Eicosapentaenoic Acid Regulates Inflammatory Pathways through Modulation of Transcripts and miRNA in Adipose Tissue of Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. RNA Extraction and cDNA Library Preparation
2.3. mRNA and miRNA Sequencing Strategy
2.4. Quality Control (QC) and Reads Alignment
2.5. Functional Analysis
2.6. Data Availability
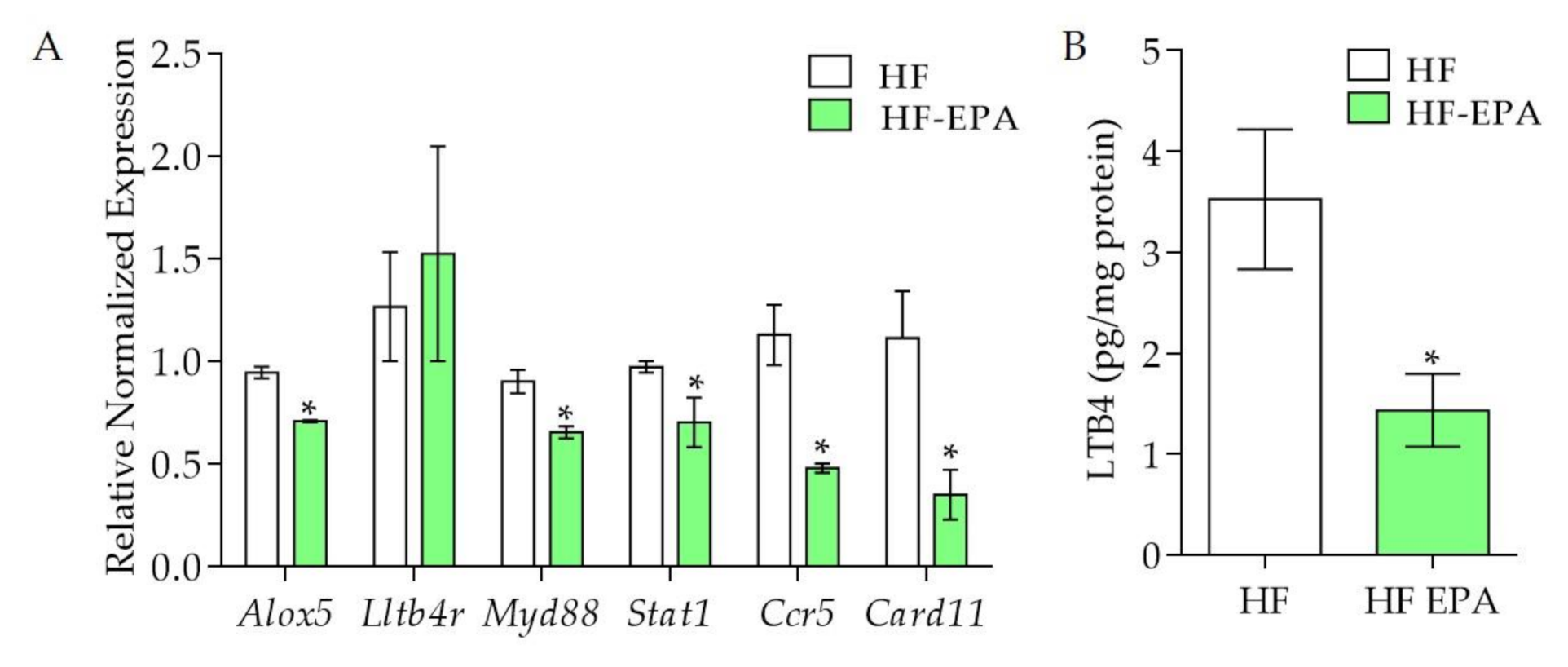
2.7. Quantitative Real-Time Polymerase Chain Reaction (q-PCR) Validation
2.8. Enzymatic Assay for LTB4 Dosage
2.9. Statistical Analyses
3. Results
3.1. RNA Sequencing Data Quality
3.2. Effects of EPA on Gene and miRNA Expression of VAT of HF Fed Mice
3.3. The Effects of EPA on Canonical Pathways and Their Genes
3.4. Further Genes and Networks
3.5. Identification of microRNAs, Validation, and Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prevalence of Obesity 2019. Available online: https://www.worldobesity.org/ (accessed on 24 April 2020).
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094-101. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic Acid Prevents and Reverses Insulin Resistance in High-Fat Diet-Induced Obese Mice via Modulation of Adipose Tissue Inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, M.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic Acid Reduces Adipocyte Hypertrophy and Inflammation in Diet-Induced Obese Mice in an Adiposity-Independent Manner. J. Nutr. 2014, 145, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijayatunga, N.N.; Pahlavani, M.; Kalupahana, N.S.; Kottapalli, K.R.; Gunaratne, P.H.; Coarfa, C.; Ramalingam, L.; Moustaid-Moussa, N. An integrative transcriptomic approach to identify depot differences in genes and microRNAs in adipose tissues from high fat fed mice. Oncotarget 2018, 9, 9246–9261. [Google Scholar] [CrossRef] [Green Version]
- Creighton, C.J.; Reid, J.G.; Gunaratne, P.H. Expression profiling of microRNAs by deep sequencing. Brief. Bioinform. 2009, 10, 490–497. [Google Scholar] [CrossRef]
- Saeed, A.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A Free, Open-Source System for Microarray Data Management and Analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Tammaro, A.; Derive, M.; Gibot, S.; Leemans, J.C.; Florquin, S.; Dessing, M.C. TREM-1 and its potential ligands in non-infectious diseases: From biology to clinical perspectives. Pharmacol. Ther. 2017, 177, 81–95. [Google Scholar] [CrossRef]
- Dalmas, E.; Toubal, A.; Alzaid, F.; Blazek, K.; Eames, H.L.; Lebozec, K.; Pini, M.; Hainault, I.; Montastier, E.; Denis, R.G.P.; et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat. Med. 2015, 21, 610–618. [Google Scholar] [CrossRef]
- Horrillo, R.; González-Périz, A.; Martínez-Clemente, M.; López-Parra, M.; Ferré, N.; Titos, E.; Morán-Salvador, E.; Deulofeu, R.; Arroyo, V.; Claria, J. 5-Lipoxygenase Activating Protein Signals Adipose Tissue Inflammation and Lipid Dysfunction in Experimental Obesity. J. Immunol. 2010, 184, 3978–3987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.-I.; Ginsberg, H.N.; et al. CCR5 Plays a Critical Role in Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance by Regulating Both Macrophage Recruitment and M1/M2 Status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, S.-C.; Chiang, E.-P.I.; Tsai, S.-Y.; Wang, F.-Y.; Pai, M.-H.; Syu, J.-N.; Cheng, C.-C.; Rodriguez, R.L.; Tang, F.-Y. Eicosapentaenoic acid induces neovasculogenesis in human endothelial progenitor cells by modulating c-kit protein and PI3-K/Akt/eNOS signaling pathways. J. Nutr. Biochem. 2014, 25, 934–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids. J. Lipid Res. 2002, 44, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Baumann, K.H.; Hessel, F.; Larass, I.; Müller, T.; Angerer, P.; Kiefl, R.; von Schacky, C. Dietary omega-3, omega-6, and omega-9 unsaturated fatty acids and growth factor and cytokine gene expression in unstimulated and stimulated monocytes. A randomized volunteer study. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Meerson, A.; Traurig, M.; Ossowski, V.; Fleming, J.M.; Mullins, M.; Baier, L.J. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia 2013, 56, 1971–1979. [Google Scholar] [CrossRef] [Green Version]
- Giroud, M.; Pisani, D.F.; Karbiener, M.; Barquissau, V.; Ghandour, R.A.; Tews, D.; Fischer-Posovszky, P.; Chambard, J.C.; Knippschild, U.; Niemi, T.; et al. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol. Metab. 2016, 5, 615–625. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, L.; Chen, X.; Gu, N.; Chen, L.; Zhu, L.; Yang, L.; Pang, L.; Guo, X.; Ji, C.; et al. IL-6 and TNF-α Induced Obesity-Related Inflammatory Response Through Transcriptional Regulation of miR-146b. J. Interferon Cytokine Rese. 2014, 34, 342–348. [Google Scholar] [CrossRef]
- Arner, E.; Mejhert, N.; Kulyté, A.; Balwierz, P.J.; Pachkov, M.; Cormont, M.; Lorente-Cebrián, S.; Ehrlund, A.; Laurencikiene, J.; Hedén, P.; et al. Adipose Tissue MicroRNAs as Regulators of CCL2 Production in Human Obesity. Diabetes 2012, 61, 1986–1993. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 2015, 21, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Bossche, J.V.D.; Ramalho, T. Macrophage Metabolism at the Crossroad of Metabolic Diseases and Cancer. Immunometabolism 2020, 2, 200022. [Google Scholar] [CrossRef]
- Brykczynska, U.; Geigges, M.; Wiedemann, S.J.; Dror, E.; Böni-Schnetzler, M.; Hess, C.; Donath, M.Y.; Paro, R. Distinct Transcriptional Responses across Tissue-Resident Macrophages to Short-Term and Long-Term Metabolic Challenge. Cell Rep. 2020, 30, 1627–1643. [Google Scholar] [CrossRef]
- Ying, W.; Wollam, J.; Ofrecio, J.M.; Bandyopadhyay, G.K.; El Ouarrat, D.; Lee, Y.S.; Oh, D.Y.; Li, P.; Osborn, O.; Olefsky, J. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J. Clin. Investig. 2017, 127, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Akoh, C.C.; Miller, C.C. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J. Lipid Res. 1991, 32, 1205–1213. [Google Scholar]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Wang, Z.; Filgueiras, L.R.; Wang, S.; Serezani, A.P.M.; Peters-Golden, M.; Jancar, S.; Serezani, C.H.; Wang, S. Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. J. Immunol. 2014, 192, 2349–2356. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.-M.; Zhang, P.; Liu, M.-H.; Chen, P.; Zhang, W.-B. MicroRNA-30e inhibits adhesion, migration, invasion and cell cycle progression of prostate cancer cells via inhibition of the activation of the MAPK signaling pathway by downregulating CHRM3. Int. J. Oncol. 2018, 54, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Gazon, H.; Barbeau, B.; Mesnard, J.-M.; Peloponese, J.-M.J. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front. Microbiol. 2018, 8, 2686. [Google Scholar] [CrossRef] [Green Version]
- Galardi, S.; Mercatelli, N.; Farace, M.G.; Ciafrè, S. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011, 39, 3892–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, K.; Yang, X.; Bam, M.; Murphy, E.A.; Nagarkatti, P.; Nagarkatti, M. MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. Int. J. Obes. 2018, 42, 1140–1150. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, H.; Pecht, T.; Shaco-Levy, R.; Harman-Boehm, I.; Kirshtein, B.; Kuperman, Y.; Chen, A.; Blüher, M.; Shai, I.; Rudich, A. Adipose Tissue Foam Cells Are Present in Human Obesity. J. Clin. Endocrinol. Metab. 2013, 98, 1173–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.R.; Midgette, Y.; Shah, R. Fish Oil Derived Omega 3 Fatty Acids Suppress Adipose NLRP3 Inflammasome Signaling in Human Obesity. J. Endocr. Soc. 2018, 3, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Amaral, F.A.; Costa, V.V.; Tavares, L.D.; Sachs, D.; Coelho, F.M.; Fagundes, C.T.; Soriani, F.M.; Silveira, T.N.; Cunha, L.D.; Zamboni, D.S.; et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B4 in a murine model of gout. Arthritis Rheum. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Andersson, N.; Strandberg, L.; Nilsson, S.; Ljungren, Ö.; Karlsson, M.K.; Mellström, D.; Lorentzon, M.; Ohlsson, C.; Jansson, J.-O. Variants of the interleukin-1 receptor antagonist gene are associated with fat mass in men. Int. J. Obes. 2009, 33, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Herder, C.; Gala, T.D.L.H.; Carstensen-Kirberg, M.; Huth, C.; Zierer, A.; Wahl, S.; Sudduth-Klinger, J.; Kuulasmaa, K.; Peretz, D.; Ligthart, S.; et al. Circulating Levels of Interleukin 1-Receptor Antagonist and Risk of Cardiovascular DiseaseHighlights. Arter. Thromb. Vasc. Boil. 2017, 37, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- A Meier, C.; Bobbioni, E.; Gabay, C.; Assimacopoulos-Jeannet, F.; Golay, A.; Dayer, J.-M. IL-1 Receptor Antagonist Serum Levels Are Increased in Human Obesity: A Possible Link to the Resistance to Leptin? J. Clin. Endocrinol. Metab. 2002, 87, 1184–1188. [Google Scholar] [CrossRef]
- Juge-Aubry, C.E.; Somm, E.; Giusti, V.; Pernin, A.; Chicheportiche, R.; Verdumo, C.; Rohner-Jeanrenaud, F.; Burger, D.; Dayer, J.-M.; Meier, C.A. Adipose tissue is a major source of interleukin-1 receptor antagonist: Upregulation in obesity and inflammation. Diabetes 2003, 52, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.; Gerdes, N.; Fritzenwanger, M.; Figulla, H.R. Circulating Levels of Interleukin-1 Family Cytokines in Overweight Adolescents. Mediat. Inflamm. 2010, 2010, 958403. [Google Scholar] [CrossRef] [PubMed]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.; Morsy, M.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]



| Ingenuity Canonical Pathway | Gene Name | −log (p-Value) | Ratio | z-Score |
|---|---|---|---|---|
| Fcγ Receptor-mediated Phagocytosis in | Fcgr1a, Hck, Fcgr3a/b, Ncf1, Syk, Pak1 | 5.520 | 0.05 | −2646 |
| Macrophages and Monocytes TREM1 Signaling | Tlr8, Tlr1, Tlr13, Lat2, Tlr7, Tlr6, Casp1 | 6.190 | 0.0933 | −2646 |
| CD28 Signaling in T Helper Cells | Card11, Syk, Pik3r5, Pak1 | 2.700 | 0.0373 | −2 |
| PI3K Signaling in B Lymphocytes | Atf3, Cd180, Pik3ap1, Blnk, Syk | 2.650 | 0.0362 | −2236 |
| Neuroinflammation Signaling Pathway | Il1rn, Tlr8, Cd200r1, Tlr1, Trem2, Csf1r, Tlr13, Tlr7, Pik3r5, Tlr6, Naip2, Pycard | 7.660 | 0.0447 | −3051 |
| Role of NFAT in Regulation of the Immune Response | Fcgr1a, Blnk, Fcgr3a/b, Syk, Pik3r5 | 3.480 | 0.0357 | −2646 |
| NF-κB Signaling | Il1rn, Card11, Tlr8, Tlr1, Tnfrsf11a, Tlr7, Pik3r5, Tlr6 | 4.400 | 0.0421 | −2828 |
| Gene Name | HF RPKM | HF-EPA RPKM | Fold Change | p Value |
|---|---|---|---|---|
| Fcgr1a | 27.683 | 3.811 | 0.137 | 0.0393 |
| Fcgr3a/b | 148.474 | 36.322 | 0.244 | 0.0449 |
| Tlr1 | 13.167 | 2.279 | 0.17 | 0.0351 |
| Tlr6 | 4.133 | 1.191 | 0.28 | 0.0460 |
| Tlr7 | 5.472 | 1.44 | 0.26 | 0.0385 |
| Tlr8 | 10.442 | 1.384 | 0.13 | 0.0296 |
| Tlr13 | 10.023 | 2.192 | 0.21 | 0.0377 |
| Il1rn | 30.568 | 1.996 | 0.065 | 0.0248 |
| Csf1r | 139.035 | 28.388 | 0.20 | 0.0293 |
| Tnfrsf11a | 6.123 | 1.284 | 0.20 | 0.0385 |
| Cd200r1 | 21.775 | 3.285 | 0.15 | 0.0173 |
| Cd180 | 27.396 | 4.421 | 0.161 | 0.0218 |
| Cd84 | 78.71 | 18.594 | 0.23 | 0.0373 |
| Ccr5 | 22.7 | 3.087 | 0.13 | 0.0421 |
| Trem2 | 254.804 | 46.296 | 0.18 | 0.0460 |
| Lat2 | 69.723 | 15.287 | 0.21 | 0.0439 |
| Gene Name | HF RPKM | HF-EPA RPKM | Fold Change | p Value |
|---|---|---|---|---|
| Hck | 16.142 | 2.941 | 0.182 | 0.0438 |
| Ncf1 | 31.825 | 7.866 | 0.247 | 0.0460 |
| Syk | 32.369 | 8.579 | 0.265 | 0.0385 |
| Pak1 | 9.562 | 2.805 | 0.293 | 0.0435 |
| Casp1 | 30.155 | 9.215 | 0.30 | 0.0385 |
| Card11 | 13.84 | 1.578 | 0.11 | 0.0276 |
| Pik3r5 | 17.611 | 4.782 | 0.27 | 0.0385 |
| Atf3 | 63.616 | 10.225 | 0.160 | 0.0385 |
| Pik3ap1 | 24.872 | 4.484 | 0.180 | 0.0293 |
| Blnk | 45.812 | 8.743 | 0.190 | 0.0248 |
| Naip2 | 4.613 | 1.316 | 0.28 | 0.0385 |
| Pycard | 49.537 | 14.658 | 0.29 | 0.0385 |
| Irf5 | 49.256 | 16.373 | 0.33 | 0.0421 |
| Alox5ap | 120.5 | 36.156 | 0.29 | 0.0473 |
| Ccl9 | 405.632 | 54.25 | 0.13 | 0.0196 |
| miRNA | RPKM HF | RPKM HF-EPA | Fold Change | p Value |
|---|---|---|---|---|
| mmu-mir-30a-5p | 11.26 | 11.85 | 0.59 | 0.0261 |
| mmu-mir-143-3p | 16.46 | 17.1 | 0.64 | 0.0041 |
| mmu-mir-125b-5p | 14.16 | 13.82 | −0.33 | 0.0226 |
| mmu-mir-146b-5p | 11.42 | 10.65 | −0.77 | 0.0173 |
| mmu-mir-181b-5p | 12.17 | 11.81 | −0.36 | 0.0296 |
| mmu-mir-221-3p | 12.38 | 11.62 | −0.76 | 0.0024 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalho, T.; Pahlavani, M.; Kalupahana, N.; Wijayatunga, N.; Ramalingam, L.; Jancar, S.; Moustaid-Moussa, N. Eicosapentaenoic Acid Regulates Inflammatory Pathways through Modulation of Transcripts and miRNA in Adipose Tissue of Obese Mice. Biomolecules 2020, 10, 1292. https://doi.org/10.3390/biom10091292
Ramalho T, Pahlavani M, Kalupahana N, Wijayatunga N, Ramalingam L, Jancar S, Moustaid-Moussa N. Eicosapentaenoic Acid Regulates Inflammatory Pathways through Modulation of Transcripts and miRNA in Adipose Tissue of Obese Mice. Biomolecules. 2020; 10(9):1292. https://doi.org/10.3390/biom10091292
Chicago/Turabian StyleRamalho, Theresa, Mandana Pahlavani, Nishan Kalupahana, Nadeeja Wijayatunga, Latha Ramalingam, Sonia Jancar, and Naima Moustaid-Moussa. 2020. "Eicosapentaenoic Acid Regulates Inflammatory Pathways through Modulation of Transcripts and miRNA in Adipose Tissue of Obese Mice" Biomolecules 10, no. 9: 1292. https://doi.org/10.3390/biom10091292








