Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis elegans via SOD-3 and DAF-16
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Pulp Preparation
2.2. Sample Preparation
2.3. Chemical Composition
2.3.1. Identification of the Constituents by LC-DAD-MS
2.3.2. Phenolic Compounds and Flavonoids
2.3.3. Lipophilic Antioxidants
2.3.4. Determination of the Ascorbic Acid Content
2.4. Antioxidant Activity
2.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH•) Free Radical Scavenging
2.4.2. Discoloration of the 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS•+) Radical
2.5. In Vivo Assays
2.5.1. Caenorhabditis Elegans Strains, Maintenance, Synchronization, and Experimental Controls
2.5.2. Toxicity
2.5.3. Number of Progeny
2.5.4. Resistance to Heat Stress
2.5.5. Resistance to Oxidative Stress
2.5.6. Lifespan Assay
2.5.7. Expression of SOD-3 and GST-4 Proteins
2.5.8. Expression of the Transcription Factor DAF-16
2.6. Statistical Analysis
3. Results
3.1. Identification of the Constituents by LC-DAD-MS
3.2. Chemical Composition
3.3. Antioxidant Activity
Neutralization of DPPH• and ABTS•+ Radicals
3.4. Assays in C. Elegans
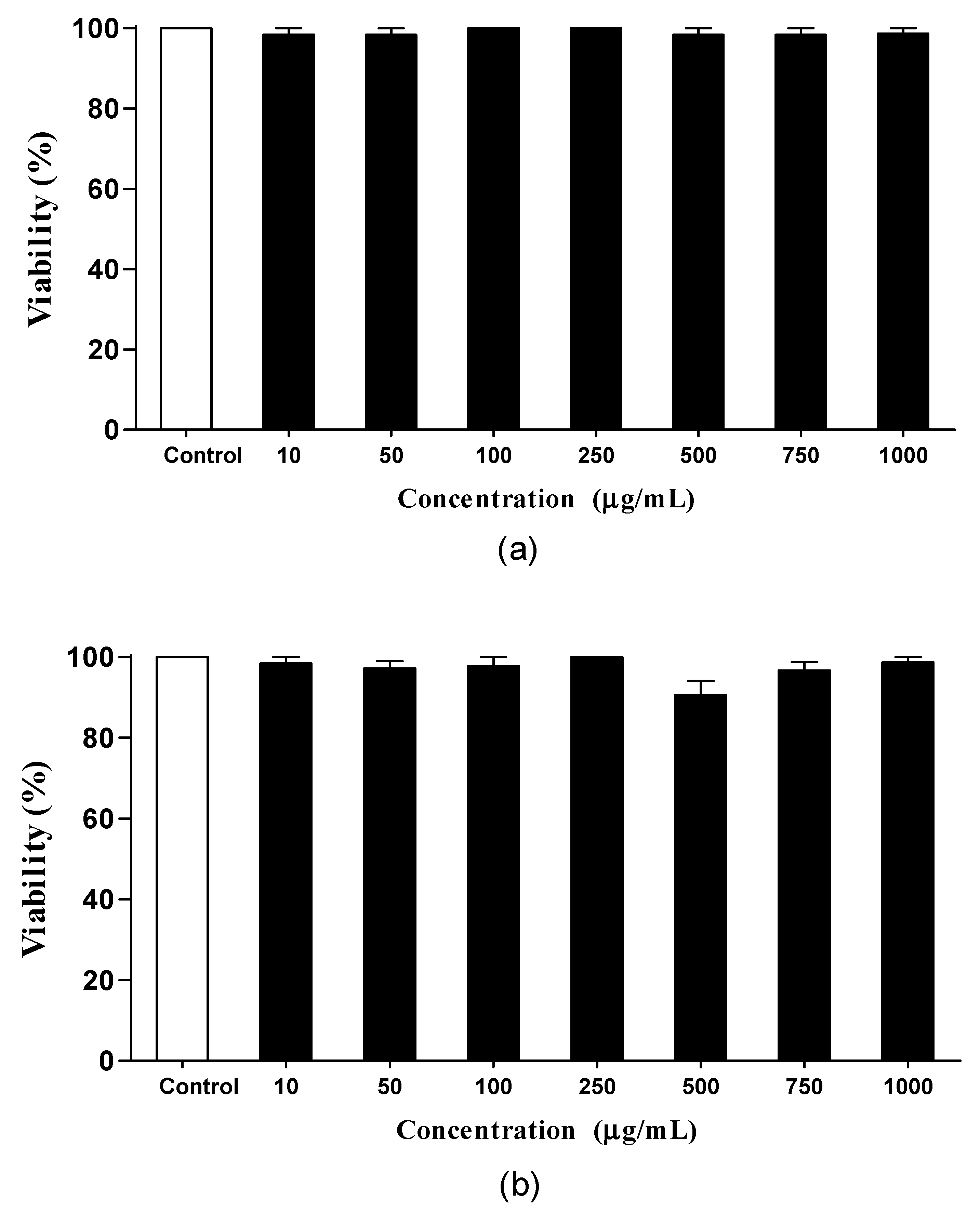
3.4.1. Toxicity
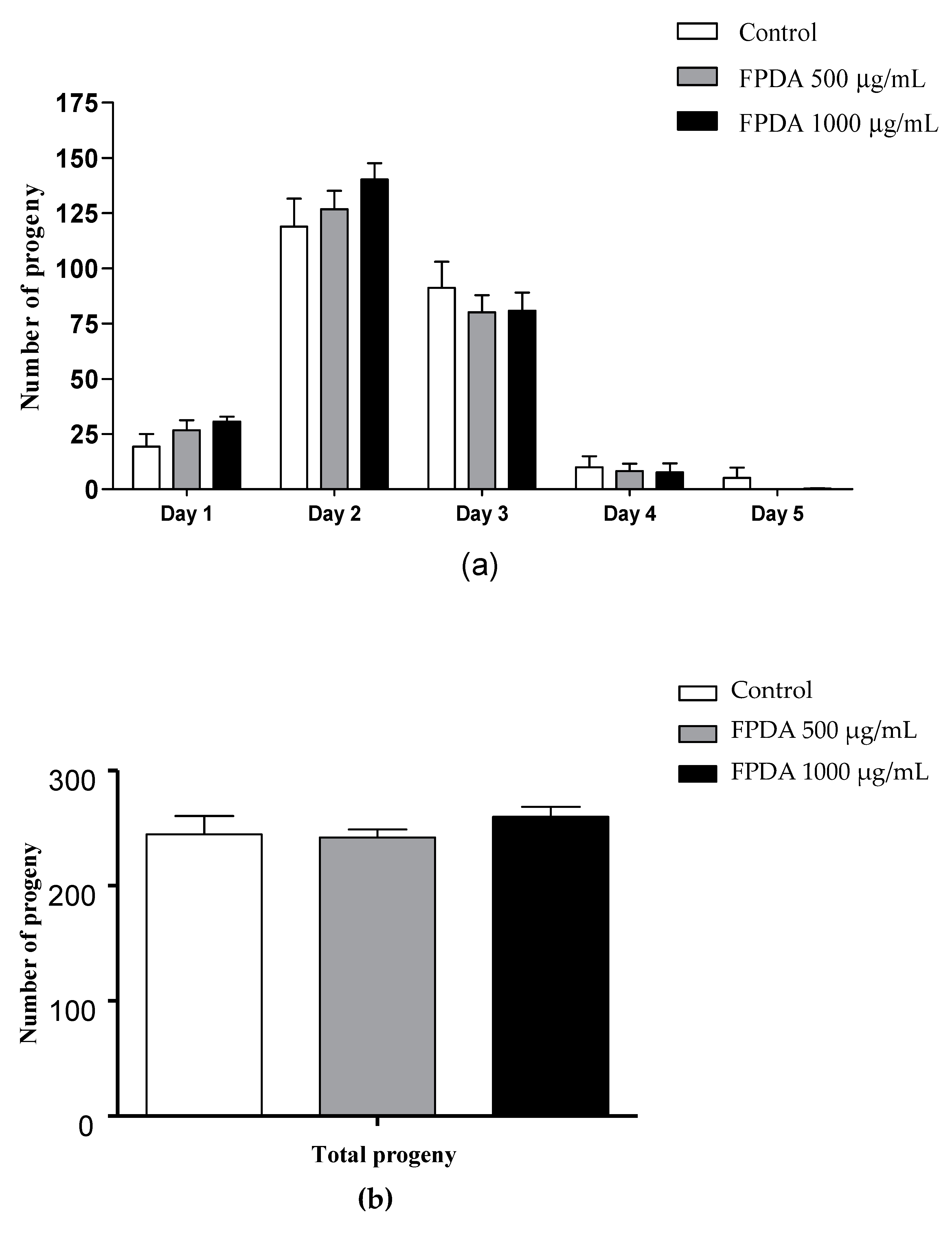
3.4.2. Number of Progeny
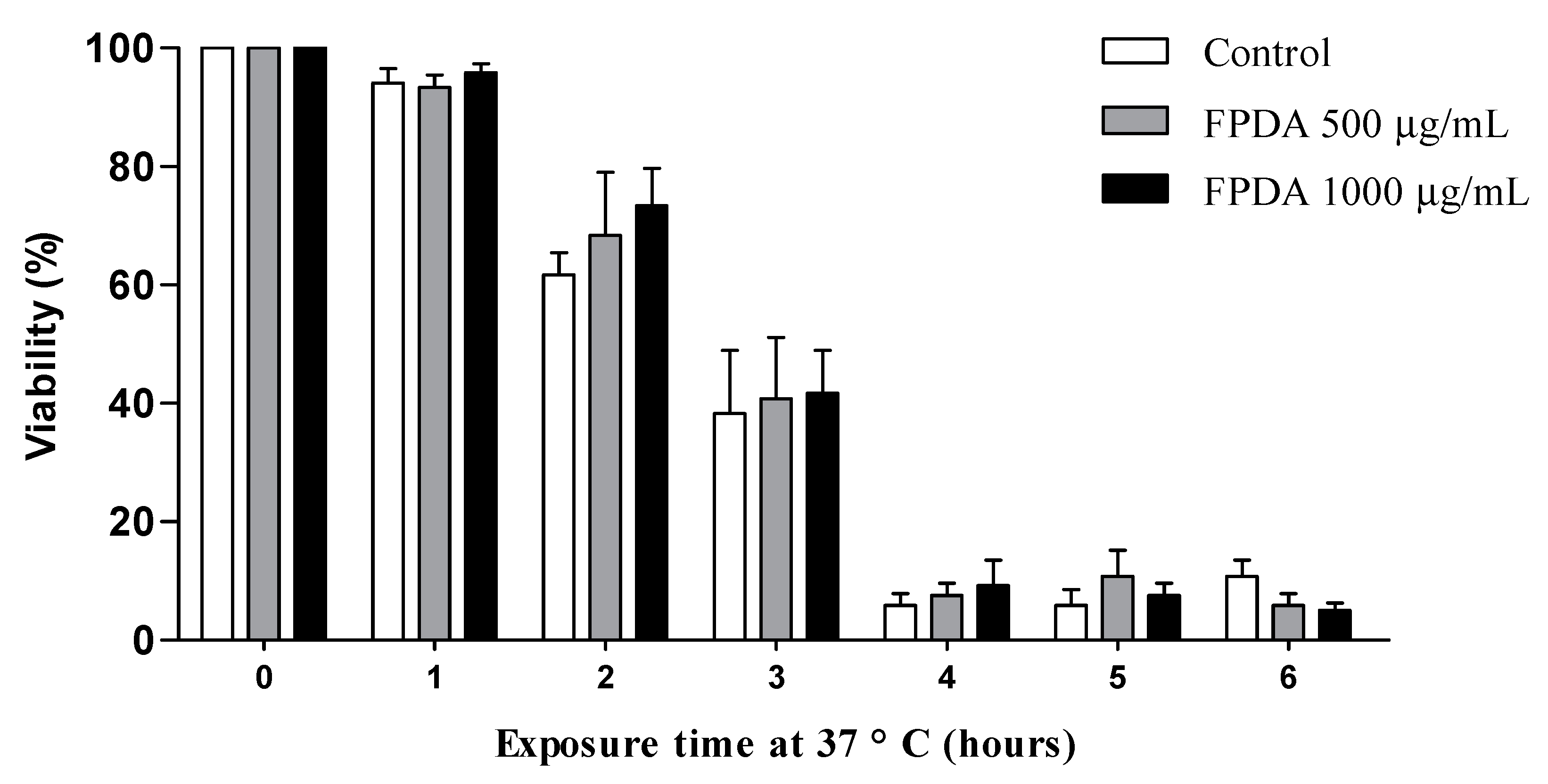
3.4.3. Resistance to Heat Stress
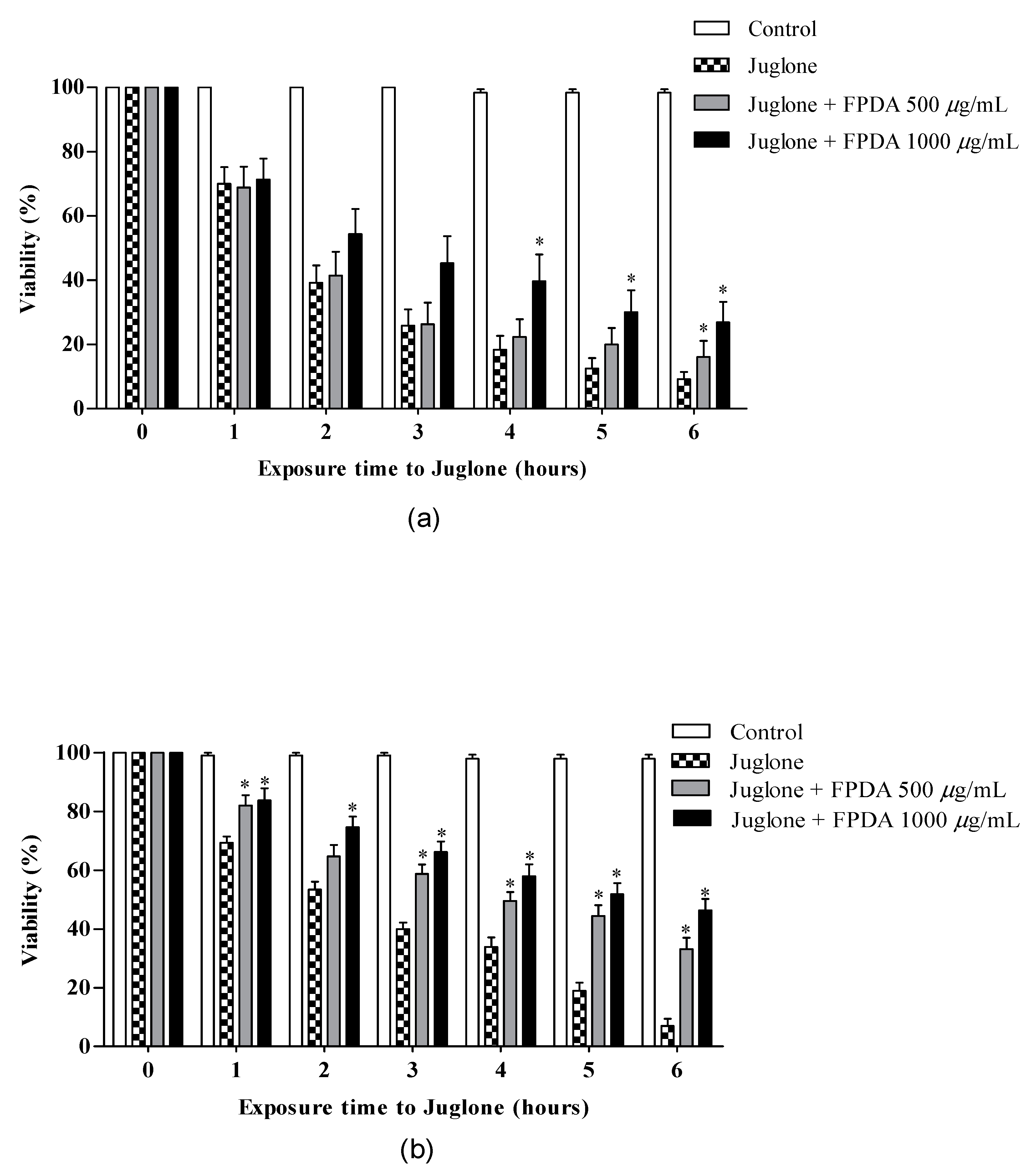
3.4.4. Resistance to Oxidative Stress
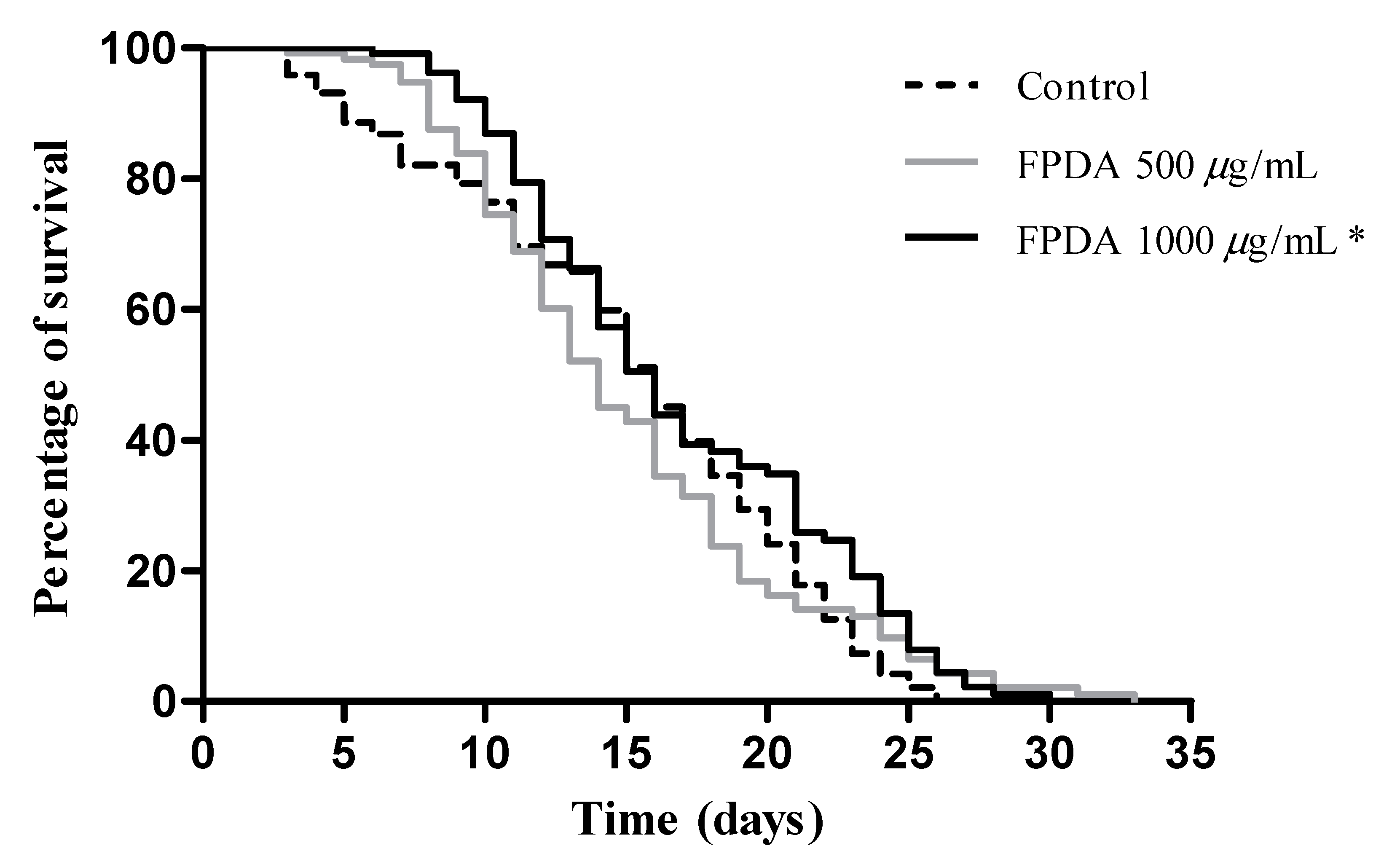
3.4.5. Lifespan
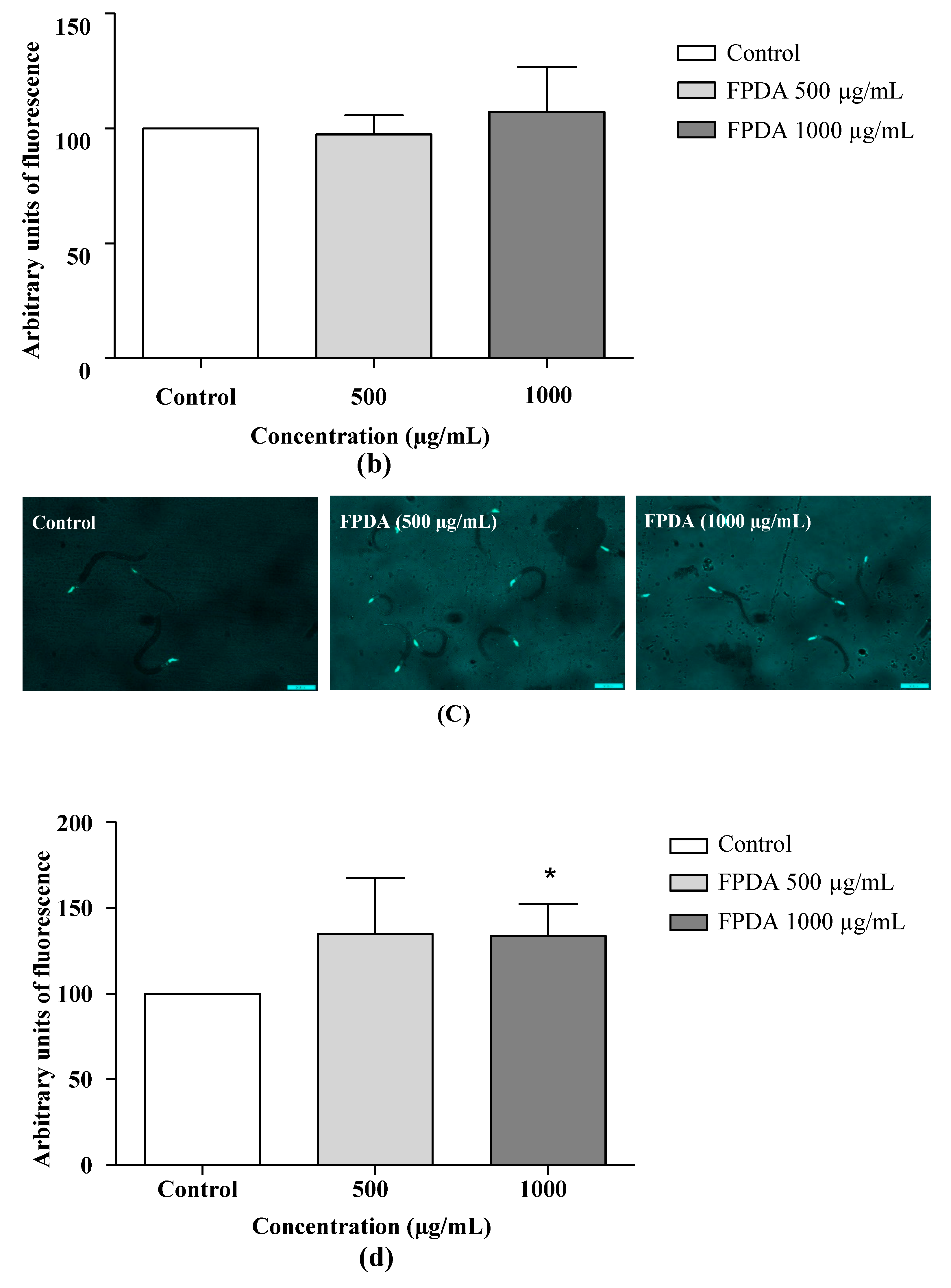
3.4.6. Expression of the SOD-3 and GST-4 Proteins
3.4.7. Expression of Transcription Factor DAF-16
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franke, J.; Barradas, A.C.S.; Borges, M.A.; Costa, M.M.; Dias, P.A.; Hoffmann, A.A.; Filho, J.C.O.; Melchiori, A.E.; Siegert, F. Fuel load mapping in the Brazilian Cerrado in support of integrated fire management. Remote Sens. Environ. 2018, 217, 221–232. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Free radicals, antioxidants and disease. Biol. Med. 2014, 6, 2–6. [Google Scholar] [CrossRef]
- Ferraz, M.C.; Parrilha, L.A.C.; Moraes, M.S.D.; Filho, J.M.; Cogo, J.C.; Santos, M.G.; Franco, L.M.; Groppo, F.C.; Puebla, P.; Feliciano, A.S.; et al. The effect of lupane triterpenoids (Dipteryx alata Vogel) in the in vitro neuromuscular blockade and myotoxicity of two snake venoms. Curr. Org. Chem. 2012, 16, 2717–2723. [Google Scholar] [CrossRef]
- Puebla, P.; Oshima-Franco, Y.; Franco, L.M.; Dos Santos, M.G.; Silva, R.V.; Rubem-Mauro, L.; San Feliciano, A. Chemical constituents of the bark of Dipteryx alata Vogel, an active species against Bothrops jararacussu Venom. Molecules 2010, 15, 8193–8204. [Google Scholar] [CrossRef]
- Marques, F.G.; Oliveira Neto, J.R.D.; Cunha, L.C.D.; Paula, J.R.D.; Bara, M.T.F. Identification of terpenes and phytosterols in Dipteryx alata (baru) oil seeds obtained through pressing. Rev. Bras. de Farmacogn. 2015, 25, 522–525. [Google Scholar] [CrossRef][Green Version]
- Souza, R.G.M.; Gomes, A.C.; De Castro, I.A.; Mota, J.F. A baru almond-enriched diet reduces abdominal adiposity and improves HDL concentrations: A randomized, placebo-controlled trial. Nutrition 2018, 55, 154–160. [Google Scholar] [CrossRef]
- Almeida Siqueira, E.M.; Marin, A.M.F.; Da Cunha, M.D.S.B.; Fustinoni, A.M.; De Sant’ana, L.P.; Arruda, S.F. Consumption of baru seeds [Dipteryx alata Vog.], a Brazilian savanna nut, prevents iron-induced oxidative stress in rats. Food Res. Int. 2012, 45, 427–433. [Google Scholar] [CrossRef]
- Bonomo, L.F.; Silva, D.N.; Boasquivis, P.F.; Paiva, F.A.; Da Costa Guerra, J.F.; Martins, T.A.F.; Torres, A.G.J.; De Paula, I.T.B.R.; Caneschi, W.L.; Jacolot, P.; et al. Açaí (Euterpe oleracea Mart.) modulates oxidative stress resistance in Caenorhabditis elegans by direct and indirect mechanisms. PLoS ONE 2014, 9, e89933. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; De Paula Oliveira, R. Guarana (Paullinia cupana) extract protects Caenorhabditis elegans models for Alzheimer disease and Huntington disease through activation of antioxidant and protein degradation pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9241308. [Google Scholar] [CrossRef]
- Tambara, A.L.; Moraes, L.D.L.S.; Dal Forno, A.H.; Boldori, J.R.; Soares, A.T.G.; De Freitas Rodrigues, C.; Mariutti, L.R.B.; Mercadante, A.Z.; Ávila, D.S.; Denardin, C.C. Purple Pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Fraguas, R.M.; Simão, A.A.; Leal, R.S.; Santos, C.M.; Rocha, D.A.; Tavares, T.S.; Marques, T.R.; Marques, T.R.; Duarte, M.H.; Marcussi, S.; et al. Chemical composition of processed baru (Dipteryx alata Vog.) almonds: Lyophilization and roasting. Afr. J. Agric. Res. 2014, 9, 1061–1069. [Google Scholar]
- Abu-Reidah, I.M.; Contreras, M.M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC-ESI-QTOF-MS-based metabolic profiling of Vicia faba L. (Fabaceae) seeds as a key strategy for characterization in foodomics. Electrophoresis 2014, 35, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Júnior, M.G.; Sousa, C.M.M.; Cavalheiro, A.J.; Lago, J.H.G.; Chaves, M.H. Phenolic derivatives from fruits of Dipteryx lacunifera Ducke and evaluation of their antiradical activities. Helv. Chim. Acta 2008, 91, 2159–2167. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Sancho, R.A.S.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian wild fruits: Nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of variun compound classes toward the Folin-Ciocalteu (F-C) reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; De La Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Nabavi, S.F.; Nabavi, S.M.; Jardim, F.R. Omega-3 polyunsaturated fatty acids and mitochondria, back to the future. Trends Food Sci. Technol. 2017, 67, 76–92. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Thirumurugan, K. Longevity promoting efficacies of different plant extracts in lower model organisms. Mech. Ageing Dev. 2018, 171, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zevian, S.C.; Yanowitz, J.L. Methodological considerations for heat shock of the nematode Caenorhabditis elegans. Methods 2014, 68, 450–457. [Google Scholar] [CrossRef]
- Furuhashi, T.; Sakamoto, K. FoxO/Daf-16 restored thrashing movement reduced by heat stress in Caenorhabditis elegans. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2014, 170, 26–32. [Google Scholar] [CrossRef]
- Mahat, D.B.; Salamanca, H.H.; Duarte, F.M.; Danko, C.G.; Lis, J.T. Mammalian heat shock response and mechanisms underlying its genome-wide transcriptional regulation. Mol. Cell 2016, 62, 63–78. [Google Scholar] [CrossRef]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Wang, E.; Wink, M. Chlorophyll enhances oxidative stress tolerance in Caenorhabditis elegans and extends its lifespan. PeerJ 2016, 4, e1879. [Google Scholar] [CrossRef]
- Sung, B.; Chung, J.W.; Bae, H.R.; Choi, J.S.; Kim, C.M.; Kim, N.D. Humulus japonicus extract exhibits antioxidative and anti aging effects via modulation of the AMPK SIRT1 pathway. Exp. Ther. Med. 2015, 9, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Islam, M.A.; Shaw, S.; Khan, I.N.; Saravi, S.S.S.; Ahmad, S.; Rehman, S.; Gupta, V.K.; et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018, 420, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M. Mechanisms underlying longevity: A genetic switch model of aging. Exp. Gerontol. 2018, 107, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Amigoni, L.; Stuknytė, M.; Ciaramelli, C.; Magoni, C.; Bruni, I.; De Noni, I.; Airoldi, C.; Regonesi, M.E.; Palmioli, A. Green coffee extract enhances oxidative stress resistance and delays aging in Caenorhabditis elegans. J. Funct. Foods 2017, 33, 297–306. [Google Scholar] [CrossRef]
- Pallauf, K.; Bendall, J.K.; Scheiermann, C.; Watschinger, K.; Hoffmann, J.; Roeder, T.; Rimbach, G. Vitamin C and lifespan in model organisms. Food Chem. Toxicol. 2013, 58, 255–263. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Braicu, C.; Mehterov, N.; Vladimirov, B.; Sarafian, V.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. Nutrigenomics in cancer: Revisiting the effects of natural compounds. Semin. Cancer Biol. 2017, 46, 84–106. [Google Scholar] [CrossRef]
- Denzel, M.S.; Lapierre, L.R.; Mack, H.I. Emerging topics in C. elegans aging research: Transcriptional regulation, stress response and epigenetics. Mech. Ageing Dev. 2019, 177, 4–21. [Google Scholar] [CrossRef]
- Hesp, K.; Smant, G.; Kammenga, J.E. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age related disease. Exp. Gerontol. 2015, 72, 1–7. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619, 1–8. [Google Scholar] [CrossRef]
- Dues, D.J.; Schaar, C.E.; Johnson, B.K.; Bowman, M.J.; Winn, M.E.; Senchuk, M.M.; Van Raamsdonk, J.M. Uncoupling of oxidative stress resistance and lifespan in long-lived isp-1 mitochondrial mutants in Caenorhabditis elegans. Free Radic. Biol. Med. 2017, 108, 362–373. [Google Scholar] [CrossRef] [PubMed]
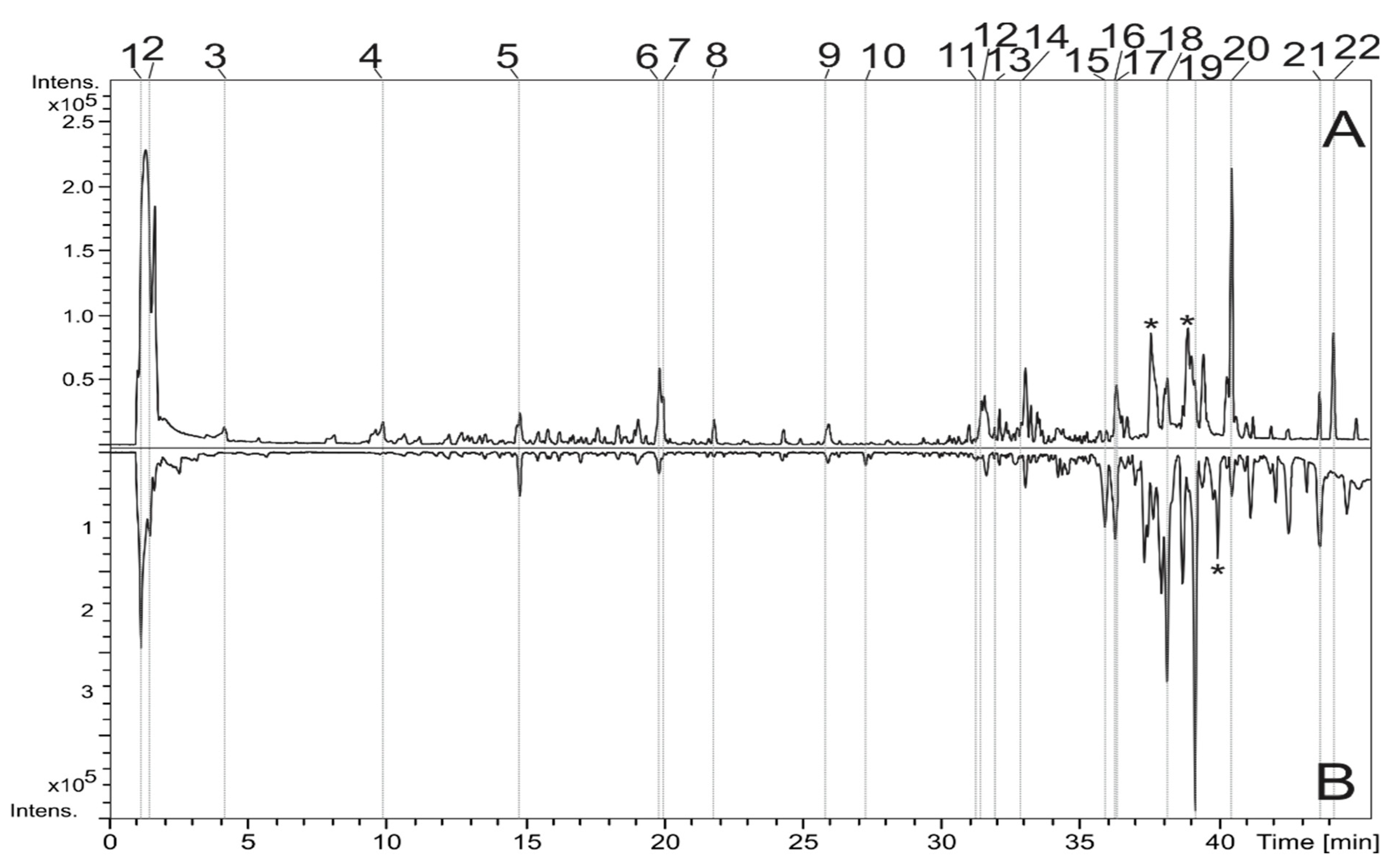


| Peak | RT (min) | Compound | UV (nm) | MF | Negative Mode (m/z) | Positive Mode (m/z) | ||
|---|---|---|---|---|---|---|---|---|
| MS [M-H]- | MS/MS | MS [M+H]+ | MS/MS | |||||
| 1 | 1.2 | di-O-hexoside | - | C12H22O11 | 341.1103 | 179 | 365.1052 a | - |
| 2 | 1.5 | Citric acid | - | C6H8O7 | 191.0206 | - | 193.0331 | |
| 3 | 4.1 | O-hexosyl protocatechuic acid | 288 | C13H16O9 | 315.0735 | 153 | ||
| 4 | 9.9 | NI | - | C17H22O12 | 417.1073 | 152 | 441.1012 a | - |
| 5 | 14.8 | Vicenin 2 | 270, 335 | C27H30O15 | 593.1544 | 503, 473, 383, 353, 325, 297 | 595.1694 | 541, 481, 457, 439, 409, 391, 379, 355, 337, 325, 295 |
| 6 | 19.9 | NI | 282 | C18H22O10 | 397.1154 | 249, 189 | 399.1301 | 223 |
| 7 | 20.0 | NI | 286, 330 | C9H16O4 | 187.0985 | - | 189.1127 | 171 |
| 8 | 21.8 | Coumaric acid derivative | 290, 318 | C24H28O12 | 507.1526 | 231, 203, 163 | 509.1626 | - |
| 9 | 25.9 | NI | 285, 335 | C16H30O6 | 317.1985 | 263, 237, 219, 171 | ||
| Luteolin | C15H10O6 | 285.0413 | 257, 239, 199, 175, 151 | 287.0564 | 153 | |||
| 10 | 27.3 | di-O-methoxy dihydroxy isoflavone | 280 | C17H14O6 | 313.0730 | - | 315.0859 | 300, 243, 167 |
| 11 | 31.5 | Fatty acid derivative | - | C18H34O5 | 329.2349 | 229, 211, 183, 171 | - | - |
| 12 | 31.7 | Diterpene | 285 | C20H28O5 | 347.1880 | 285, 259 | 349.2005 | 285, 239, 187, 161 |
| 13 | 32.1 | NI | 285 | C21H30O6 | 377.1983 | 333, 301, 263 | 379.2130 | 361, 283, 213, 161 |
| 14 | 33.1 | NI | 285 | C22H32O6 | 391.2133 | 287, 191 | 393.2267 | 315, 297, 269, 213, 199, 171, 161 |
| 15 | 35.9 | NI | - | C21H30O4 | - | - | 347.2220 | 287, 269, 251, 187, 163 |
| 16 | 36.3 | NI | - | C19H28O3 | 303.1966 | 252, 205 | ||
| 17 | 36.3 | NI | - | C21H30O4 | - | - | 347.2219 | 287, 269, 243, 187, 163 |
| 18 | 38.2 | NI | - | C45H94N6O17 | - | - | 991.6737 | |
| 19 | 39.2 | NI | - | C22H34O4 | - | - | 361.2372 | 301, 283, 245, 199, 171 |
| 20 | 40.5 | Fatty acid derivative | - | C20H30O2 | 301.2186 | - | - | - |
| 21 | 43.6 | Hexadecanoic acid | - | C16H32O2 | 255.2341 | |||
| 22 | 44.2 | Octadecenoic acid | - | C18H34O2 | 281.2491 | |||
| Samples | DPPH• | ABTS•+ |
|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | |
| Ascorbic acid | 2.65 ± 0.20 | 1.43 ± 0.09 |
| BHT | 14.58 ± 2.15 | 10.15 ± 0.94 |
| FPDA | 2306.33 ± 101.83 | 416.0 ± 28.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, N.R.; Araújo, L.C.A.d.; Rocha, P.d.S.d.; Agarrayua, D.A.; Ávila, D.S.; Carollo, C.A.; Silva, D.B.; Estevinho, L.M.; de Picoli Souza, K.; dos Santos, E.L. Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis elegans via SOD-3 and DAF-16. Biomolecules 2020, 10, 1106. https://doi.org/10.3390/biom10081106
Leite NR, Araújo LCAd, Rocha PdSd, Agarrayua DA, Ávila DS, Carollo CA, Silva DB, Estevinho LM, de Picoli Souza K, dos Santos EL. Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis elegans via SOD-3 and DAF-16. Biomolecules. 2020; 10(8):1106. https://doi.org/10.3390/biom10081106
Chicago/Turabian StyleLeite, Natasha Rios, Laura Costa Alves de Araújo, Paola dos Santos da Rocha, Danielle Araujo Agarrayua, Daiana Silva Ávila, Carlos Alexandre Carollo, Denise Brentan Silva, Leticia Miranda Estevinho, Kely de Picoli Souza, and Edson Lucas dos Santos. 2020. "Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis elegans via SOD-3 and DAF-16" Biomolecules 10, no. 8: 1106. https://doi.org/10.3390/biom10081106
APA StyleLeite, N. R., Araújo, L. C. A. d., Rocha, P. d. S. d., Agarrayua, D. A., Ávila, D. S., Carollo, C. A., Silva, D. B., Estevinho, L. M., de Picoli Souza, K., & dos Santos, E. L. (2020). Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis elegans via SOD-3 and DAF-16. Biomolecules, 10(8), 1106. https://doi.org/10.3390/biom10081106










