Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ tf/J Mouse Model of Autism
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Drugs and Biochemical Reagents
2.3. Behavioral Assessments
2.3.1. Three-Chamber Test (TCB)
2.3.2. Marble Burying Test (MBB)
2.3.3. Nestlet-Shredding Test (NST)
2.3.4. Elevated Plus Maze Test (EPMT)
2.3.5. Open Field Test (OFT)
2.4. Biochemical Assessments
2.4.1. Brain Collection and Tissue Preparation for AChE Activity Assessment
2.4.2. Immunofluorescence Staining of Iba-1
2.5. Assessment of Cerebral Acetylcholinesterase (AChE) Activity in BTBR Mice
2.6. Statistics
3. Results
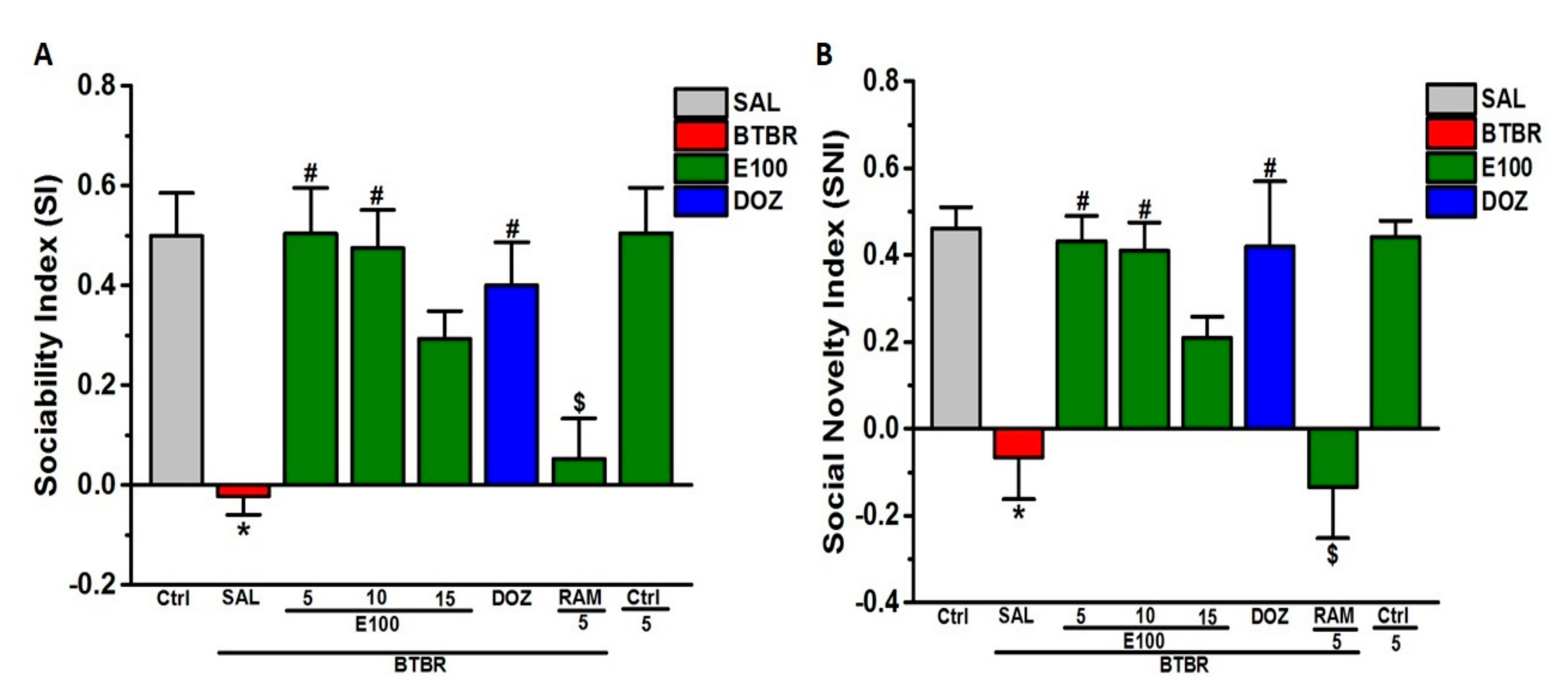
3.1. Effects of E100 on Sociability and Social Novelty Impairments of BTBR Mouse in TCB
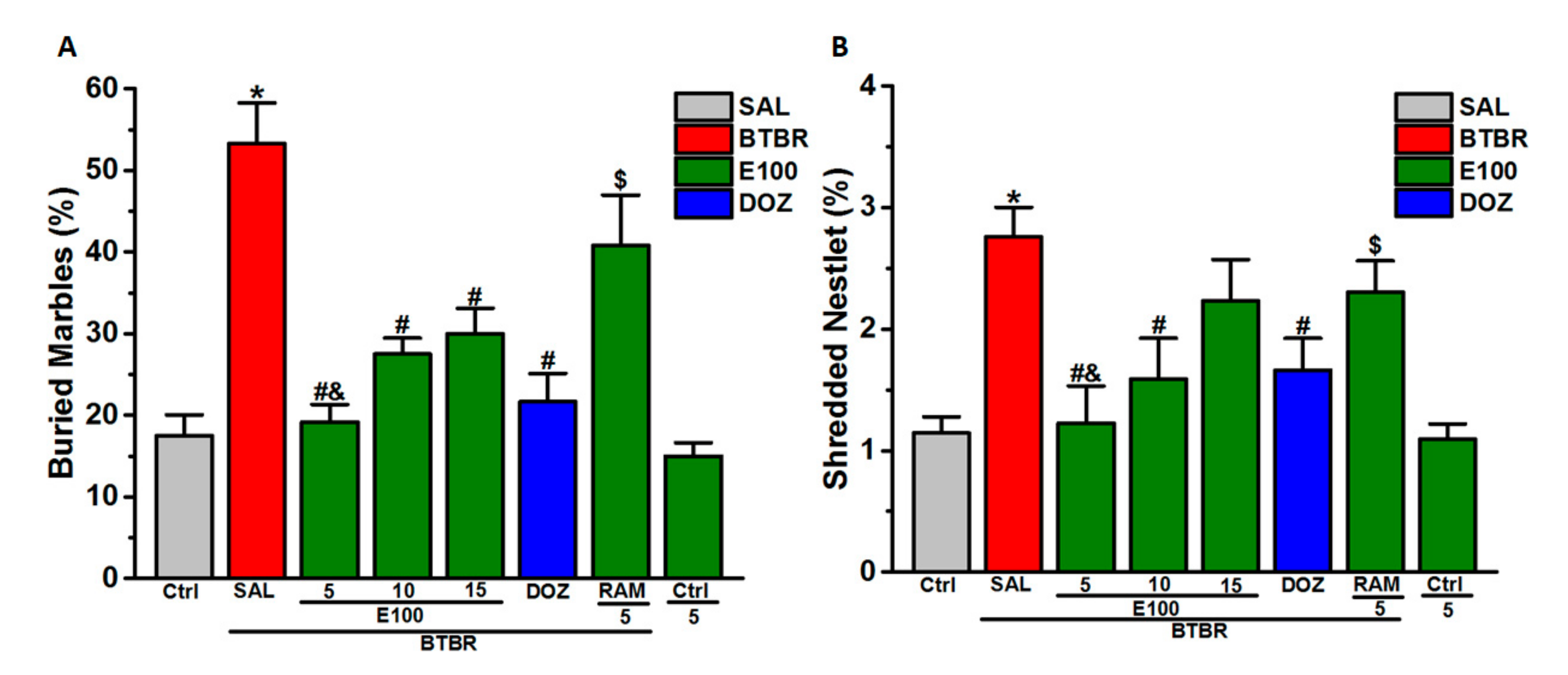
3.2. Effects E100 on Stereotyped Repetitive and Obsessive-Compulsive Behaviors of BTBR Mouse in MBB and NST
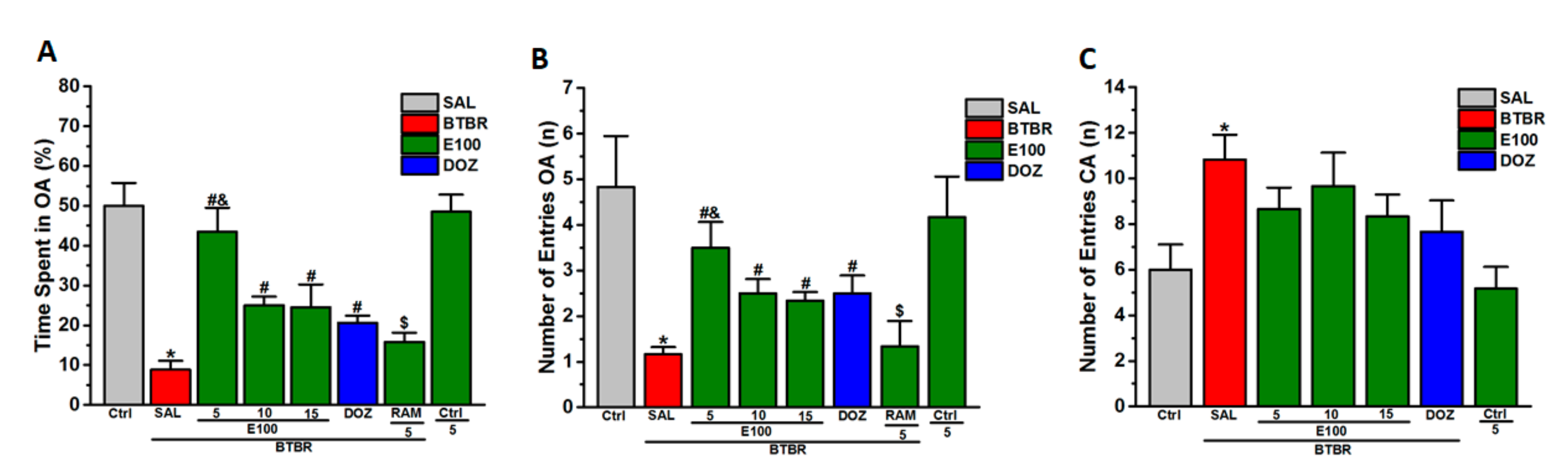
3.3. Effects of E100 on Anxiety Levels and Locomotor Activity of BTBR Mice in EPMT
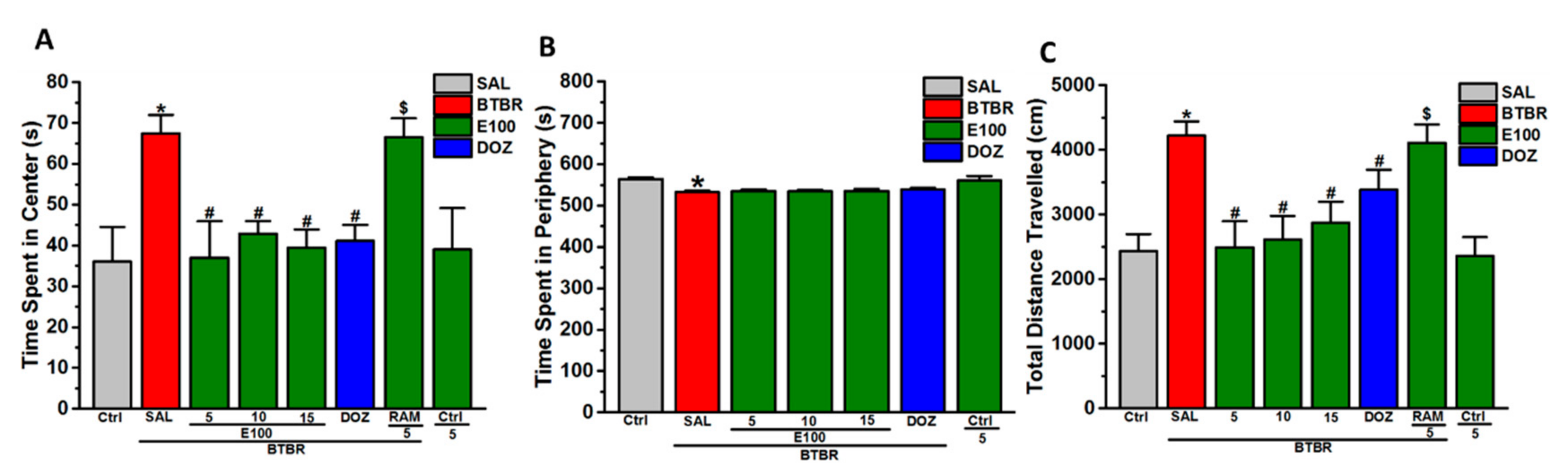
3.4. Effects of E100 on Anxiety-Related Behavior and Locomotor Activity Parameters of BTBR Mice in OFT
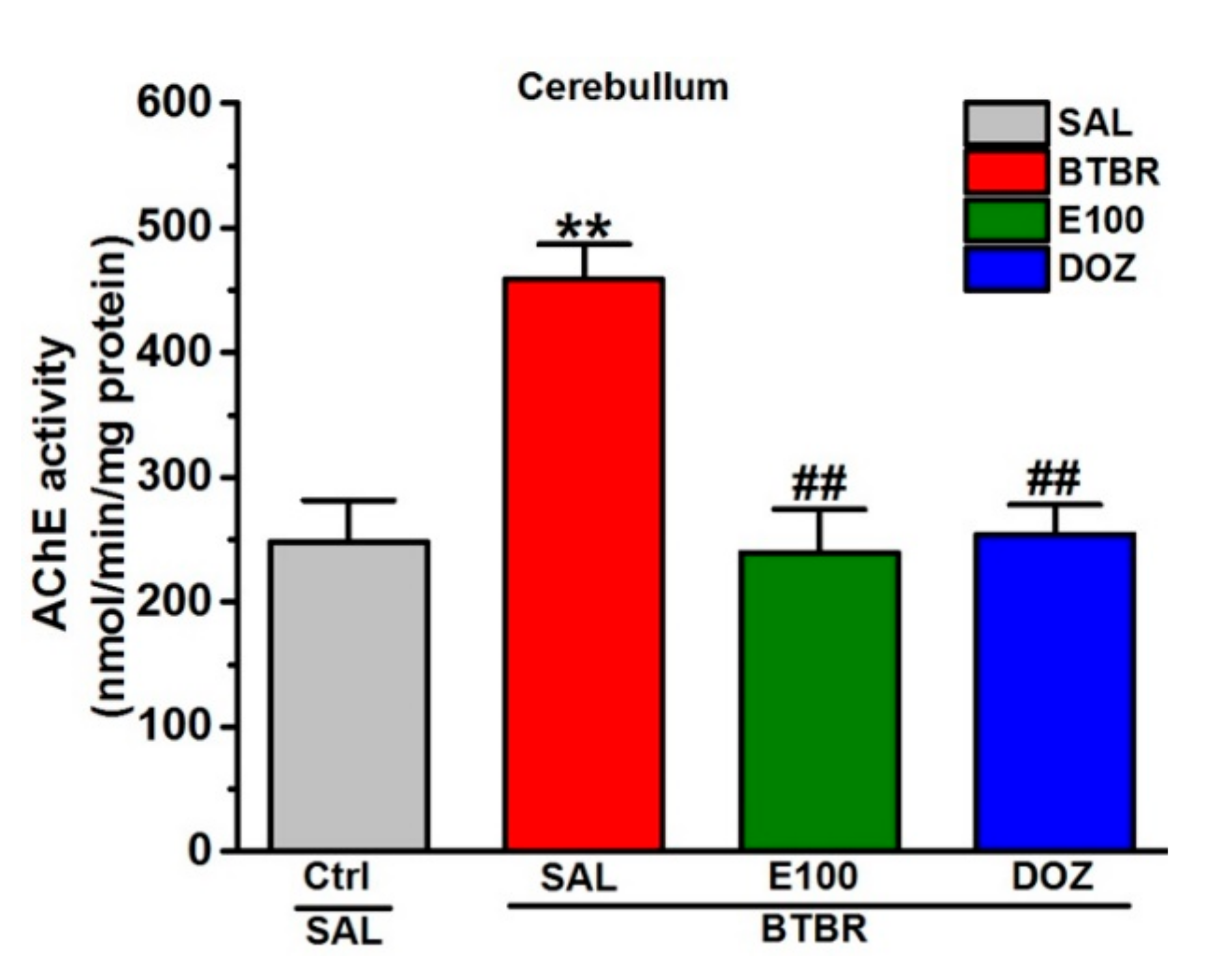
3.5. Effects of E100 on Cerebellar Acetylcholine Esterase Activity of BTBR Mice
3.6. Effects of E100 on Cerebellar Activated Microglial Cells of BTBR Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lord, C.; Cook, E.H.; Leventhal, B.L.; Amaral, D.G. Autism spectrum disorders. Neuron 2000, 28, 355–363. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Sheldrick, R.C.; Carter, A. State-level trends in the prevalence of autism spectrum disorder (ASD) from 2000 to 2012: A reanalysis of findings from the autism and developmental disabilities network. J. Autism Dev. Disord. 2018, 48, 3086–3092. [Google Scholar] [CrossRef]
- Xu, G.; Strathearn, L.; Liu, B.; Bao, W. Prevalence of autism spectrum disorder among us children and adolescents, 2014–2016. JAMA 2018, 319, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, D.A.; Jones, J.H.; Sweeney, J.A.; E Ragozzino, M. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav. Brain Res. 2012, 227, 64–72. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Łażewska, R.; Kieć-Kononowicz, K.; et al. The dual-active histamine H3 receptor antagonist and acetylcholine esterase inhibitor E100 ameliorates stereotyped repetitive behavior and neuroinflammmation in sodium valproate induced autism in mice. Chem. Biol. Interact. 2019, 312, 108775. [Google Scholar] [CrossRef]
- Kemper, T.; Bauman, M.L. Neuropathology of infantile autism. J. Neuropathol. Exp. Neurol. 1998, 57, 645–652. [Google Scholar] [CrossRef]
- Friedman, S.D.; Shaw, D.W.W.; Artru, A.A.; Dawson, G.; Petropoulos, H.; Dager, S.R. Gray and white matter brain chemistry in young children with autism. Arch. Gen. Psychiatry 2006, 63, 786–794. [Google Scholar] [CrossRef]
- Mukaetova-Ladinska, E.B. Silent lives: Why do we fail community-dwelling people with dementia? Age Ageing 2017, 46, 341–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Rinne, J.; Kuokkanen, K.; Eriksson, K.S.; Sallmen, T.; Kalimo, H.; Relja, M. Neuronal histamine deficit in Alzheimer’s disease. Neuroscience 1997, 82, 993–997. [Google Scholar] [CrossRef]
- Sadek, B.; Stark, H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology 2016, 106, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Sadek, B.; Saad, A.; Sadeq, A.; Jalal, F.; Stark, H. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav. Brain Res. 2016, 312, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Arrang, J.-M.; Garbarg, M.; Schwartz, J.-C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983, 302, 832–837. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Roland, B.L.; Wilson, S.J.; Jiang, X.; Pyati, J.; Huvar, A.; Jackson, M.R.; Erlander, M.G. Cloning and functional expression of the human histamine H3 receptor. Mol. Pharmacol. 1999, 55, 1101–1107. [Google Scholar] [CrossRef]
- Berlin, M.; Boyce, C.W.; Ruiz, M.D.L. Histamine H3 receptor as a drug discovery target. J. Med. Chem. 2011, 54, 26–53. [Google Scholar] [CrossRef]
- Parmentier, R.; Anaclet, C.; Guhennec, C.; Brousseau, E.; Bricout, D.; Giboulot, T.; Bozyczko-Coyne, D.; Spiegel, K.; Ohtsu, H.; Williams, M.; et al. The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders. Biochem. Pharmacol. 2007, 73, 1157–1171. [Google Scholar] [CrossRef]
- Alachkar, A.; Khan, N.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Histamine H3 receptor antagonist E177 attenuates amnesia induced by dizocilpine without modulation of anxiety-like behaviors in rats. Neuropsychiatr. Dis. Treat. 2019, 15, 531–542. [Google Scholar] [CrossRef]
- Alachkar, A.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The histamine H3 receptor antagonist E159 reverses memory deficits induced by dizocilpine in passive avoidance and novel object recognition paradigm in rats. Front. Pharmacol. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Azimullah, S.; Ojha, S.; Beiram, R.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The neuroprotective effects of histamine H3 receptor antagonist E177 on pilocarpine-induced status epilepticus in rats. Molecules 2019, 24, 4106. [Google Scholar] [CrossRef] [PubMed]
- Baronio, D.; Castro, K.; Gonchoroski, T.; De Melo, G.M.; Nunes, G.D.F.; Bambini-Junior, V.; Gottfried, C.; Riesgo, R. Effects of an H3R antagonist on the animal model of autism induced by prenatal exposure to valproic acid. PLoS ONE 2015, 10, e0116363. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Jayaprakash, P.; Azimullah, S.; Ojha, S.K.; Al-Houqani, M.; Jalal, F.Y.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The histamine H3R antagonist DL77 attenuates autistic behaviors in a prenatal valproic acid-induced mouse model of autism. Sci. Rep. 2018, 8, 13077. [Google Scholar] [CrossRef]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.; Beiram, R.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; et al. The dual-active histamine H3 receptor antagonist and acetylcholine esterase inhibitor e100 alleviates autistic-like behaviors and oxidative stress in valproic acid induced autism in mice. Int. J. Mol. Sci. 2020, 21, 3996. [Google Scholar] [CrossRef]
- Shah, A.; Wing, L. Psychological approaches to chronic catatonia-like deterioration in autism spectrum disorders. Int. Rev. Neurobiol. 2006, 72, 245–264. [Google Scholar] [CrossRef]
- Wang, L.; Almeida, L.E.F.; Spornick, N.A.; Kenyon, N.; Kamimura, S.; Khaibullina, A.; Nouraie, M.; Quezado, Z. Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology 2015, 232, 4303–4316. [Google Scholar] [CrossRef]
- Chen, R.; Davis, L.K.; Guter, S.; Wei, Q.; Jacob, S.; Potter, M.H.; Cox, N.J.; Cook, E.H.; Sutcliffe, J.S.; Li, B. Leveraging blood serotonin as an endophenotype to identify de novo and rare variants involved in autism. Mol. Autism 2017, 8, 14. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- A Ellenbroek, B.; Ghiabi, B. Do Histamine receptor 3 antagonists have a place in the therapy for schizophrenia? Curr. Pharm. Des. 2015, 21, 3760–3770. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The cerebellum, sensitive periods, and autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, L.; Depino, A.M. Altered peripheral and central inflammatory responses in a mouse model of autism. Autism Res. 2014, 7, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Smith, S.E.P.; Malkova, N.; Tse, D.; Su, Y.; Patterson, P.H. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009, 23, 116–123. [Google Scholar] [CrossRef]
- Miller, H.L.; E Ragozzino, M.; Cook, E.H.; Sweeney, J.A.; Mosconi, M.W. Cognitive set shifting deficits and their relationship to repetitive behaviors in autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 805–815. [Google Scholar] [CrossRef]
- Gadad, B.S.; Hewitson, L.; Young, K.A.; German, D.C. Neuropathology and animal models of autism: Genetic and environmental factors. Autism Res. Treat. 2013, 2013, 731935. [Google Scholar] [CrossRef]
- Fernández, M.; Sierra-Arregui, T.; Peñagarikano, O. The cerebellum and autism: More than motor control. In Behavioral Neuroscience; IntechOpen: London, UK, 2019. [Google Scholar]
- Kuder, K.J.; Łażewska, D.; Latacz, G.; Schwed, J.S.; Karcz, T.; Stark, H.; Karolak-Wojciechowska, J.; Kieć-Kononowicz, K. Chlorophenoxy aminoalkyl derivatives as histamine H3R ligands and antiseizure agents. Bioorganic Med. Chem. 2016, 24, 53–72. [Google Scholar] [CrossRef]
- Łażewska, D.; Jończyk, J.; Bajda, M.; Szałaj, N.; Wieckowska, A.; Panek, D.; Moore, C.; Kuder, K.J.; Malawska, B.; Kieć-Kononowicz, K. Cholinesterase inhibitory activity of chlorophenoxy derivatives—Histamine H3 receptor ligands. Bioorganic Med. Chem. Lett. 2016, 26, 4140–4145. [Google Scholar] [CrossRef]
- Khan, N.; Saad, A.; Nurulain, S.M.; Darras, F.H.; Decker, M.; Sadek, B. The dual-acting H3 receptor antagonist and AChE inhibitor UW-MD-71 dose-dependently enhances memory retrieval and reverses dizocilpine-induced memory impairment in rats. Behav. Brain Res. 2016, 297, 155–164. [Google Scholar] [CrossRef]
- Sadek, B.; Khan, N.; Darras, F.H.; Pockes, S.; Decker, M. The dual-acting AChE inhibitor and H3 receptor antagonist UW-MD-72 reverses amnesia induced by scopolamine or dizocilpine in passive avoidance paradigm in rats. Physiol. Behav. 2016, 165, 383–391. [Google Scholar] [CrossRef]
- Sadek, B.; Bahi, A.; Schwed, J.S.; Walter, M.; Stark, H. Anxiolytic and antidepressant-like activities of the novel and potent non-imidazole histamine H3 receptor antagonist ST-1283. Drug Des. Dev. Ther. 2014, 8, 627–637. [Google Scholar] [CrossRef]
- Bahi, A.; Sadek, B.; Nurulain, S.M.; Łażewska, D.; Kieć-Kononowicz, K. The novel non-imidazole histamine H3 receptor antagonist DL77 reduces voluntary alcohol intake and ethanol-induced conditioned place preference in mice. Physiol. Behav. 2015, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 50978. [Google Scholar] [CrossRef]
- Kim, J.-W.; Seung, H.; Kwon, K.J.; Ko, M.J.; Lee, E.J.; Oh, H.A.; Choi, C.S.; Kim, K.C.; Gonzales, E.L.; You, J.S.; et al. Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS ONE 2014, 9, e104927. [Google Scholar] [CrossRef]
- Bahi, A. Individual differences in elevated plus-maze exploration predicted higher ethanol consumption and preference in outbred mice. Pharmacol. Biochem. Behav. 2013, 105, 83–88. [Google Scholar] [CrossRef]
- Bahi, A. Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress 2013, 16, 441–451. [Google Scholar] [CrossRef]
- Bahi, A.; Dreyer, J.-L. Hippocampus-specific deletion of tissue plasminogen activator “tPA” in adult mice impairs depression- and anxiety-like behaviors. Eur. Neuropsychopharmacol. 2012, 22, 672–682. [Google Scholar] [CrossRef]
- Bahi, A.; Dreyer, J.-L. Chronic psychosocial stress causes delayed extinction and exacerbates reinstatement of ethanol-induced conditioned place preference in mice. Psychopharmacology 2014, 231, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.E.; Javed, H.; Azimullah, S.; Khair, S.B.A.; Ojha, S. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Dev. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Khair, S.B.A.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- A McCloy, R.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Pichat, P.; Pruniaux, M.-P.; Beeské, S.; Lopez-Grancha, M.; Genet, E.; Terranova, J.-P.; Castro, A.; Sánchez, J.A.; Black, M.; et al. SAR110894, a potent histamine H3-receptor antagonist, displays procognitive effects in rodents. Pharmacol. Biochem. Behav. 2012, 102, 203–214. [Google Scholar] [CrossRef]
- McTighe, S.M.; Neal, S.J.; Lin, Q.; Hughes, Z.A.; Smith, D.G. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS ONE 2013, 8, e62189. [Google Scholar] [CrossRef]
- Riedel, G.; Kang, S.; Choi, D.; Platt, B. Scopolamine-induced deficits in social memory in mice: Reversal by donepezil. Behav. Brain Res. 2009, 204, 217–225. [Google Scholar] [CrossRef]
- Galici, R.; Boggs, J.D.; Aluisio, L.; Fraser, I.C.; Bonaventure, P.; Lord, B.; Lovenberg, T.W. JNJ-10181457, a selective non-imidazole histamine H3 receptor antagonist, normalizes acetylcholine neurotransmission and has efficacy in translational rat models of cognition. Neuropharmacology 2009, 56, 1131–1137. [Google Scholar] [CrossRef]
- Karvat, G.; Kimchi, T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 2013, 39, 831–840. [Google Scholar] [CrossRef]
- Amodeo, D.A.; Yi, J.; Sweeney, J.A.; E Ragozzino, M. Oxotremorine treatment reduces repetitive behaviors in BTBR T+ tf/J mice. Front. Synaptic Neurosci. 2014, 6, 17. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Alshammari, M.A.; Al-Zahrani, K.S.; Bakheet, S.A. Increased oxidative stress in the cerebellum and peripheral immune cells leads to exaggerated autism-like repetitive behavior due to deficiency of antioxidant response in BTBR T+ tf/J mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Benno, R.; Smirnova, Y.; Vera, S.; Liggett, A.; Schanz, N. Exaggerated responses to stress in the BTBR T+ tf/J mouse: An unusual behavioral phenotype. Behav. Brain Res. 2009, 197, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Pobbe, R.L.; Defensor, E.B.; Pearson, B.L.; Bolivar, V.J.; Blanchard, D.C.; Blanchard, R.J. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav. Brain Res. 2011, 216, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Pérez, A.; Holloway, L.P.; Barbaro, R.P.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.; Lauder, J.M.; et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Yang, M.; Turner, S.M.; Katz, A.M.; Bell, D.B.; I Koenig, J.; Crawley, J.N. Low stress reactivity and neuroendocrine factors in the BTBR T+ tf/J mouse model of autism. Neuroscience 2010, 171, 1197–1208. [Google Scholar] [CrossRef]
- Yang, M.; Clarke, A.M.; Crawley, J.N. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur. J. Neurosci. 2009, 29, 1663–1677. [Google Scholar] [CrossRef]
- Chadman, K.K. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol. Biochem. Behav. 2011, 97, 586–594. [Google Scholar] [CrossRef]
- Schmitt, U.; Hiemke, C. Combination of open field and elevated plus-maze: A suitable test battery to assess strain as well as treatment differences in rat behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1998, 22, 1197–1215. [Google Scholar] [CrossRef]
- Lucki, I.; Dalvi, A.; Mayorga, A.J. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 2001, 155, 315–322. [Google Scholar] [CrossRef]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of neuroinflammation in autism spectrum disorder and the emergence of brain histaminergic system. Lessons also for BPSD? Front. Pharmacol. 2020, 11, 833. [Google Scholar] [CrossRef]
- Rodriguez, J.I.; Kern, J.K. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol. 2011, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, N.; Kluetzman, K.; Mendoza, A.; Bolivar, V.J.; Reilly, A.; Jolly, J.K.; Lawrence, D.A. The maternal autoimmune environment affects the social behavior of offspring. J. Neuroimmunol. 2013, 258, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE 2011, 6, e20912. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Z. The roles of histamine and its receptor ligands in central nervous system disorders: An update. Pharmacol. Ther. 2017, 175, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, S.; Fabbrizio, P.; Amadio, S.; Napoli, G.; Verdile, V.; Morello, G.; Iemmolo, R.; Aronica, E.; Cavallaro, S.; Volonté, C. Histamine regulates the inflammatory profile of SOD1-G93A microglia and the histaminergic system is dysregulated in amyotrophic lateral sclerosis. Front. Immunol. 2017, 8, 1689. [Google Scholar] [CrossRef] [PubMed]
- Barata-Antunes, S.; Cristóvão, A.C.; Pires, J.; Rocha, S.M.; Bernardino, L. Dual role of histamine on microglia-induced neurodegeneration. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 764–769. [Google Scholar] [CrossRef]
- Frick, L.; Rapanelli, M.; Abbasi, E.; Ohtsu, H.; Pittenger, C.M. Histamine regulation of microglia: Gene-environment interaction in the regulation of central nervous system inflammation. Brain Behav. Immun. 2016, 57, 326–337. [Google Scholar] [CrossRef]
- Parada, E.; Egea, J.; Buendia, I.; Negredo, P.; Cunha, A.C.; Cardoso, S.; Soares, M.P.; López, M.G. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid. Redox Signal. 2013, 19, 1135–1148. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, N.; Jayaprakash, P.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ tf/J Mouse Model of Autism. Biomolecules 2020, 10, 1251. https://doi.org/10.3390/biom10091251
Eissa N, Jayaprakash P, Stark H, Łażewska D, Kieć-Kononowicz K, Sadek B. Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ tf/J Mouse Model of Autism. Biomolecules. 2020; 10(9):1251. https://doi.org/10.3390/biom10091251
Chicago/Turabian StyleEissa, Nermin, Petrilla Jayaprakash, Holger Stark, Dorota Łażewska, Katarzyna Kieć-Kononowicz, and Bassem Sadek. 2020. "Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ tf/J Mouse Model of Autism" Biomolecules 10, no. 9: 1251. https://doi.org/10.3390/biom10091251
APA StyleEissa, N., Jayaprakash, P., Stark, H., Łażewska, D., Kieć-Kononowicz, K., & Sadek, B. (2020). Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-Like Behaviors in BTBR T+ tf/J Mouse Model of Autism. Biomolecules, 10(9), 1251. https://doi.org/10.3390/biom10091251





