Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors †
Abstract
:1. Introduction
2. Materials and Methods
2.1. ASC Culture
2.2. Hydrogel Synthesis
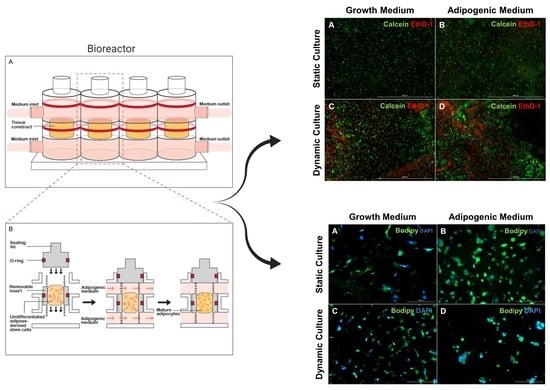
2.3. Cell Encapsulation and Bioreactor Set-Up
2.4. Construct Culture and Adipogenesis
2.5. Live/Dead Staining
2.6. Alamar Blue Staining
2.7. Neutral Lipid Staining
2.8. Quantitative Polymerase Chain Reaction (qPCR)
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
3.1. Cell Viability and Morphology
3.2. Increase in Lipid Accumulation in Cultures Maintained in Adipogenic Medium
3.3. Confirmation of Adipogenic Differentiation Based on Lineage-Specific Gene Expression
3.4. Production of Adipokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal Interact. 2006, 6, 376–378. [Google Scholar] [PubMed]
- Mattar, P.; Bieback, K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth- Associated Tissue Mesenchymal Stromal Cells. Front. Immunol. 2015, 6, 560. [Google Scholar] [CrossRef] [Green Version]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526. [Google Scholar] [CrossRef] [PubMed]
- Guillen, M.I.; Platas, J.; Del Caz, M.D.P.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Stannus, O.P.; Jones, G.; Quinn, S.J.; Cicuttini, F.M.; Dore, D.; Ding, C. The association between leptin, interleukin-6, and hip radiographic osteoarthritis in older people: A cross-sectional study. Arthritis Res. Ther. 2010, 12, R95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003, 48, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Juslin, S.; Nieminen, R.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res. Ther. 2011, 13, R184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, W.; Litherland, G.J.; Elias, M.S.; Kitson, G.I.; Cawston, T.E.; Rowan, A.D.; Young, D.A. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann. Rheum. Dis. 2012, 71, 455. [Google Scholar] [CrossRef] [PubMed]
- De Boer, T.N.; Van Spil, W.E.; Huisman, A.M.; Polak, A.A.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; Mastbergen, S.C. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr. Cartil. 2012, 20, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Krasnokutsky, S.; Belitskaya-Lévy, I.; Bencardino, J.; Samuels, J.; Attur, M.; Regatte, R.; Rosenthal, P.; Greenberg, J.; Schweitzer, M.; Abramson, S.B.; et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011, 63, 2983–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, I.H.; Burmester, G.R.; Backhaus, M.; Althoff, C.E.; Hermann, K.G.; Scheel, A.K.; Werner, C.; Knetsch, T.; Schoenharting, M. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann. Rheum. Dis. 2008, 67, 19. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.; Sokolove, J.; Sharpe, O.; Erhart, J.; Chandra, P.; Lahey, L.; Lindstrom, T.; Hwang, I.; Boyer, K.; Andriacchi, T.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festa, A.; D’Agostino, R., Jr.; Williams, K.; Karter, A.J.; Mayer-Davis, E.J.; Tracy, R.P.; Haffner, S.M. The relation of body fat mass and distribution to markers of chronic inflammation. Int. J. Obes. 2001, 25, 1407. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Dahaghin, S.; Bierma-Zeinstra, S.M.A.; Koes, B.W.; Hazes, J.M.W.; Pols, H.A.P. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann. Rheum. Dis. 2007, 66, 916. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, E.; Nelissen, R.G.; Ioan-Facsinay, A.; Stojanovic-Susulic, V.; Degroot, J.; Van Osch, G.; Middeldorp, S.; Huizinga, T.W.J.; Kloppenburg, M. Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann. Rheum. Dis. 2010, 69, 761. [Google Scholar] [CrossRef] [Green Version]
- Ballegaard, C.; Riis, R.G.C.; Bliddal, H.; Christensen, R.; Henriksen, M.; Bartels, E.M.; Lohmander, L.S.; Hunter, D.J.; Bouert, R.; Boesen, M. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: A cross-sectional study. Osteoarthr. Cartil. 2014, 22, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Gao, Y.-H.; Dong, N.; Zhao, C.-W.; Huang, Y.-F.; Liu, J.-G.; Qi, X. Differential expression of adipokines in the synovium and infrapatellar fat pad of osteoarthritis patients with and without metabolic syndrome. Connect. Tissue Res. 2019, 60, 611–618. [Google Scholar] [CrossRef]
- Strong, A.; Gimble, J.; Bunnell, B. Analysis of the Pro- and Anti-Inflammatory Cytokines Secreted by Adult Stem Cells during Differentiation. Stem Cells Int. 2015. [Google Scholar] [CrossRef] [Green Version]
- Chiellini, C. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol. Biol. 2008, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-Regulation of C/EBPα and PPARγ Controls the Transcriptional Pathway of Adipogenesis and Insulin Sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.-S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halvorsen, Y.-D.C.; Bond, A.; Sen, A.; Franklin, D.M.; Lea-Currie, Y.R.; Sujkowski, D.; Ellis, P.N.; Wilkison, W.O.; Gimble, J.M. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: Biochemical, cellular, and molecular analysis. Metabolism 2001, 50, 407–413. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Elabd, C.; Delteil, F.; Astier, J.; Vernochet, C.; Saint-Marc, P.; Guesnet, J.; Guezennec, A.; Amri, E.-Z.; Dani, C.; et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem. Biophys. Res. Commun. 2004, 315, 255–263. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Chang, W. Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells From the Infrapatellar Fat Pad. Am. J. Sports Med. 2017. [Google Scholar] [CrossRef]
- Delany, J.P.; Floyd, Z.E.; Zvonic, S.; Smith, A.; Gravois, A.; Reiners, E.; Wu, X.; Kilroy, G.; Lefevre, M.; Gimble, J.M. Proteomic analysis of primary cultures of human adipose-derived stem cells: Modulation by Adipogenesis. Mol. Cell. Proteom. MCP 2005, 4, 731. [Google Scholar] [CrossRef] [Green Version]
- Tangchitphisut, P.; Srikaew, N.; Numhom, S.; Tangprasittipap, A.; Woratanarat, P.; Wongsak, S.; Kijkunasathian, C.; Hongeng, S.; Murray, I.R.; Tawonsawatruk, T. Infrapatellar Fat Pad: An Alternative Source of Adipose-Derived Mesenchymal Stem Cells. Arthritis 2016. [Google Scholar] [CrossRef] [Green Version]
- Lopa, S.; Colombini, A.; De Girolamo, L.; Sansone, V.; Moretti, M. New Strategies in Cartilage Tissue Engineering for Osteoarthritic Patients: Infrapatellar Fat Pad as an Alternative Source of Progenitor Cells. J. Biomater. Tissue Eng. 2011, 1, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Lopa, S.; Colombini, A.; Stanco, D.; De Girolamo, L.; Sansone, V.; Moretti, M. Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment. Eur. Cells Mater. 2014, 27, 298–311. [Google Scholar]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Lin, H.; Lozito, T.P.; Alexander, P.G.; Gottardi, R.; Tuan, R.S. Stem Cell-Based Microphysiological Osteochondral System to Model Tissue Response to Interleukin-1β. Mol. Pharm. 2014, 11, 2203–2212. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Li, Z.; Li, E.N.; Li, X.; Del Duke, C.J.; Shen, H.; Hao, T.; O’Donnell, B.; Bunnell, B.A.; Goodman, S.B.; et al. Osteochondral Tissue Chip Derived From iPSCs: Modeling OA Pathologies and Testing Drugs. Front. Bioeng. Biotechnol. 2019, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Cheng, A.W.-M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part A 2014, 20, 2402. [Google Scholar] [CrossRef] [Green Version]
- Aldridge, A.; Kouroupis, D.; Churchman, S.; English, A.; Ingham, E.; Jones, E. Assay validation for the assessment of adipogenesis of multipotential stromal cells—A direct comparison of four different methods. Cytotherapy 2013, 15, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Bucher, N.L.; Farmer, S.R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell. Biol. 1996, 16, 4128. [Google Scholar] [CrossRef] [Green Version]
- Satish, L.; Krill-Burger, J.M.; Gallo, P.H.; Etages, S.D.; Liu, F.; Philips, B.J.; Ravuri, S.; Marra, K.G.; LaFramboise, W.A.; Kathju, S.; et al. Expression analysis of human adipose-derived stem cells during in vitro differentiation to an adipocyte lineage. BMC Med Genom. 2015, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Flynn, L.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials 2007, 28, 3834–3842. [Google Scholar] [CrossRef]
- Stacey, D.; Hanson, S.; Lahvis, G.; Gutowski, K.; Masters, K. In vitro Adipogenic Differentiation of Preadipocytes Varies with Differentiation Stimulus, Culture Dimensionality, and Scaffold Composition. Tissue Eng. Part A 2009, 15, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Parameswaran, V.; Cicuttini, F.; Burgess, J.; Zhai, G.; Quinn, S.; Jones, G. Association between leptin, body composition, sex and knee cartilage morphology in older adults: The Tasmanian older adult cohort (TASOAC) study. Ann. Rheum. Dis. 2008, 67, 1256. [Google Scholar] [CrossRef]
- Grad, S.; Eglin, D.; Alini, M.; Stoddart, M. Physical Stimulation of Chondrogenic Cells In Vitro: A Review. Clin. Orthop. Relat. Res.® 2011, 469, 2764–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothrauff, B.B.; Sasaki, H.; Kihara, S.; Overholt, K.J.; Gottardi, R.; Lin, H.; Fu, F.H.; Tuan, R.S.; Alexander, P.G. Point-of-Care Procedure for Enhancement of Meniscal Healing in a Goat Model Utilizing Infrapatellar Fat Pad–Derived Stromal Vascular Fraction Cells Seeded in Photocrosslinkable Hydrogel. Am. J. Sports Med. 2019, 47, 3396–3405. [Google Scholar] [CrossRef]
- Kouroupis, D. CD10/Neprilysin Enrichment in Infrapatellar Fat Pad–Derived Mesenchymal Stem Cells Under Regulatory-Compliant Conditions: Implications for Efficient Synovitis and Fat Pad Fibrosis Reversal. Am. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Willman, M.A.; Perucca Orfei, C.; Colombini, A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar fat pad-derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation. Sci. Rep. 2019, 9, 10864. [Google Scholar] [CrossRef] [Green Version]
- Bohnsack, M.; Meier, F.; Walter, G.; Hurschler, C.; Schmolke, S.; Wirth, C.; Rühmann, O. Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: A neurohistological approach to anterior knee pain syndrome. Arch. Orthop. Trauma Surg. 2005, 125, 592–597. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, Z.; Yao, Y.; Wei, Z.R.; Wang, D.; Deng, C. Diabetic Human Adipose-Derived Stem Cells Accelerate Pressure Ulcer Healing by Inducing Angiogenesis and Neurogenesis. Stem Cells Dev. 2019, 28, 319. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, D.; Liu, Z.; Jin, W.; Huang, G.; Wei, Z.; Wang, D.; Deng, C. Diabetes-induced glucolipotoxicity imparis wound healing ability of adipose-derived stem cells-through the miR-1248/CITED2/HIF-1 alpha pathway. Aging Us 2020, 12, 6947–6966. [Google Scholar]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein-Wieringa, I.R.; De Lange-Brokaar, B.J.E.; Yusuf, E.; Andersen, S.N.; Kwekkeboom, J.C.; Kroon, H.M.; Van Osch, G.J.V.M.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Nelissen, R.G.H.H.; et al. Inflammatory Cells in Patients with Endstage Knee Osteoarthritis: A Comparison between the Synovium and the Infrapatellar Fat Pad. J. Rheumatol. 2016, 43, 771. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, T.; Chano, T.; Inoue, K.; Matsusue, Y. Cytokine production in the infrapatellar fat pad: Another source of cytokines in knee synovial fluids. Ann. Rheum. Dis. 2003, 62, 108. [Google Scholar] [CrossRef] [Green Version]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Feijt, C.; De Clerck, L.; Verhaar, J.A.N.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Somville, J.; Kloppenburg, M.; Van Osch, G.J.V.M. Cytokine production by infrapatellar fat pad can be stimulated by interleukin 1β and inhibited by peroxisome proliferator activated receptor α agonist. Ann. Rheum. Dis. 2012, 71, 1012. [Google Scholar] [CrossRef] [PubMed]




| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| PPARγ | AGGCGAGGGCGATCTTG | CCCATCATTAAGGAATTCATGTCATA |
| ADIPOQ | AACATGCCCATTCGCTTTAC | AGAGGCTGACCTTCACATCC |
| LEP | GAAGACCACATCCACACACG | AGCTCAGCCAGACCCATCTA |
| AGCACCATAACCTTAGATGGGG | CGTGGAAGTGACGCCTTTCA | |
| PL1N | ACAAGTTCAGTGAGGTAG | CCTTGGTTGAGGAGACAG |
| LPL | GAGATTTCTCTGTATGGCACTG | CTGCAAATGAGACACTTTCTC |
| Genes | Monolayer | Static | Dynamic | |||
|---|---|---|---|---|---|---|
| Growth Medium | Adipogenic Medium | Growth Medium | Adipogenic Medium | Growth Medium | Adipogenic Medium | |
| PPAR-γ | 1.06 ± 3.8 × 10−2 | 2.96 × 103 ± 2.90 × 103 | 1.01 ± 1.0 × 10−2 | 18.58 ± 7.83 | 1.01 ± 1.0 × 10−2 | 6.91 × 101 ± 3.19 × 101 |
| APN | 1.09 ± 7.4 × 10−2 | 4.18 × 106 ± 3.62 × 106 | 1.01 ± 1.0 × 10−2 | 4.45 × 104 ± 3.07 × 104 | 1.41 ± 4.0 × 10−1 | 2.44 × 103 ± 1.40 × 103 |
| PL1N | 1.03 ± 4.2 × 10−2 | 7.49 × 103 ± 4.28 × 103 | 1.07 ± 7.0 × 10−2 | 1.67 × 103 ± 1.42 × 103 | 1.03 ± 3.0 × 10−2 | 6.70 × 101 ± 3.3.7 × 101 |
| LPL | 1.48 ± 2.4 × 10−1 | 1.92 × 106 ± 1.86 × 106 | 1.00 ± 2.53 × 10−5 | 4.67 × 103 ± 4.43 × 103 | 1.16 ± 1.5 × 10−1 | 5.16 × 102 ± 1.65 × 102 |
| FABP4 | 1.48 ± 4.3 × 10−1 | 2.94 × 106 ± 2.67 × 106 | 1.02 ± 9.97 × 10−6 | 9.24 × 103 ± 8.97 × 103 | 1.06 ± 5.0 × 10−2 | 9.01 × 103 ± 5.75 × 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O'Donnell, B.T.; Al-Ghadban, S.; Ives, C.J.; L'Ecuyer, M.P.; Monjure, T.A.; Romero-Lopez, M.; Li, Z.; Goodman, S.B.; Lin, H.; Tuan, R.S.; et al. Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors. Biomolecules 2020, 10, 1070. https://doi.org/10.3390/biom10071070
O'Donnell BT, Al-Ghadban S, Ives CJ, L'Ecuyer MP, Monjure TA, Romero-Lopez M, Li Z, Goodman SB, Lin H, Tuan RS, et al. Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors. Biomolecules. 2020; 10(7):1070. https://doi.org/10.3390/biom10071070
Chicago/Turabian StyleO'Donnell, Benjamen T., Sara Al-Ghadban, Clara J. Ives, Michael P. L'Ecuyer, Tia A. Monjure, Monica Romero-Lopez, Zhong Li, Stuart B. Goodman, Hang Lin, Rocky S. Tuan, and et al. 2020. "Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors" Biomolecules 10, no. 7: 1070. https://doi.org/10.3390/biom10071070
APA StyleO'Donnell, B. T., Al-Ghadban, S., Ives, C. J., L'Ecuyer, M. P., Monjure, T. A., Romero-Lopez, M., Li, Z., Goodman, S. B., Lin, H., Tuan, R. S., & Bunnell, B. A. (2020). Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors. Biomolecules, 10(7), 1070. https://doi.org/10.3390/biom10071070