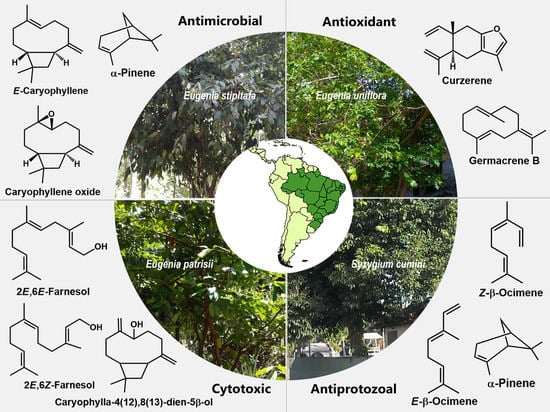
Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities
Abstract
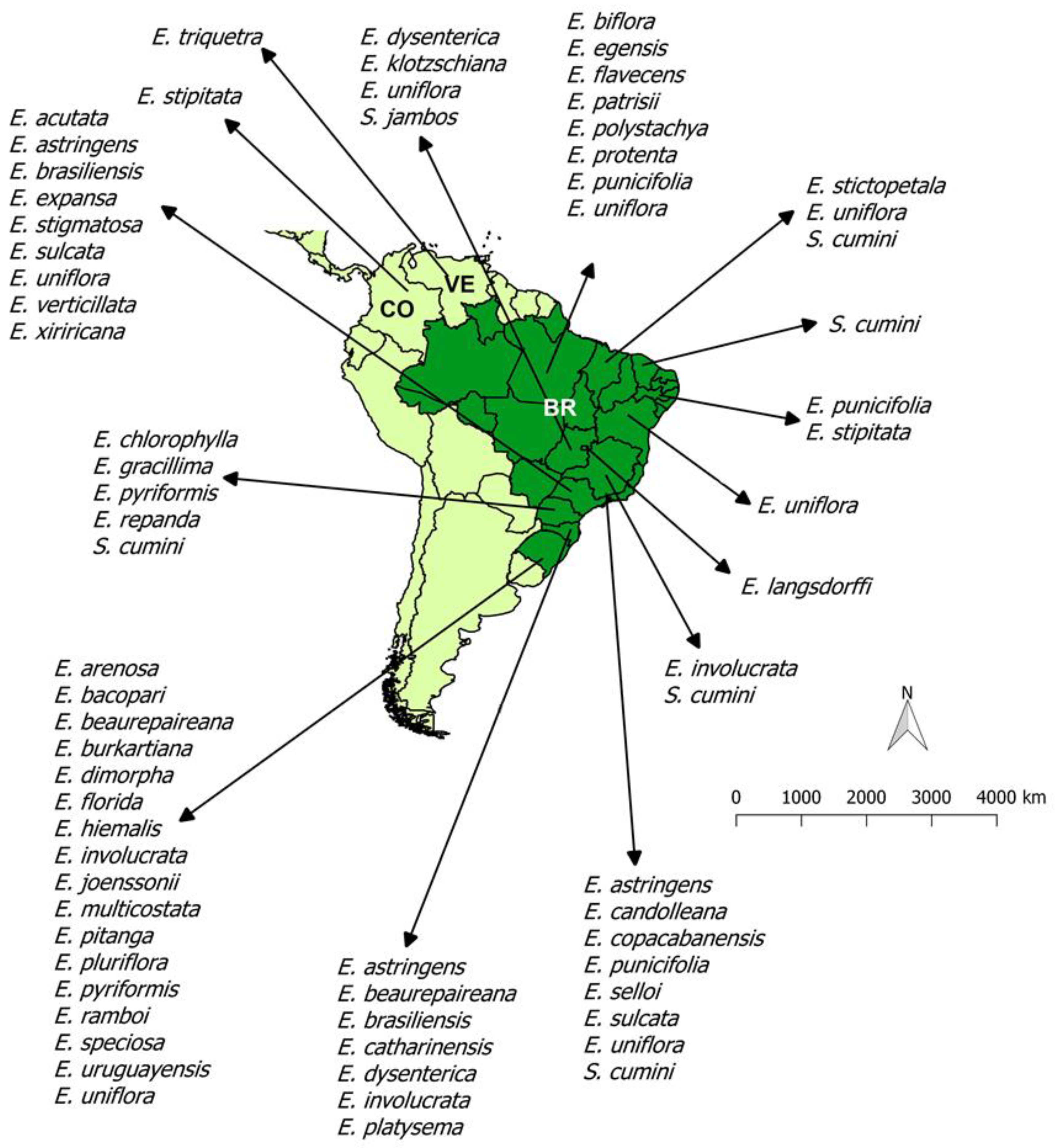
1. Introduction
2. Bibliographic Search Criteria
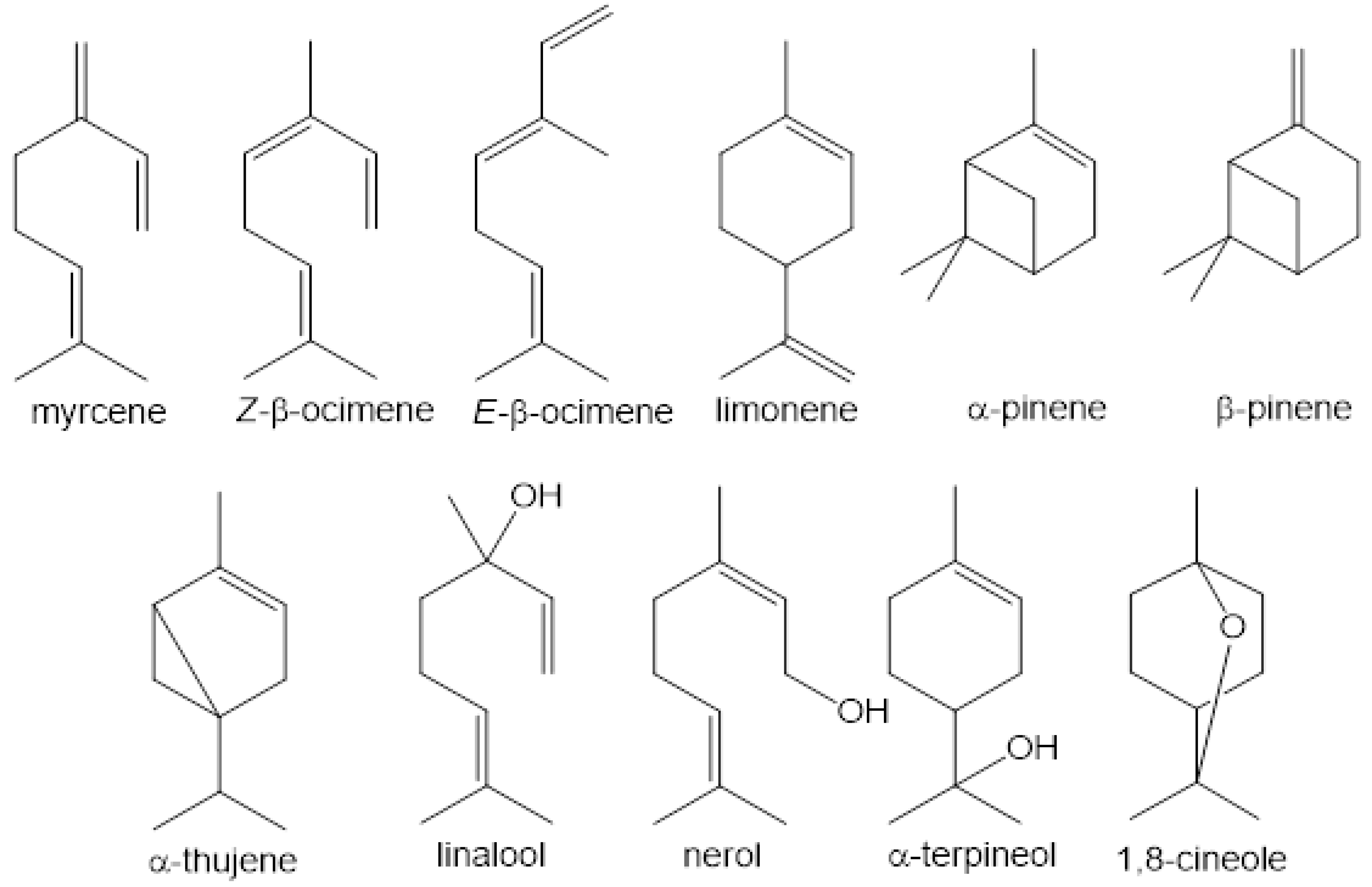
3. Volatile Profiles
4. Seasonal Variation of Oil Composition
5. Differences in Oil Composition and Extractions Methods
6. Biological Activities
6.1. Antibacterial and Antifungal Activity
6.2. Acetylcholinesterase Inhibition
6.3. Cytotoxic Activity
6.4. Antiprotozoal Activity
6.5. Antioxidant Aactivity
6.6. Other Biological Activities
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2:2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ACh | Acetylcholine |
| AChE | Acetylcholine esterase |
| BHA | Butylated hydroxyanisole |
| BR | Brazil |
| CO | Colombia |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl (radical) |
| ED50 | Median Effective dose |
| EOs | Essential oils |
| GC–MS | Gas chromatography–mass spectrometry |
| HCA | Hierarchical Cluster Analysis |
| HD | Hydrodistillation |
| IC50 | Median inhibitory concentration |
| LC50 | Median lethal concentration |
| mg.AA/g | Milligram of ascorbic acid per gram |
| mg.BHAE/mL | Milligram BHA equivalente per gram |
| mgTE/g | Milligram trolox equivalente per gram |
| MIC | Minimum inhibitory concentration |
| MMC | Minimum microbicide concentration |
| NI | Unidentified compound |
| NMR | Nuclear magnetic resonance |
| PCA | Principal cluster analysis |
| SD | Steam distillation |
| SFE | Supercritical fluid extraction |
| SPME | Solid Phase Micro Extraction |
| spp. | Species (plural) |
| TLC | Thin-layer chromatography |
| VE | Venezuela |
Appendix A
| Species | Occurrence | Essential Oil | Primary Components (>5%) | Essential Oil Bioactivity | Ref. |
|---|---|---|---|---|---|
| E. acutata | Valinhos, São Paulo, Brazil | Leaf (SD) | E-caryophyllene (26.93%), δ-cadinene (6.01%), germacrene D (5.29%) | — | [60] |
| E. arenosa | Manuel Viana, Rio Grande do Sul, Brazil | Leaf (HD) | farnesyl acetate (70.4%), aromadendrene (11.7%), globulol (7.1%) | — | [32] |
| E. astringens | Macaé, Rio de Janeiro, Brazil | Leaf (HD) | α-pinene (15.8%), 1-epi-cubenol (11.9%), α-eudesmol (10.6%), selin-11-en-4α-ol (9.4%) | — | [28] |
| E. astringens | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (HD) b | α-pinene (19.7-34.4%), β-pinene (20.6-34.1%), E-caryophyllene (3.6-11.7%) | — | [26] |
| E. astringens | Juréia, São Paulo, Brazil | Leaf (HD) | α-pinene (24.7%), E-caryophyllene (14.6%), β-pinene (11.0%), myrcene (7.7%) | — | [27] |
| E. astringens | Santo Amaro da Imperatriz, Santa Catarina, Brazil | Leaf (HD) | viridiflorol (17.7%), β-pinene (13.2%), α-pinene (11.2%), aromadendrene (6.9%) | Antibacterial, broth dilution assay (Staphylococcus aureus, MIC 119.2 µg/mL; Pseudomonas aeruginosa, MIC 447.0 µg/mL; Escherichia coli, MIC 477.0 µg/mL) | [29] |
| E. bacopari | Dom Pedro de Alcantara, Rio Grande do Sul, Brazil | Leaf (HD) | δ-cadinene (15.8%), aromadendrene (12.2%), viridiflorene (7.9%), globulol (6.8%) | — | [59] |
| E. beaurepaireana | Morrinhos do Sul, Rio Grande do Sul, Brazil | Leaf (HD) | bicyclogermacrene (14.3%), germacrene D (8.6%), δ-cadinene (7.2%), E-caryophyllene (6.4%) | — | [30] |
| E. beaurepaireana | Santo Amaro da Imperatriz, Santa Catarina, Brazil | Leaf (HD) | E-caryophyllene (8.0%), bicyclogermacrene (7.2%), valencene (5.5%), δ-cadinene (4.9%), spathulenol (4.9%), viridiflorol (4.9%) | Antibacterial, broth dilution assay (Staphylococcus aureus, 1110 µg/mL; Pseudomonas aeruginosa, MIC 278.3 µg/mL; Escherichia coli, MIC 556.6 µg/mL) | [29] |
| E. biflora | Belém, Pará, Brazil | Leaf (HD) | caryophyllene oxide (28.6%), E-caryophyllene (16.8%), τ--murrolol (8.4%), 1-epi-cubenol (6.4%), α-cadinol (6.2%) | — | [24] |
| E. biflora | Belém, Pará, Brazil | Leaf (HD) | caryophyllene oxide (20.5%), E-caryophyllene (11.4%), epi-α-murrolol (7.6%), 1-epi-cubenol (6.6%), α-cadinol (6.3%) | — | [24] |
| E. biflora | Belém, Pará, Brazil | Leaf (HD) | E-caryophyllene (9.8%), globulol (9,8%), germacrene B (7.9%), α-cadinol (7.5%) | — | [24] |
| E. biflora | Belém, Pará, Brazil | Leaf (HD) | α-cadinol (14.7%), τ-murrolol (8.3%), caryophyllene oxide (7.5%), copaborneol (5.6%) | — | [24] |
| E. biflora | Maracanã, Pará, Brazil | Leaf (HD) | E-caryophyllene (15.4%), germacrene D (6.6%), α-copaene (6.0%), bicyclogermacrene (5.8%) | — | [25] |
| E. biflora | Maracanã, Pará, Brazil | Leaf (HD) | β-pinene (27.8%), α-pinene (27.3%), limonene (6.7%) | — | [25] |
| E. brasiliensis | Blumenau, Santa Catarina, Brazil | Leaf (HD) | β-pinene (10.4%), α-pinene (10.3%), spathulenol (7.7%), τ-cadinol (7.1%) | — | [32] |
| E. brasiliensis | Florianópolis, Santa Catarina, Brazil | Leaf (HD) b | α-pinene (1.77-15.94%), β-pinene (2.98-11.24%), spathulenol (8.10-18.17%), 1-epi-cubenol (4.83-7.46%), τ-cadinol (10.38-15.30%) | Antibacterial, broth dilution assay (Staphylococcus saprophyticus, MIC 500-1000 µg/mL; Escherichia coli, MIC 1000 µg/mL; Pseudomonas aeruginosa, MIC 500-1000 µg/mL) Antioxidant (DPPH IC50 > 500 μg/mL; β-carotene/linoleic acid, 1.67-14.05%; iron reducing power, 60.37-94.32 mg AA/g) Enzyme inhibitory (acetylcholinesterase, IC50 >1000 μg/mL) | [71] |
| E. brasiliensis | Moji-Guaçu, São Paulo, Brazil | Leaf Purple Variety (HD) | α-pinene (18.8%), β-pinene (11.0%), 1,8-cineol (9.6%), limonene (8.6%), τ-cadinol (6.8%) | — | [33] |
| E. brasiliensis | Moji-Guaçu, São Paulo, Brazil | Leaf Yellow Variety (HD) | α-pinene (33.5%), 1,8-cineol (28.2%), β-pinene (14.4%), myrcene (5.5%) | — | [33] |
| E. brasiliensis | Moji-Guaçu, São Paulo, Brazil | Fruit Yellow Variety (HD) | α-pinene (15.4%), myrcene (10.7%), α–terpineol (10.2%), β-pinene (9.3%) | — | [33] |
| E. brasiliensis | Santo Amaro da Imperatriz, Santa Catarina, Brazil | Leaf (HD) | spathulenol (12.7%), τ-cadinol (8.7%), viridiflorol (7.1%), 1-epi-cubenol (6.3%), α-cadinol (6.6%) | Antibacterial, broth dilution assay (Staphylococcus aureus, MIC 156.2 µg/mL; Pseudomonas aeruginosa, MIC 624.9 µg/mL; Escherichia coli, MIC 624.9 µg/mL) | [29] |
| E. brasiliensis | São Paulo, São Paulo, Brazil | Leaf (HD) b | spathulenol (7.0-18.0%), trans−α−bergamotene (2.6-19.0%), α−thujene (4.0-11.5%), β−selinene (2.3-8.5%) | — | [72] |
| E. brasiliensis | Moji-Guaçu, São Paulo, Brazil | Fruit Purple Variety (HD) | caryophyllene oxide (22.2%), α-cadinol (10.4%), τ-cadinol (9.9%), β-bisabolene (9.6%) | — | [33] |
| E. burkartiana | Parque do Turvo, Rio Grande do Sul, Brazil | Leaf (HD) | bicyclogermacrene (14.2%), germacrene D (8.8%), E-caryophyllene (7.8%), 3E-hexenol (6.7%) | — | [59] |
| E. candolleana | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (SD) | 1-epi-cubenol (77.59%), δ-elemene (13.87%), muurola-4,10(14)-dien-1β-ol (8.68%), globulol (5.52%), α-cadinol (5.26%) | — | [60] |
| E. catharinensis | Blumenau, Santa Catarina, Brazil | Leaf (HD) | ethyl palmitate (10.5%), trans-α-bergamotene (6.5%), β-selinene (5.9%) | — | [59] |
| E. chlorophylla | Curitiba, Paraná, Brazil | Fine Stem (HD) | caryophyllene oxide (17.2%), τ-muurolol (16.8%), globulol (16.5%), α-cadinol (12.1%), 1-epi-cubenol (10.9%) | Antimicrobial, agar diffusion assay and broth dilution assay (Kocuria rhizophila, MIC 500 µg/mL; Staphylococcus aureus, MIC 500 µg/mL; Streptococcus mutans, MIC 50.0 µg/mL; Streptococcus sobrinus, MIC 50.0 µg /mL; Candida tropicalis, MIC 50.0 µg/mL) | [34] |
| E. chlorophylla | Curitiba, Paraná, Brazil. | Leaf (HD) | globulol (22.5%), 1-10-di-epi-cubenol, (9.8%), τ-muurolol (8.1%), caryophyllene oxide (6.4%) | Antimicrobial, agar diffusion assay and broth dilution assay (Kocuria rhizophila, MIC 500, 50.0 µg /mL; Staphylococcus aureus, MIC 500, 50.0 µg/mL; Streptococcus mutans, MIC 50.0 µg/mL; Streptococcus sobrinus, MIC 50.0 µg/mL; Candida albicans, MIC 50.0 µg/mL; Candida tropicalis, MIC 50.0 µg/mL) | [34] |
| E. chlorophylla | Curitiba, Paraná, Brazil | Leaf at flowering stage (HD) | globulol (18.9%), α-cadinol (9.4%), 1-epi-cubenol (8.1%), E-caryophyllene (8.1%), caryophyllene oxide (5.8%) | Antimicrobial, agar diffusion assay and broth dilution assay (Kocuria rhizophila, MIC 500 µg/mL; Staphylococcus aureus, MIC 500 µg/mL; Streptococcus mutans, MIC 50.0 µg/mL; Streptococcus sobrinus, MIC 50.0 µg/mL; Candida albicans, MIC 50.0 µg/mL; Candida tropicalis, 50.0 µg/mL) | [34] |
| E. chlorophylla | Curitiba, Paraná, Brazil | Flower (HD) | E-caryophyllene (12.8%), α-cadinol (10.1%), caryophyllene oxide (8.9%), τ-muurolol (8.5%) | Antimicrobial, agar diffusion assay and broth dilution assay (Kocuria rhizophila, MIC 500 µg/mL; Staphylococcus aureus, MIC 500 µg/mL; Streptococcus mutans, MIC 50.0 µg/mL; Streptococcus sobrinus, MIC 50.0 µg/mL; Enterococcus faecalis, MIC 1000 µg/mL) | [34] |
| E. copacabanensis | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (SD) | 1,10-di-epi-cubenol (14.24%), caryophyllene oxide (6.75%), epi-α-cadinol (5.03%), γ-cadinene (4.17%) | — | [60] |
| E. copacabanensis | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (HD) | β-pinene (50.4%), α-pinene (20.2%), E-caryophyllene (10.3%) | — | [89] |
| E. dimorpha | Morro Santana, Rio Grande do Sul, Brazil | Leaf (HD) | α-pinene (22.4%), α-humulene (12.9%), 1,8-cineole (9.9%) | — | [59] |
| E. dysenterica | Catalão, Goiás, Brazil | Leaf (HD) | E-caryophyllene (14.8%), α-humulene (11.0%), α-terpineol (6.1%), limonene (5.5%), δ-cadinene (5.8%), caryophyllene oxide (5.4%) | Antibacterial, broth dilution assay (Cryptococcus neoformans var neoformans, MIC 250 µg/mL; Cryptococcus neoformans var gattii, MIC 250 μg/mL) | [35] |
| E. dysenterica | Senador Canedo Santa Catarina, Brazil | Leaf (HD) | E-caryophyllene (18.0%), β-pinene (9.3%), α-pinene (9.0%), limonene (7.8%), Z-β-ocimene (5.9%) | — | [36] |
| E. dysenterica | Campo Alegre de Goiás, Goiás, Brazil | Leaf (HD) | E-caryophyllene (24.0%), δ-cadinene (13.0%), α-copaene (9.6%), α-pinene (6.4%), caryophyllene oxide (4.5%) | — | [36] |
| E. egensis | Marabá, Pará, Brazil | Aerial parts (HD) | 5-hydroxy-Z-calamenene (35.8%), E-caryophyllene (8.9%), trans-muurola-3,5-diene (5.9%), trans-calamenene (6.1%), trans-cadin-1,4-diene (6.3%), ledol (5.0%) | Antioxidant, DPPH assay (79.6%, 216.5 mgTE/mL, 177.6 mgBHAE/mL) Cytotoxic (HCT-116 human colorectal carcinoma, IC50 > 25 μg/mL; MRC5 human fibroblast IC50 > 25 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 > 25 μg/mL; SKMEL-19 human melanoma, IC50 > 25 μg/mL) | [37] |
| E. expansa | Peruíbe, São Paulo, Brazil | Leaf (HD) | spathulenol (12.1%), E-caryophyllene (9.2%), caryophyllene oxide (8.7%), α-copaene (5.0%) | — | [32] |
| E. flavescens | Maracanã, Pará, Brazil | Leaf (HD) | germacrene D (14.5%), bicyclogermacrene (11.7%), δ-cadinene (5.7%) | — | [25] |
| E. flavescens | Parauapebas, Pará, Brazil | Aerial parts (HD) | E-γ-bisabolene (35.0%), β-bisabolene (34.7%), E-iso-γ-bisabolene (5.1%) | Antioxidant, DPPH assay (45.1%, 122.6 mgTE/mL, 100.6 mgBHAE/mL) Cytotoxic (HCT-116 human colorectal carcinoma, IC50 13.99 μg/mL; MRC5 human fibroblast IC50 14.0 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 > 25 μg/mL; SKMEL-19 human melanoma, IC50 >25 μg/mL) | [37] |
| E. florida | Derrubadas, Rio Grande do Sul, Brazil | Leaf (HD) | E-caryophyllene (14.5%), β-elemene (11.8%), α-copaene (7.9%), α-cadinol (5.8%) | — | [61] |
| E. gracillima | Porto Rico, Paraná, Brazil | Leaf (HD) | globulol (8.7%), viridiflorene (6.9%), epi-globulol (6.8%), spathulenol (5.9%) | — | [61] |
| E. hiemalis | Morro Santana, Rio Grande do Sul, Brazil | Leaf (HD) | bicyclogermacrene (37.7%), E-caryophyllene (7.4%), germacrene D (7.0%), globulol (5.8%) | — | [62] |
| E. involucrata | Urupema, Santa Catarina, Brazil | Leaf (SFE) | β-elemene (41.8%), bicyclogermacrene (28.4%), E-caryophyllene (6.7%), germacrene D (7.4%) | Antioxidant (β-carotene/ linoleic acid assay, at 1 mg/mL, 93.8%) | [38] |
| E. involucrata | Urupema, Santa Catarina, Brazil | Leaf (HD) | β-elemene (42.4%), bicyclogermacrene (23.0%), E-caryophyllene (13.4%) | Antioxidant (β-carotene/ linoleic acid assay, at 1 mg/mL, 6.41%) | [38] |
| E. involucrata | Uberlândia, Minas Gerais, Brazil | Leaf (HD) | spathulenol (21.4%), bicyclogermacrene (19.3%), E-caryophyllene (8.6%), caryophyllene oxide (7.7%) | Antibacterial, broth dilution assay (Streptococcus mitis, MIC 400 µg/mL; S. sanguinis, MIC 400 μg/mL; Prevotella nigrescens, MIC 100 µg/mL; Porphyromonas gingivalis, MIC 100 μg/mL) | [39] |
| E. involucrata | Pelotas, Rio Grande do Sul, Brazil, | Fruit (HD) | E-caryophyllene (10.1%), spathulenol (7.8%), β-bisabolene (7.2%), γ-eudesmol (6.0%) | Antioxidant, DPPH assay (TLC method) | [40] |
| E. joenssonii | Morro Santana, Rio Grande do Sul, Brazil | Leaf (HD) | 5-epi-paradisiol (8.4%), δ-selinene (7.9%), β-selinene (7.2%), neointermedeol (6.3%), α-cadinol (5.3%) | — | [59] |
| E. klotzschiana | Portelândia, Goiás, Brazil | Leaf (HD) | α-copaene (10.6%), α-humulene (5.5%), spathulenol (8.7%), caryophyllene oxide (7.4%), τ-muurolol (5.4%) α-cadinol (6.2%) | — | [41] |
| E. klotzschiana | Portelândia, Goiás, Brazil | Leaf (HD) | β-bisabolene (14.0%), spathulenol (10.9%), caryophyllene oxide (6.3%), bicyclogermacrene (5.4%) | — | [41] |
| E. klotzschiana | Portelândia, Goiás, Brazil | Leaf (HD) | β-bisabolene (17.4%), germacrene D (13.3%), α-humulene (10.2%), α-trans-bergamotene (10.1%), spathulenol (7.2%) | — | [41] |
| E. klotzschiana | Portelândia, Goiás, Brazil | Flower (HD) | α-trans-bergamotene (29.9%), germacrene D (12.1%), β-bisabolene (10.2%) | — | [41] |
| E. langsdorffi | Brasília, Distrito Federal, Brazil | Leaf (HD) | epi-longipinanol (13.6%), γ-eudesmol (12.3%), limonene (11.8%), 10-epi-γ-eudesmol (10.6%), maaliol (6.2%) | Acaricidal (Tetranychus urticae, Fumigation LC50 1.79 µL/L; residual contact LC50 21.90 µL/L) | [42] |
| E. langsdorffi | Brasília, Distrito Federal, Brazil | Fruit (HD) | 10-epi-γ-eudesmol (35.7%), 1,10-di-epi-cubenol (15.6%), caryophyllene oxide (7.5%), epi-longipinanol (7.3%), isolongifolan-7-α-ol (7.1%) | Acaricidal (Tetranychus urticae, Fumigation LC50 3.06 µL/L; residual contact LC50 12.25 µL/L) | [42] |
| E. multicostata | Dom Pedro de Alcântara, Rio Grande do Sul, Brazil | Leaf (HD) | α-pinene (16.1%), spathulenol (10.7%), epi-globuol (7.8%), β-pinene (7.3%) | — | [32] |
| E. patrisii | Maracanã, Pará, Brazil | Leaf (HD) | trans-cadin-1,4-diene (16.5%), trans-muurola-3,5-diene (13.3%), E-caryophyllene (11.1%), α-cubebene (9.8%) | — | [25] |
| E. patrisii | São Geraldo do Araguaia, Pará, Brazil | Aerial parts (HD) | 2E,6E-Farnesol (34.5%), 2E,6Z-Farnesol (23.2%), caryophylla-4(12)-8(13)-dien-5β-ol (15.6%) | Antioxidant, DPPH assay (40.9%, 111.2 mgTE/mL, 91.3 mgBHAE/mL) Cytotoxic (HCT-116 human colorectal carcinoma, IC50 16.4 μg/mL; MRC5 human Fibroblast IC50 18.1 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 > 25 μg/mL; SKMEL-19 human melanoma, IC50 > 25 μg/mL) | [37] |
| E. pitanga | Manuel Viana, Rio Grande do Sul, Brazil | Leaf (HD) | germacrene D (29.3%), bicyclogermacrene (22.4%), E-β-ocimene (10.5%), E-caryophyllene (5.1%) | — | [32] |
| E. platysema | Blumenau, Santa Catarina, Brazil | Leaf (HD) | β-selinene (17.9%), allo-aromadendrene (12.6%), 7-epi-α-selinene (10.4%) | — | [27] |
| E. pluriflora | Aparados da Serra, Rio Grande do Sul, Brazil | Leaf (HD) | E-nerolidol (24.6%), α-pinene (24.0%), 1,8-cineole (12.7%), α-terpineol (5.8%) | — | [27] |
| E. polystachya | Parauapebas, Pará, Brazil | Aerial parts (HD) | germacrene D (18.4%), ishwarane (15.7%), 7-epi-α-selinene (7.5%), bicyclogermacrene (5.1%), α-ylangene (5.0%) | Antioxidant, DPPH assay (11.5%) Cytotoxic (HCT-116 human colorectal carcinoma, IC50 10.3 μg/mL; MRC5 human fibroblast IC50 > 25 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 > 25 μg/mL; SKMEL-19 human melanoma, IC50 > 25 μg/mL) | [37] |
| E. protenta | Maracanã, Pará, Brazil | Leaf (HD) | germacrene D (15.1%), β-elemene (12.8%), δ-cadinene (9.3%), E-caryophyllene (8.3%), bicyclogermacrene (5.9%), α-cadinol (5.5%) | — | [25] |
| E. protenta | Santarém Novo, Pará, Brazil | Leaf (HD) | dimethylxanthoxylin (73.2%), limonene (5.9%) | — | [23] |
| E. protenta | Santarém Novo, Pará, Brazil | Leaf (HD) | dimethylxanthoxylin (83.0%) | — | [23] |
| E. protenta | Santarém Novo, Pará, Brazil | Leaf (HD) | selin-11-en-4α-ol (18.3%), β-elemene (16.9%), β-selinene (5.6%), δ-cadinene (5.5%), γ-selinene (5.1%) | — | [23] |
| E. protenta | Magalhães Barata, Pará, Brazil | Leaf (HD) | selin-11-en-4α-ol (14.4%), β-elemene (12.3%), α-selinene (6.5%), cubenol (5.7%) | — | [23] |
| E. protenta | Maracanã, Pará, Brazil | Leaf (HD) | β-elemene (9.2%), germacrene D (7.8%), α-cadinol (8.1%) | — | [23] |
| E. protenta | Maracanã, Pará, Brazil | Leaf (HD) | germacrene D (15.1%), bicyclogermacrene (11.8%), δ-cadinene (5.8%), α-copaene (5.1%), E-caryophyllene (5.1%) | — | [23] |
| E. protenta | Maracanã, Pará, Brazil | Leaf (HD) | δ-cadinene (15.4%), germacrene D (11.5%), δ-elemene (8.5%), E-caryophyllene (7.9%), α-copaene (6.2%) | — | [23] |
| E. protenta | Algodoal island, Maracanã, Pará, Brazil | Leaf (HD) | germacrene D (15.6%), δ-cadinene (8.5%), α-cadinol (6.9%), bicyclogermacrene (6.8%), τ-muurolol (5.9%), E-caryophyllene (5.7%) | — | [23] |
| E. punicifolia | Serra Negra, Pernambuco, Brazil | Leaf (HD) | linalool (44.0%), E-caryophyllene (22.7%), α-terpineol (8.8%) | — | [43] |
| E. punicifolia | Brejo da Madre de Deus, Pernambuco, Brazil | Leaf (HD) | linalool (61.2%), E-caryophyllene (16.2%), α-terpineol (6.7%) | — | [43] |
| E. punicifolia | Maracanã, Pará, Brazil | Leaf (HD) | E-caryophyllene (9.9%), bicyclogermacrene (8.7%), E-β-ocimene (5.5%), germacrene D (5.4%) | — | [25] |
| E. punicifolia | Macaé, Rio de Janeiro, Brazil | Leaf (HD) | α-cadinol (10.6%), 10-epi-γ-eudesmol (10.2%), paradisiol (9;0%), 7-epi-α-selinene (6.8%) | — | [28] |
| E. pyriformis | Porto Alegre, Rio Grande do Sul, Brazil | Leaf (HD) | α-cadinol (14.0%), δ-cadinene (12.4%), τ-cadinol (11.9%), bicyclogermacrene (10.2%) | — | [30] |
| E. pyriformis | Curitiba, Paraná, Brazil | Leaf (HD) b | β-pinene (0.0-25.7%), limonene (0.2-22.0%), caryophyllene oxide (3.9-21.3%), 1,8-cineole (0.6-14.7%) | — | [44] |
| E. pyriformis | Curitiba, Paraná, Brazil | Flower (HD) | E-caryophyllene (22.8%), germacrene D (15.3%), bicyclogermacrene (8.4%), α-cadinol (7.2%), spathulenol (6.8%) | — | [44] |
| E. pyriformis | Curitiba, Paraná, Brazil | Fruits (HD) | caryophyllene oxide (16.2%), limonene (12.4%), α-terpineol (5.4%), α-cadinol (5.4%) | — | [44] |
| E. ramboi | Torres, Rio Gande do Sul, Brazil | Leaf (HD) | β-elemene (10.6%), bicyclogermacrene (9.7%), E-caryophyllene (6.2%) | — | [27] |
| E. repanda | Porto Rico, Paraná, Brazil | Leaf (HD) | E-caryophyllene (16.3%), α-humulene (10.2%), bicyclogermacrene (9.4%), germacrene D (7.5%), β-elemene (7.3%) | — | [61] |
| E. selloi | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (HD) b | bicyclogermacrene (15.2-24.3%), germacrene D (5.7-18.7%), E-caryophyllene (6.7-12.5%), viridiflorol (3.5-9.1%) | — | [26] |
| E. speciosa | Itapuã, Rio Grande do Sul, Brazil | Leaf (HD) | α-pinene (47.3%), limonene (23.0%), γ-terpinene (2.5%) | — | [32] |
| E. stictopetala | São Luis, Maranhão, Brazil | Leaf (HD) | 𝛾-elemene (17.48%), E-caryophyllene (16.46%), bicyclogermacrene (8.11%), 𝛽-pinene (7.08%), germacrene D (5.64%) | Larvicidal (Aedes aegypti, LC50 230 μg/mL) | [63] |
| E. stigmatosa | Peruibe, São Paulo, Brazil | Leaf (HD) | physeteric acid (90.5%) | — | [62] |
| E. stipitata | São Miguel island, Açores, Portugal | Leaf (HD) | E-caryophyllene (22.7%), caryophyllene oxide (15.4%), α-pinene (14.1%), 4,8-α-epoxy-caryophyllene (10.9%) a | Antibacterial, agar diffusion assay (Staphylococcus aureus, 14 mm; Pseudomonas aeruginosa, 11 mm; Listeria monocytogenes, 12 mm) | [77] |
| E. stipitata | Recife, Pernambuco, Brazil | Fruit (SPE) | germacrene D (38.3%), β-pinene (15.2%), α-pinene (10.4%), NI (12.6%) | — | [90] |
| E. stipitata | Florencia-Caquetá, Colombia | Fruit (SD) | limonene (81.0%), myrcene (5.5%) | — | [91] |
| E. sulcata | Restinga de Jurubatiba, Rio de Janeiro, Brazil | Leaf (HD) | E-caryophyllene (24.6%), α-pinene (17.2%), β-pinene (10.9%), 1,8-cineole (5.6%), α-humulene (5.1%) | Enzyme inhibitory (acetylcholinesterase, IC50 4.66 μg/mL); Insecticidal (Dysdercus peruvianus and Oncopeltus fasciatus) | [45,46] |
| E. sulcata | Jureia, São Paulo, Brazil | Leaf (HD) | α-pinene (34.2%), E-caryophyllene (15.0%), globulol (11.8%), spathulenol (7.0%) | — | [32] |
| E. sulcata | Restinga de Jurubatiba, Rio de Janeiro, Brazil | Fine stem (HD) | E-caryophyllene (18.8%), spathulenol (8.8%), calamenene (7.0%), γ-cadinene (5.8%), α-humulene (5.6%), caryophyllene oxide (5.6%) | — | [46] |
| E. sulcata | Macaé, Rio de Janeiro, Brazil | Leaf (HD) | 1,8-cineole (19.0%), α-pinene (16.9%), β-pinene (14.5%), caryophyllene oxide (9.8%) | — | [28] |
| E. triquetra | Pueblo Hondo, Táchira, Venezuela | Leaf (HD) | linalool (17.5%), limonene (16.9%), α-pinene (11.6%), β-pinene (8.7%) | Larvicidal (Aedes aegypti, LC50 64.8 ppm) | [65] |
| E. uruguayensis | Torres, Rio Grande do Sul, Brazil | Leaf (HD) | α-pinene (23.5%), β-pinene (11.8%), E-caryophyllene (9.5%), caryophyllene oxide (6.4%) | — | [27] |
| E. uniflora | Pelotas, Rio Grande do Sul, Brazil | Leaf (HD) | germacrene B (21.2%), selin-1,3,7-(11)-trien-8-one epoxide (19.3%), E-caryophyllene (12.6%), germacrene A (11.6%), germacrene D (11.4%), selina-1,3,7-(11)-trien-8-one (9.7%) | Antimicrobial, agar diffusion assay and broth dilution assay (Staphylococcus aureus, MIC 800 μg/mL; Listeria monocytogenes, MIC 1040 μg/mL; Candida lipolytica, MIC 93.7 μg/mL; C. guilliermondii, MIC 109.4 μg/mL). Antioxidant (DPPH radical scavenging assay, IC50 833 μg/mL; ABTS IC50 8.1 μg/mL). Hepatoprotective effect in rats | [57,88] |
| E. uniflora | São Lourenço do Sul, Rio Grande do Sul, Brazil | Leaf (HD) | NI (38.28%), E-caryophyllene (8.18%), spathulenol (7.71%), 9,10-dehydro-iso-longifolene (6.21%), viridiflorol (5.78%), allo-aromadendrene (5.66%), dihydro-cis-α-copaene-8-ol (5.24%), δ-cadinene (5.19%), τ-cadinol (5.08%) | Antibacterial, broth dilution assay (Enterobacter aerogenes MIC 3.125%; Salmonella typhimurium (MIC 3.125%) | [78] |
| E. uniflora | São Luís, Maranhão, Brazil | Leaf (HD) | curzerene (47.3%), γ-elemene (14.2%), trans-β-elemenone (10.4%), β-elemene (5.5%) | Antileishmanial (L. amazonensis promastigotes, IC50 3.04 μg/mL; L. amazonensis amastigotas, IC50 1.92 μg/mL) | [47] |
| E. uniflora | Osasco, São Paulo, Brazil | Leaf (SD) | atractilone (26.78%), curzerene (17.96%), germacrene B (9.31%) | Antimicrobial, agar diffusion assay and broth dilution assay (Streptococcus equi, MIC 7500 μg/mL; Staphylococcus epidermis, MIC 7500 μg/mL; Candida dubliniensis, MIC 230 μg/mL;C. tropicalis, MIC 900 μg/mL; C. albicans, MIC 1800 μg/mL; C. glabrata, MIC 930 μg/mL; C. parapsilosis, MIC 3750 μg/mL; C. grubii serotype A, MIC 450 μg/mL; C. gattii serotype C, MIC 1800 μg/mL; C. gattii serotype B, MIC 220 μg/mL; C. neoformans serotype D, MIC 110 μg/mL; Saccharomyces cerevisiae, MIC 220 μg/mL) | [51] |
| E. uniflora | Águas de Santa Bárbara, São Paulo, Brazil | Leaf (SD) | selina-1,3,7-(11)-trien-8-one (34.0%), selina-1,3,7-(11)-trien-8-one epoxide (17.0%), germacrene B (10.5%), curzerene (5.5%), E-caryophyllene (5.0%) | — | [53] |
| E. uniflora | Botucatu, São Paulo, Brazil | Leaf (HD) | selina-1,3,7-(11)-trien-8-one (30.1%), selina-1,3,7-(11)-trien-8-one epoxide (21.89%), E-caryophyllene (6.1%) | Antibacterial, broth dilution assay (Staphylococcus aureus, MIC 55,600.0 μg/mL; Staphylococcus aureus – methicillin resistant, MIC 56,000.0 μg/mL; Staphylococcus aureus – methicillin sensitive, MIC 50,800.0 μg/mL; Salmonella spp., MIC 92,400.0 μg/mL; Salmonella enteritidis, MIC 92,400.0 μg/mL; Salmonella typhimurium, MIC 92,400.0 μg/mL; Escherichia coli, MIC 84,300.0 μg/mL; Pseudomonas aeruginosa, MIC 92,400.0 μg/mL) | [76] |
| E. uniflora | Seropédica, Rio de Janeiro, Brazil | Young leaf (HD) | germacrone (37.86%), curzerene (16.6%), germacrene B (13.59%), E-caryophyllene (6.02%) | — | [92] |
| E. uniflora | Seropédica, Rio de Janeiro, Brazil | Old leaf (HD) | curzerene (22.37%), furanodiene (18.99%), germacrene B (14.39%), E-caryophyllene (9.35%) | — | [92] |
| E. uniflora | Anápolis, Goiás, Brazil | Aerial parts (HD) b | selina-1,3,7-(11)-trien-8-one (43.0%), selina-1,3,7-(11)-trien-8-one epoxide (20.0-29.0%), spathulenol (7.5-10.0%) | — | [54] |
| E. uniflora | Belém, Pará, Brazil | Aerial parts (HD) | germacrone (32.8%), curzerene (30.0%), germacrene B (15.6%) | — | [55] |
| E. uniflora | Santarém, Pará, Brazil | Leaf (HD) | selina-1,3,7(11)-trien-8-one (32.6%), selina-1,3,7(11)-trien-8-one epoxide (30.4%), E-caryophyllene (5.4%), germacrene B (5.0%) | Antioxidant, DPPH radical scavenging assay (30.3%, 153.5 mg.TE/mL); Antioxidant, β-carotene/linoleic acid assay at 1 mg/mL, (10.8%) | [48] |
| E. uniflora | Belém, Pará, Brazil | Leaf (HD) | selina-1,3,7(11)-trien-8-one (43.1%), selina-1,3,7(11)-trien-8-one epoxide (21.7%), germacrene B (5.9%) | Antioxidant, DPPH radical scavenging assay (35.3%, 178.8 mg.ET/mL); Antioxidant, β-carotene/linoleic acid assay at 1 mg/mL (23.0%); Cytotoxic (HCT- human colon carcinoma, IC50 16.26 μg/mL; MRC5 human fibroblast IC50 10.27 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 12.60 μg/mL; SKMEL-19 human melanoma, IC50 12.20 μg/mL) | [48] |
| E. uniflora | Belém, Pará, Brazil | Leaf (HD) | caryophyllene oxide (18.1%), selina-1,3,7(11)-trien-8-one (18.1%), selina-1,3,7(11)-trien-8-one epoxide (16.0%), β-elemene (8.9%), E-caryophyllene (6.1%) | Antioxidant, DPPH assay (45.1%, 228.3 mg.ET/mL); Antioxidant, β-carotene/linoleic acid assay at 1mg/mL (16.8%); Cytotoxic (HCT-116 human colon carcinoma, IC50 > 25 μg/mL; MRC5 human fibroblast IC50 > 25 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 > 25 μg/mL; SKMEL-19 human melanoma, IC50 >25 μg/mL) | [48] |
| E. uniflora | Bahia, Brazil | Leaf (SPME) | germacrene B (9.5%), γ-elemene (8.5%), β-elemene (7.4%), E-caryophyllene (7.1%), germacrene D (5.5%), γ-muurolene (5.3%), germacrone (5.1%) | — | [58] |
| E. uniflora | Belém, Pará, Brazil | Leaf (HD) | curzerene (50.6%), germacrene B (5.3%) | Antioxidant, DPPH assay (42.8%, 217.0 mg.ET/mL); Antioxidant, β-carotene/linoleic acid assay at 1mg/mL (20.1%) Cytotoxic (HCT-116 human colon carcinoma, IC50 9.28 μg/mL; MRC5 human fibroblast IC50 14.95 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 8.73 μg/mL; SKMEL-19 human melanoma, IC50 15.42 μg/mL) | [48] |
| E. uniflora | Belém, Pará, Brazil | Leaf (HD) b | curzerene (34.4-53.1%), germacrone (0.2–10.5%), globulol (1.5–7.4%), germacrene B (0.1–7.5%), spathulenol (0.5–7.0%), viridiflorol (0.8–6.2%), β-elemene (1.8–5.8%) | Antioxidant, DPPH assay (42.6-64.2%, 186.9-400.3 mg.ET/g) | [73] |
| E. uniflora | Belém, Pará, Brazil | Leaf (HD) | germacrene B (18.4%), curzerene (13.4%), E-caryophyllene (9.1%), γ-elemene (7.8%), β-elemene (7.4%) | Antioxidant, DPPH assay (40.6%, 205.6 mg.ET/mL); Antioxidant β-carotene/linoleic acid (26.3%); Cytotoxic (HCT-116 human colon carcinoma, IC50 > 25 μg/mL; MRC5 human fibroblast IC50 > 25 μg/mL; AGP-01 human gastric adenocarcinoma, IC50 > 25 μg/ML; SKMEL-19 human melanoma, IC50 >25 μg/mL) | [48] |
| E. uniflora | Bahia, Brazil | Leaf (SPME) | germacrene B (9.0%), γ-elemene (8.1%), γ-muurolene (7.7%), germacrene D (6.1%), β-elemene (5.0%) | — | [58] |
| E. uniflora | Bahia, Brazil | Leaf (SPME) | germacrene B (9.6%), β-elemene (9.3%), γ-elemene (8.0%), germacrene D (6.5%), E-caryophyllene (5.2%), γ-muurolene (5.0%) | — | [58] |
| E. uniflora | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (HD) | atractilone, 3-furanoeudesmene | Antinociceptive (ED50 218.6 mg/kg) and hypothermic in mouse model | [52] |
| E. uniflora | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (HD) | curzerene (50.2%), β-elemene (5.9%) | — | [50] |
| E. uniflora | Goiás, Brazil | Leaf (HD) | germacrene B (21.6%), curzerene (20.5%), germacrone (17.3%), E-caryophyllene (8.7%) | Antifungal, broth dilution assay (Paracoccidioides brasiliensis, MIC 500 µg/mL; MFC 500 µg/mL) | [49] |
| E. uniflora | Goiás, Brazil | Leaf (HD) | curzerene (42.6%), germacrone (13.5%), germacrene D (8.8%), germacrene A (7.4%), E-caryophyllene (7.0%) | Antifungal, broth dilution assay (Paracoccidioides brasiliensis, MIC 62.5 µg/mL; MFC 125 µg/mL) | [49] |
| E. uniflora | Goiás, Brazil | Leaf (HD) | selin-1,3,7-(11)-trien-8-one (48.2%), selin-1,3,7-(11)-trien-8-one epoxide (19.3%), atractilone (11.7%), germacrene B (5.9%) | Antifungal, broth dilution assay (Paracoccidioides brasiliensis, MIC 250 µg/mL; MFC 250 µg/mL) | [49] |
| E. uniflora | Pelotas, Rio Grande do Sul, Brazil | Fruits (HD) | hexadecanoic acid (11.7%), E-β-ocimene (7.4%), α-selinene (7.2%), germacrene B (7.2%), bicyclogermacrene (5.2%) | Antioxidant, DPPH assay (TLC method) | [40] |
| E. verticillata | Caraguatatuba, São Paulo, Brazil | Leaf (HD) | valerianol (28.1%), 10-epi-γ-eudesmol (12.6%), E-caryophyllene (10.9%), α-selinene (6.1%), δ-cadinene (5.7%) | Enzyme inhibitory (acetylcholinesterase, IC50 67.3 μg/mL) | [64] |
| E. xiriricana | Peruíbe, São Paulo, Brazil | Leaf (HD) | spathulenol (15.4%), β-pinene (14.1%), globulol (8.6%), α-pinene (7.8%) | — | [32] |
| S. cumini | Rio de Janeiro, Rio de Janeiro, Brazil | Leaf (HD) | α-pinene (22.2%), Z-β-ocimene (10.2%), E-caryophyllene (9.45%), limonene (7.3%), α-terpineol (7.00%), E-β-ocimene (5.88%), α-humulene (5.5%) | Anti-inflammatory, lipopolysaccharide-induced pleurisy model, an eosinophil was inhibited in 67% | [66] |
| S. cumini | São Luis, Maranhão, Brazil | Leaf (HD) | α-pinene (31.85%), Z-β-ocimene (28.98%), E-β-ocimene (11.71%), β-pinene (5.57%), E-caryophyllene (5.02%) | Antileishmanial (Leishmania amazonensis promastigotes, IC50 60 μg/mL); Molluscicide (Biomphalaria glabrata, LC50 90 μg/mL) | [67] |
| S. cumini | Crato, Ceará, Brazil | Leaf (HD) | α-pinene (48.1%), E-nerolidol (8.7%), nerol (7.1%), nonanol (6.8%) a | Antibacterial, broth dilution assay (Staphylococcus aureus, MIC 128 µg/mL; Escherichia coli MIC > 1024 μg/mL) | [68] |
| S. cumini | Crato, Ceará, Brazil | Leaf (HD) | α-pinene (30.0%), E-β-ocimene (26.8%), β-ocimene (11.1%), β-pinene (8.3%), l imonene (6.5%), β-Fenchol (7.3%) a | — | [69] |
| S. cumini | Juiz de Fora, Minas Gerais, Brazil | Leaf (HD) | α-humulene (25.24%), E-caryophyllene (16.00%), α-terpineol (9.08%), globulol (5.23%) | Anti-inflammatory; Antibacterial (Mycobacterium bovis) | [70] |
| S. cumini | Porto Rico, Paraná, Brazil | Seed (HD) b | E-caryophyllene (6.4-42.5%), α-humulene (6.6-22.2%), caryophyllene oxide (5.3-37.3%), humulene epoxide II (2.1-17.1%) | — | [93] |
| S. jambos | Rio Verde, Goiás, Brazil | Leaf (HD) b | E-caryophyllene (0–10.86%), α-humulene (0.0-7.07%), α-zingiberene (0.97–17.73%), hydroxytoluenebuthyled (3.44–32.82%), caryophyllenyl alcohol (1.48–17.14%), caryolan-8-ol (0–10.75%), caryophyllene oxide (0–5.05%), tujopsan-2-α-ol (1.01–12.19%), heneicosane (1.73–18.0%) a | — | [74] |
Appendix B




References
- Parnell, J.A.N.; Craven, L.A.; Biffin, E. Matters of Scale: Dealing with One of the Largest Genera of Angiosperms. In Reconstructing the Tree of Life: Taxonomy and Systematics of Species Rich Taxa; Hodkinson, T.R., Parnell, J.A.N., Eds.; Taylor and Francis: Boca Raton, IL, USA, 2007; pp. 251–273. ISBN 9780429128097. [Google Scholar]
- THE ANGIOSPERM PHYLOGENY GROUP. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Mazine, F.F.; Faria, J.E.Q.; Giaretta, A.; Vasconcelos, T.; Forest, F.; Lucas, E. Phylogeny and biogeography of the hyper-diverse genus Eugenia (Myrtaceae: Myrteae), with emphasis on E. sect. Umbellatae, the most unmanageable clade. TAXON 2018, 67, 752–769. [Google Scholar] [CrossRef]
- Craven, L.; Biffin, E. An infrageneric classification of Syzygium (Myrtaceae). Blumea Biodivers. Evol. Biogeogr. Plants 2010, 55, 94–99. [Google Scholar] [CrossRef]
- Van Der Merwe, M.M.; Van Wyk, A.E.E.; Botha, A.M. Molecular phylogenetic analysis of Eugenia L. (Myrtaceae), with emphasis on southern African taxa. Plant Syst. Evol. 2004, 251, 21–34. [Google Scholar] [CrossRef]
- Wilson, P.G. Conspectus of the genus Eugenia (Myrtaceae) in the Philippines. Gard. Bull. Singapore. 2009, 60, 399–410. [Google Scholar]
- Gressler, E.; Pizo, M.A.; Morellato, L.P.C. Polinização e dispersão de sementes em Myrtaceae do Brasil. Braz. J. Bot. 2006, 29, 509–530. [Google Scholar] [CrossRef]
- Siani, A.; Sampaio, A.L.F.; Souza, M.C.; Henriques, M.G.M.O.; Ramos, M.F. Óleos essenciais: Potencial antiinflamatório. Biotecnol. Ciência e Desenvolv 2000, 16, 38–46. [Google Scholar]
- De Queiroz, J.M.G.; Suzuki, M.C.M.; Motta, A.P.R.; Nogueira, J.M.R.; De Carvalho, E.M. Aspectos populares e científicos do uso de espécies de Eugenia como fitoterápico. Rev. Fitos 2015, 9, 87–100. [Google Scholar] [CrossRef]
- De Souza, A.M.; De Oliveira, C.F.; Oliveira, V.B.; Betim, F.C.M.; Miguel, O.G.; Miguel, M.D. Traditional Uses, Phytochemistry, and Antimicrobial Activities of Eugenia Species – A Review. Planta Medica 2018, 84, 1232–1248. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Baider, C.; Bernardini, B. Syzygium (Myrtaceae): Monographing a taxonomic giant via 22 coordinated regional revisions. PeerJ Prepr. 2016, 4, e1930v1. [Google Scholar] [CrossRef]
- Soh, W.K. Taxonomy of Syzygium. In The Genus Syzygium: Syzygium cumini and Other Underutilized Species; Nair, K.N., Ed.; Taylor & Francis: New York, NY, USA, 2017; pp. 1–5. ISBN 9788578110796. [Google Scholar]
- Myrtaceae in Lista de Espécies da Flora do Brasil. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 30 July 2020).
- Cavalcante, P.B. Frutas Comestíveis na Amazônia; 7th ed.; Museu Paraense Emilio Goeldi: Belém, Brazil, 2010. [Google Scholar]
- Sujanapal, P.; Kunhikannan, C. The Genus Syzygium in Western Ghats. In The Genus Syzygium: Syzygium cumini and Other Underutilized Species; Nair, K.N., Ed.; Taylor & Francis: New York, NY, USA, 2017; pp. 15–56. ISBN 9781315118772. [Google Scholar]
- Mala Ranghoo-Sanmukhiya, V.; Chellan, Y.; Govinden-Soulange, J.; Lambrechts, I.; Stapelberg, J.; Crampton, B.G.; Lall, N. Biochemical and phylogenetic analysis of Eugenia and Syzygium species from Mauritius. J. Appl. Res. Med. Aromat. Plants 2019, 12, 21–29. [Google Scholar] [CrossRef]
- Lima, L.A.; Siani, A.C.; Brito, F.A.; Sampaio, A.L.F.; Henriques, M.D.G.; Riehl, C.A. Correlation of anti-inflammatory activity with phenolic content in the leaves of Syzygium cumini (l.) skeels (myrtaceae). Química Nova 2007, 30, 860–864. [Google Scholar] [CrossRef]
- de Melo, R.R.; de Araújo, É.R.S.; da Silva, A.A.L.; Randau, K.P.; de Azevedo Ximenes, E.C.P. Características farmacobotânicas, químicas e biológicas de Syzygium malaccense (L.) Merr.& l. M. Perry. Rev. Bras. Farmacol 2009, 90, 298–302. [Google Scholar]
- Sujanapal, P.; Sankaran, K.V. Syzygium aqueum (Burm. f.) Alston. In Common Plants of Maldives; Sujanapal, P., Sankaran, K.V., Eds.; Food and Agriculture Organization of the United Nations; Kerala Forest Research Institute: Bangkok, Thailand, 2016; p. 258. ISBN 9789251092958. [Google Scholar]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. Analysis of Volatile Compounds by Advanced Analytical Techniques and Multivariate Chemometrics. Chem. Rev. 2017, 117, 6399–6422. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, M.E.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- Zoghbi, M.; Guilhon, G.; Sarges, F.; Pereira, R.; Oliveira, J. Chemical variability of the volatiles from the leaves of Eugenia protenta McVaugh (Myrtaceae) growing wild in the North of Brazil. Biochem. Syst. Ecol. 2011, 39, 660–665. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Fernandes, H.A.; Da Silva, A.R.C.; Alves, N.S.F.; Setzer, W.N.; Da Silva, J.K.R.; Maia, J.G.S. Variability in the Chemical Composition of Eugenia biflora Essential Oils from the Brazilian Amazon. Nat. Prod. Commun. 2019, 14, 1934578–19892439. [Google Scholar] [CrossRef]
- Pereira, R.A.; Zoghbi, M.D.G.B.; Bastos, M.D.N.D.C. Essential Oils of Twelve Species of Myrtaceae Growing Wild in the Sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants 2010, 13, 440–450. [Google Scholar] [CrossRef]
- Defaveri, A.C.A.; Sato, A.; Borré, L.B.; Aguiar, D.L.M.; Gil, R.A.S.S.; Arruda, R.C.O.; Riehl, C.A.S. Eugenia neonitida Sobral and Eugenia rotundifolia Casar. (Myrtaceae) essential oils: Composition, seasonality influence, antioxidant activity and leaf histochemistry. J. Braz. Chem. Soc. 2011, 22, 1531–1538. [Google Scholar] [CrossRef]
- Apel, M.A.; Limberger, R.P.; Sobral, M.; Henriques, A.T.; Ntalani, H.; Vérin, P.; Menut, C.; Bessiere, J.-M. Chemical Composition of the Essential Oils from Southern Brazilian Eugenia Species. Part III. J. Essent. Oil Res. 2002, 14, 259–262. [Google Scholar] [CrossRef]
- Ramos, M.F.D.S.; Monteiro, S.D.S.; Da Silva, V.P.; Nakamura, M.J.; Siani, A.C. Essential Oils From Myrtaceae Species of the Brazilian Southeastern Maritime Forest (Restinga). J. Essent. Oil Res. 2010, 22, 109–113. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Dalmarco, E.M.; Wisniewski, A.; Simionatto, E.L.; Dalmarco, J.B.; Pizzolatti, M.G.; Brighente, I.M.C.; Wisniewski, A. Chemical composition and antibacterial activity of essential oils of Eugenia species. J. Nat. Med. 2009, 63, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Apel, M.A.; Sobral, M.; Schapoval, E.; Henriques, A.T.; Menut, C.; Bessiere, J.-M. Chemical Composition of the Essential Oils of Eugenia beaurepaireana and Eugenia pyriformis: Section Dichotomae. J. Essent. Oil Res. 2004, 16, 191–192. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Pietrovski, E.F.; Gomig, F.; Falkenberg, D.D.B.; Cabrini, D.A.; Otuki, M.F.; Pizzollati, M.G.; Brighente, I.M.C. Topical antiinflammatory activity and chemical composition of the epicuticular wax from the leaves of Eugenia beaurepaireana (Myrtaceae). Braz. J. Pharm. Sci. 2009, 45, 171–176. [Google Scholar] [CrossRef][Green Version]
- Apel, M.A.; Sobral, M.; Schapoval, E.; Henriques, A.T.; Menut, C.; Bessiere, J.-M. Essential Oils from Eugenia Species—Part VII: Sections Phyllocalyx and Stenocalyx. J. Essent. Oil Res. 2004, 16, 135–138. [Google Scholar] [CrossRef]
- Moreno, P.R.H.; Lima, M.E.L.; Sobral, M.; Young, M.C.M.; Cordeiro, I.; Apel, M.A.; Limberger, R.P.; Henriques, A.T. Essential oil composition of fruit colour varieties of Eugenia brasiliensis Lam. Sci. Agricola 2007, 64, 428–432. [Google Scholar] [CrossRef]
- Stefanello, M.; Élida, A.; Cervi, A.C.; Ito, I.Y.; Salvador, M.; Wisniewski, A.; Simionatto, E.L.; Wisniewski, A. Chemical Composition and Antimicrobial Activity of Essential Oils of Eugenia chlorophylla (Myrtaceae). J. Essent. Oil Res. 2008, 20, 75–78. [Google Scholar] [CrossRef]
- Costa, T.R.; Fernandes, O.F.; Santos, S.D.C.; Oliveira, C.M.; Lião, L.M.; Ferri, P.H.; Paula, J.R.; Ferreira, H.D.; Sales, B.H.; Silva, M.D.R.R. Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil. J. Ethnopharmacol. 2000, 72, 111–117. [Google Scholar] [CrossRef]
- Duarte, A.R.; Naves, R.R.; Santos, S.D.C.; Seraphin, J.C.; Ferri, P.H. Genetic and environmental influence on essential oil composition of Eugenia dysenterica. J. Braz. Chem. Soc. 2010, 21, 1459–1467. [Google Scholar] [CrossRef]
- Da Da Silva, J.K.R.; Andrade, E.H.A.; Barreto, L.H.; Da Silva, N.C.F.; Ribeiro, A.F.; Montenegro, R.C.; Maia, J.G.S. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Ciarlini, J.; Marangoni, A.; Bolzan, A. Selectivity of supercritical CO2 extraction and atmospheric pressure techniques for the major volatile compounds of Eugenia involucrata leaves from Southern Brazil. Food Bioprod. Process. 2017, 106, 29–34. [Google Scholar] [CrossRef]
- Sousa, R.M.F.; De Morais, S.A.; Vieira, R.B.; Napolitano, D.R.; Guzman, V.B.; Moraes, T.S.; Cunha, L.C.; Martins, C.H.G.; Chang, R.; De Aquino, F.J.T.; et al. Chemical composition, cytotoxic, and antibacterial activity of the essential oil from Eugenia calycina Cambess. leaves against oral bacteria. Ind. Crop. Prod. 2015, 65, 71–78. [Google Scholar] [CrossRef]
- Marin, R.; Apel, M.A.; Limberger, R.P.; Raseira, M.C.B.; Pereira, J.F.M.; Zuanazzi, J.Â.S.; Henriques, A.T. Volatile Components and Antioxidant Activity from some Myrtaceous Fruits cultivated in Southern Brazil. Lat. Am. J. Pharm. 2008, 27, 172–177. [Google Scholar]
- Carneiro, N.S.; Alves, C.C.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; et al. Chemical composition, antioxidant and antibacterial activities of essential oils from leaves and flowers of Eugenia klotzschiana Berg (Myrtaceae). Anais da Academia Brasileira de Ciências 2017, 89, 1907–1915. [Google Scholar] [CrossRef]
- De Moraes, M.M.; Da Câmara, C.A.G.; Dos Santos, M.L.; Fagg, C.W. Essential oil composition of Eugenia langsdorffii O. Berg.: Relationships between some terpenoids and toxicity against Tetranychus urticae. J. Braz. Chem. Soc. 2012, 23, 1647–1656. [Google Scholar] [CrossRef]
- De Oliveira, R.; Dias, I.; Câmara, C. Estudo comparativo do óleo essencial de Eugenia punicifolia (HBK) DC. de diferentes localidades de Pernambuco. Rev. Bras. de Farm. 2005, 15, 39–43. [Google Scholar] [CrossRef][Green Version]
- Stefanello, M.É.A.; Junior, A.W.; Simionatto, E.L.; Cervi, A.C. Composição Química e Variação Sazonal dos Óleos Essenciais de Eugenia pyriformis (Myrtaceae). Lat. Am. J. Pharm. 2009, 28, 449–453. [Google Scholar]
- Gonzalez, M.S.; Lima, B.G.; Oliveira, A.F.; Nunes, D.D.; Fernandes, C.P.; Santos, M.G.; Tietbohl, L.A.; Mello, C.B.; Rocha, L.; Feder, D. Effects of essential oil from leaves of Eugenia sulcata on the development of agricultural pest insects. Rev. Bras. de Farm. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Lima, B.G.; Tietbohl, L.A.C.; Fernandes, C.P.; Cruz, R.A.S.; da Botas, G.S.; Santos, M.G.; Silva-Filho, M.V.; Rocha, L. Chemical composition of essential oils and anticholinesterasic activity of Eugenia sulcata spring ex mart. Lat. Am. J. Pharm. 2012, 31, 152–155. [Google Scholar]
- Rodrigues, K.A.D.F.; Amorim, L.V.; De Oliveira, J.M.G.; Noleto, C.; Moraes, D.F.C.; Andrade, E.H.A.; Maia, J.G.S.; Carneiro, S.M.P.; Carvalho, F.A.D.A. Eugenia uniflora L. Essential Oil as a Potential Anti-Leishmania Agent: Effects on Leishmania amazonensis and Possible Mechanisms of Action. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.B.; Pinto, L.C.; Da Costa, J.S.; Da Silva, A.R.C.; Mourão, R.; Montenegro, R.C.; Da Silva, J.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.P.; Filho, E.D.G.A.; Silva, L.M.A.; Santos, S.D.C.; Passos, X.S.; Silva, M.D.R.R.; Seraphin, J.C.; Ferri, P.H. Influence of fruit biotypes on the chemical composition and antifungal activity of the essential oils of Eugenia uniflora leaves. J. Braz. Chem. Soc. 2010, 21, 851–858. [Google Scholar] [CrossRef]
- Melo, R.M.; Corrêa, V.F.S.; Amorim, A.C.L.; Miranda, A.L.P.; Rezende, C.M. Identification of impact aroma compounds in Eugenia uniflora L. (Brazilian Pitanga) leaf essential oil. J. Braz. Chem. Soc. 2007, 18, 179–183. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Souza, E.D.; Mariane, B.; Pascon, R.; Vallim, M.A.; Martins, R.C.C.; Baroli, A.A.; Carvalho, B.A.; Soares, M.G.; Santos, R.T.; et al. Chemical and Biological Evaluation of Essential Oils from Two Species of Myrtaceae — Eugenia uniflora L. and Plinia trunciflora (O. Berg) Kausel. Molecules 2011, 16, 9827–9837. [Google Scholar] [CrossRef] [PubMed]
- Amorim, A.C.L.; Lima, C.K.F.; Hovell, A.M.C.; Miranda, A.L.P.; Rezende, C.M. Antinociceptive and hypothermic evaluation of the leaf essential oil and isolated terpenoids from Eugenia uniflora L. (Brazilian Pitanga). Phytomedicine 2009, 16, 923–928. [Google Scholar] [CrossRef]
- Gallucci, S.; Neto, A.P.; Porto, C.; Barbizan, D.; Costa, I.; Marques, K.; Benevides, P.; Figueiredo, R. Essential Oil of Eugenia uniflora L.: An Industrial Perfumery Approach. J. Essent. Oil Res. 2010, 22, 176–179. [Google Scholar] [CrossRef]
- Costa, D.P.; Santos, S.D.C.; Seraphin, J.C.; Ferri, P.H. Seasonal variability of essential oils of Eugenia uniflora leaves. J. Braz. Chem. Soc. 2009, 20, 1287–1293. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Da Silva, M.H.L.; Zoghbi, M.G.B. A new chemotype of Engenia uniflora L. from North Brazil. J. Essent. Oil Res. 1999, 11, 727–729. [Google Scholar] [CrossRef]
- Victoria, F.N.; de Siqueira Brahm, A.; Savegnago, L.; Lenardão, E.J. Involvement of serotoninergic and adrenergic systems on the antidepressant-like effect of E. uniflora L. leaves essential oil and further analysis of its antioxidant activity. Neurosci. Lett. 2013, 544, 105–109. [Google Scholar] [CrossRef]
- Victoria, F.N.; Lenardão, E.J.; Savegnago, L.; Perin, G.; Jacob, R.G.; Alves, D.; Da Silva, W.P.; Motta, A.D.S.D.; Nascente, P.D.S. Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.R.R.; Nunes, E.C.; Dos Santos, F.; Bastos, L.P.; Costa, M.A.; Rodrigues, F.D.M.; De Andrade, J.B. Discrimination of Eugenia uniflora L. biotypes based on volatile compounds in leaves using HS-SPME/GC–MS and chemometric analysis. Microchem. J. 2017, 130, 79–87. [Google Scholar] [CrossRef]
- Apel, M.A.; Limberger, R.P.; Sobral, M.; Henriques, A.T.; Ntalani, H.; Menut, C.; Bassiere, J.-M. Chemical Composition of the Essential Oils from Southern Brazilian Eugenia Species Part II. J. Essent. Oil Res. 2002, 14, 163–166. [Google Scholar] [CrossRef]
- Nakamura, M.J.; Monteiro, S.S.; Bizarri, C.; Siani, A.C.; Ramos, M.F.D.S. Essential oils of four Myrtaceae species from the Brazilian southeast. Biochem. Syst. Ecol. 2010, 38, 1170–1175. [Google Scholar] [CrossRef]
- Apel, M.A.; Sobral, M.; Henriques, A.T.; Menut, C.; Bessière, J.-M. Chemical Composition of the Essential Oils from Southern Brazilian Eugenia Species. Part IV: Section Racemulosae. J. Essent. Oil Res. 2002, 14, 290–292. [Google Scholar] [CrossRef]
- Apel, M.A.; Sobral, M.; Schapoval, E.; Henriques, A.T.; Menut, C.; Bessiere, J.-M. Chemical Composition of the Essential Oils of Eugenia hyemalis and Eugenia stigmatosa. Part VI: Section Biflorae. J. Essent. Oil Res. 2004, 16, 437–439. [Google Scholar] [CrossRef]
- Noleto-Dias, C.; Alves, L.P.L.; Rodrigues, K.A.D.F.; Brito, M.C.A.; Rosa, C.D.S.; Amaral, F.M.M.D.; Monteiro, O.D.S.; Andrade, E.H.D.A.; Maia, J.G.S.; Moraes, D.F.C. Chemical Composition and Larvicidal Activity of Essential Oils Extracted from Brazilian Legal Amazon Plants against Aedes aegypti L. (Diptera: Culicidae). Evidence-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Souza, A.D.; Lopes, E.M.C.; Silva, M.C.D.; Cordeiro, I.; Young, M.C.M.; Sobral, M.E.G.; Moreno, P.R.H. Chemical composition and acetylcholinesterase inhibitory activity of essential oils of Myrceugenia myrcioides (Cambess.) O. Berg and Eugenia riedeliana O. Berg, Myrtaceae. Rev. Bras. Farmacogn. 2010, 20, 175–179. [Google Scholar] [CrossRef]
- Mora, F.D.; Avila, J.L.; Rojas, L.B.; Ramírez, R.; Usubillaga, A.; Segnini, S.; Carmona, J.; Silva, B. Chemical composition and larvicidal activity of Eugenia triquetra essential oil from Venezuelan Andes. Nat. Prod. Commun. 2010, 5, 1934578–1000500633. [Google Scholar] [CrossRef]
- Siani, A.C.; Souza, M.C.; Henriques, M.; Ramos, M.F.D.S. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Boil. 2013, 51, 881–887. [Google Scholar] [CrossRef]
- Dias, C.N.; Rodrigues, K.A.F.; Carvalho, F.A.A.; Carneiro, S.M.P.; Maia, J.G.S.; Andrade, E.H.A.; Moraes, D.F.C. Molluscicidal and Leishmanicidal Activity of the Leaf Essential Oil of Syzygium cumini (L.) Skeels from Brazil. Chem. Biodivers 2013, 10, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Aquino, P.E.; Júnior, J.G.; Cristo, J.S.; Filho, M.A.V.; Moura, F.F.; Ferreira, N.M.; Silva, M.K.; Nascimento, E.M.; Correia, F.M.; et al. Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. J. Photochem. Photobiol. B Boil. 2017, 174, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sobral-Souza, C.E.; Leite, N.F.; Da Cunha, F.A.B.; Pinho, A.I.; Albuquerque, R.S.; Carneiro, J.N.; Menezes, I.R.; Da Costa, J.G.M.; Franco, J.L.; Coutinho, H.D.M. Cytoprotective effect against mercury chloride and bioinsecticidal activity of Eugenia jambolana Lam. Arab. J. Chem. 2014, 7, 165–170. [Google Scholar] [CrossRef]
- Machado, R.R.P.; Jardim, D.F.; Souza, A.R.; Scio, E.; Fabri, R.L.; Carpanez, A.G.; Grazul, R.M.; de Mendonça, J.P.R.F.; Lesche, B.; Aarestrup, F.M. The effect of essential oil of Syzygium cumini on the development of granulomatous inflammation in mice. Rev. Bras. Farmacogn. 2013, 23, 488–496. [Google Scholar] [CrossRef]
- Siebert, D.A.; Tenfen, A.; Yamanaka, C.N.; De Cordova, C.M.M.; Scharf, D.R.; Simionatto, E.; Alberton, M.D. Evaluation of seasonal chemical composition, antibacterial, antioxidant and anticholinesterase activity of essential oil from Eugenia brasiliensis Lam. Nat. Prod. Res. 2014, 29, 289–292. [Google Scholar] [CrossRef]
- Lima, N.P.; Cerqueira, S.H.F.; Fávero, O.A.; Romoff, P.; Lago, J.H.G. Composition and Chemical Variation of the Essential Oil from Leaves Of Eugenia brasiliensis Lam. And Eugenia sp. (Myrtaceae). J. Essent. Oil Res. 2008, 20, 223–225. [Google Scholar] [CrossRef]
- Da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef]
- Rezende, W.P.; Borges, L.L.; Alves, N.M.; Ferri, P.H.; Paula, J.R. Chemical variability in the essential oils from leaves of Syzygium jambos. Rev. Bras. de Farm. 2013, 23, 433–440. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. ChemInform Abstract: Antimicrobial Natural Products: An Update on Future Antibiotic Drug Candidates. Chemin 2010, 41, 238–254. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Probst, I.D.S.; Andrade, B.F.M.T.; Alves, F.C.B.; Albano, M.; Cunha, M.; Doyama, J.T.; Rall, V.L.; Junior, A.F. In vitro Antibacterial and Chemical Properties of Essential Oils Including Native Plants from Brazil against Pathogenic and Resistant Bacteria. J. Oleo Sci. 2015, 64, 289–298. [Google Scholar] [CrossRef]
- Medeiros, J.; Medeiros, N.; Medeiros, H.; Davin, L.B.; Lewis, N.G. Composition of the Bioactive Essential Oils from the Leaves of Eugenia stipitata McVaugh ssp. sororia from the Azores. J. Essent. Oil Res. 2003, 15, 293–295. [Google Scholar] [CrossRef]
- Becker, N.A.; Volcão, L.M.; Camargo, T.M.; Freitag, R.A.; Ribeiro, G.A. Biological properties of Eugenia uniflora L. essential oil: Chemical composition and antimicrobial activity. VITTALLE Revista de Ciências da Saúde 2017, 29, 22–30. [Google Scholar] [CrossRef]
- Bhattacharjee, A. Anticholinesterase potential of phytoextracts and their bioactive compounds: A promising therapeutic agent against Alzheimer’s disorder. MOJ Tumor Res. 2018, 1, 83–87. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Tirillini, B.; Akhtar, M.S.; Dimitriu, L.; Bricchi, E.; Bertuzzi, G.; Venanzoni, R. Essential Oil with Anticancer Activity: An Overview. In Anticancer Plants: Natural Products and Biotechnological Implements; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; Volume 2, pp. 207–231. [Google Scholar]
- Kaiser, M.; Mäser, P.; Tadoori, L.P.; Ioset, J.-R.; Brun, R. Antiprotozoal Activity Profiling of Approved Drugs: A Starting Point toward Drug Repositioning. PLoS ONE 2015, 10, e0135556. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Monzote, L.; Alarcón, O.; Setzer, W.N. Antiprotozoal activity of essential oils. Agric. Consp. Sci. 2012, 77, 167–175. [Google Scholar]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1–28. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Orčić, D.; Lesjak, M.; Šibul, F.; Zheljazkov, V.; Cantrell, C.L. Essential Oils as Powerful Antioxidants: Misconception or Scientific Fact? In ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2016; Volume 1218, pp. 187–208. [Google Scholar]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Victoria, F.; Anversa, R.; Savegnago, L.; Lenardão, E. Essential oils of E. uniflora leaves protect liver injury induced by acetaminophen. Food Biosci. 2013, 4, 50–57. [Google Scholar] [CrossRef]
- Arruda, R.D.C.D.O.; Victório, C.P. Leaf Secretory Structure and Volatile Compounds of Eugenia copacabanensis Kiaersk. (Myrtaceae). J. Essent. Oil Res. 2011, 23, 1–6. [Google Scholar] [CrossRef]
- Franco, M.R.B.; Shibamoto, T. Volatile Composition of Some Brazilian Fruits: Umbu-caja (Spondias citherea), Camu-camu (Myrciaria dubia), Araça-boi (Eugenia stipitata), and Cupuaçu (Theobroma grandiflorum). J. Agric. Food Chem. 2000, 48, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Oliveros, A.; Morales Pérez, A.L. Composition of arazá (Eugenia stipitata McVaugh) seed essential oil. Momentos Cienc. 2011, 8, 126–130. [Google Scholar]
- Santos, F.R.; Braz-Filho, R.; Castro, R.N. Influência da idade das folhas de Eugenia uniflora L. na composição química do óleo essencial. Quim. Nova 2015, 38, 762–768. [Google Scholar] [CrossRef]
- Scharf, D.R.; Simionatto, E.; Kassuya, C.A.L.; Stefanello, M.; Élida, A. Essential Oil from Eugenia jambolana Seeds: Chemical Composition and Changes During Storage. J. Essent. Oil Bear. Plants 2016, 19, 2077–2082. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, J.S.; da Cruz, E.d.N.S.; Setzer, W.N.; da Silva, J.K.d.R.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules 2020, 10, 1155. https://doi.org/10.3390/biom10081155
da Costa JS, da Cruz EdNS, Setzer WN, da Silva JKdR, Maia JGS, Figueiredo PLB. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules. 2020; 10(8):1155. https://doi.org/10.3390/biom10081155
Chicago/Turabian Styleda Costa, Jamile S., Ellen de Nazaré S. da Cruz, William N. Setzer, Joyce Kelly do R. da Silva, José Guilherme S. Maia, and Pablo Luis B. Figueiredo. 2020. "Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities" Biomolecules 10, no. 8: 1155. https://doi.org/10.3390/biom10081155
APA Styleda Costa, J. S., da Cruz, E. d. N. S., Setzer, W. N., da Silva, J. K. d. R., Maia, J. G. S., & Figueiredo, P. L. B. (2020). Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules, 10(8), 1155. https://doi.org/10.3390/biom10081155