 | |||
| Degree of myristoylation, % | 20 | 80 | 40–60 | 98 | |||
| Protein | Degree of Myristoylation, % | |||||
|---|---|---|---|---|---|---|
| Loading Fraction | 400 mM NaCl | 300 mM NaCl | 200 mM NaCl | 100 mM NaCl | 0 mM NaCl | |
| Recoverin | 96 | - | - | 10 | 50 | 99 |
| NCALD | 97 | - | - | 10 | 50 | 99 |
| NCS-1 | 50 | 10 | 30 | 70 | 85 | 96 |
| GCAP-1 | 75 | - | 2 | 15 | 85 | 98 |
| GCAP-2 | 80 | - | 5 | 20 | 90 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladimirov, V.I.; Baksheeva, V.E.; Mikhailova, I.V.; Ismailov, R.G.; Litus, E.A.; Tikhomirova, N.K.; Nazipova, A.A.; Permyakov, S.E.; Zernii, E.Y.; Zinchenko, D.V. A Novel Approach to Bacterial Expression and Purification of Myristoylated Forms of Neuronal Calcium Sensor Proteins. Biomolecules 2020, 10, 1025. https://doi.org/10.3390/biom10071025
Vladimirov VI, Baksheeva VE, Mikhailova IV, Ismailov RG, Litus EA, Tikhomirova NK, Nazipova AA, Permyakov SE, Zernii EY, Zinchenko DV. A Novel Approach to Bacterial Expression and Purification of Myristoylated Forms of Neuronal Calcium Sensor Proteins. Biomolecules. 2020; 10(7):1025. https://doi.org/10.3390/biom10071025
Chicago/Turabian StyleVladimirov, Vasiliy I., Viktoriia E. Baksheeva, Irina V. Mikhailova, Ramis G. Ismailov, Ekaterina A. Litus, Natalia K. Tikhomirova, Aliya A. Nazipova, Sergei E. Permyakov, Evgeni Yu. Zernii, and Dmitry V. Zinchenko. 2020. "A Novel Approach to Bacterial Expression and Purification of Myristoylated Forms of Neuronal Calcium Sensor Proteins" Biomolecules 10, no. 7: 1025. https://doi.org/10.3390/biom10071025






