Cardioprotective Effects of Dietary Flaxseed Post-Infarction Are Associated with Changes in MicroRNA Expression
Abstract
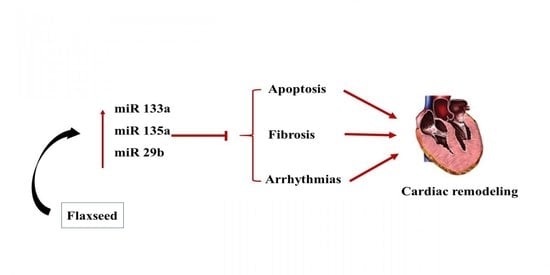
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Biological Sample Collection and Analysis
2.3. Dietary Fatty Acid Analysis
2.4. Western Blot Analysis for Collagen 1A1 and the Na+-Ca2+ Exchanger (NCX)
2.5. MicroRNA Detection in Cardiac Tissue
2.6. Statistical Analysis
3. Results
3.1. Dietary Fatty Acid Analysis
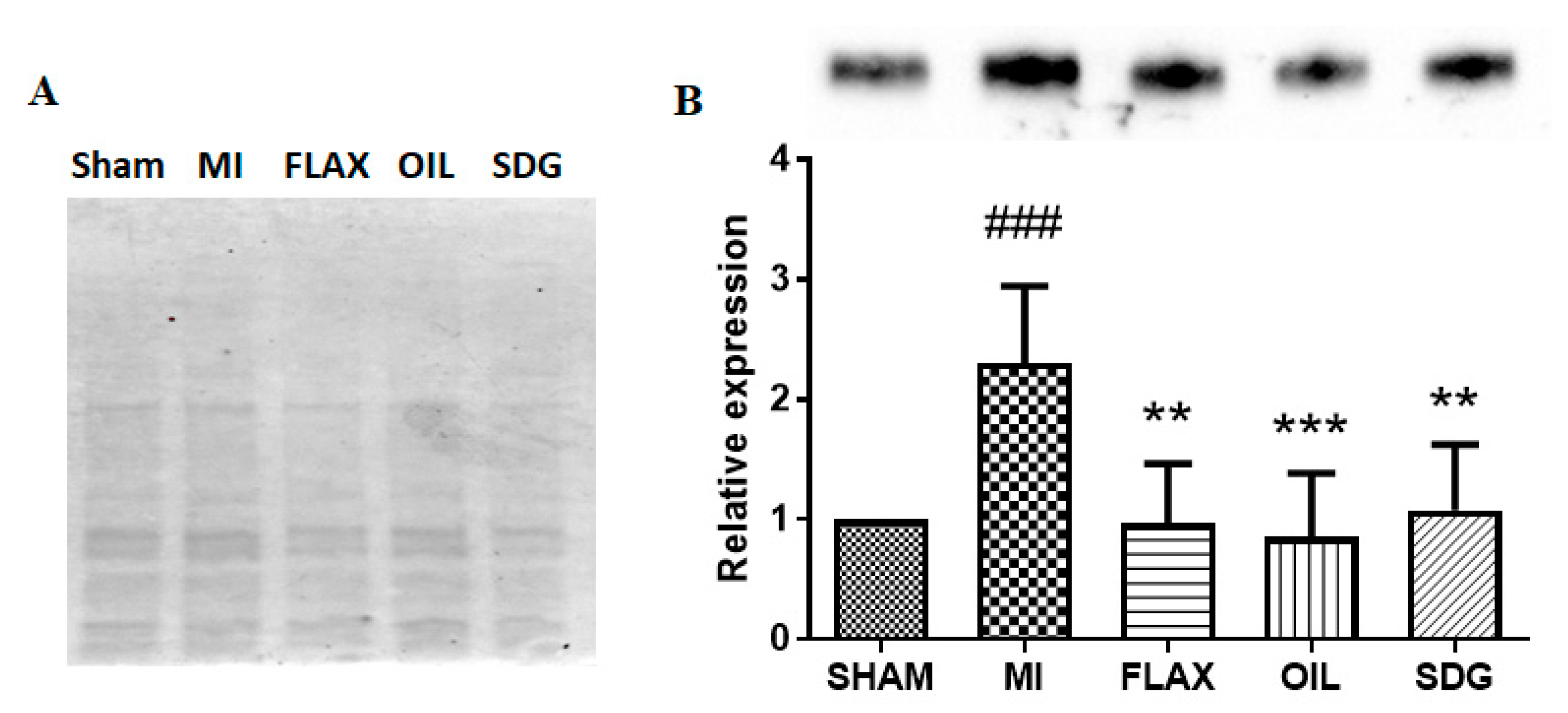
3.2. The Effect of Dietary Flaxseed and Its Components on the Expression of Collagen I
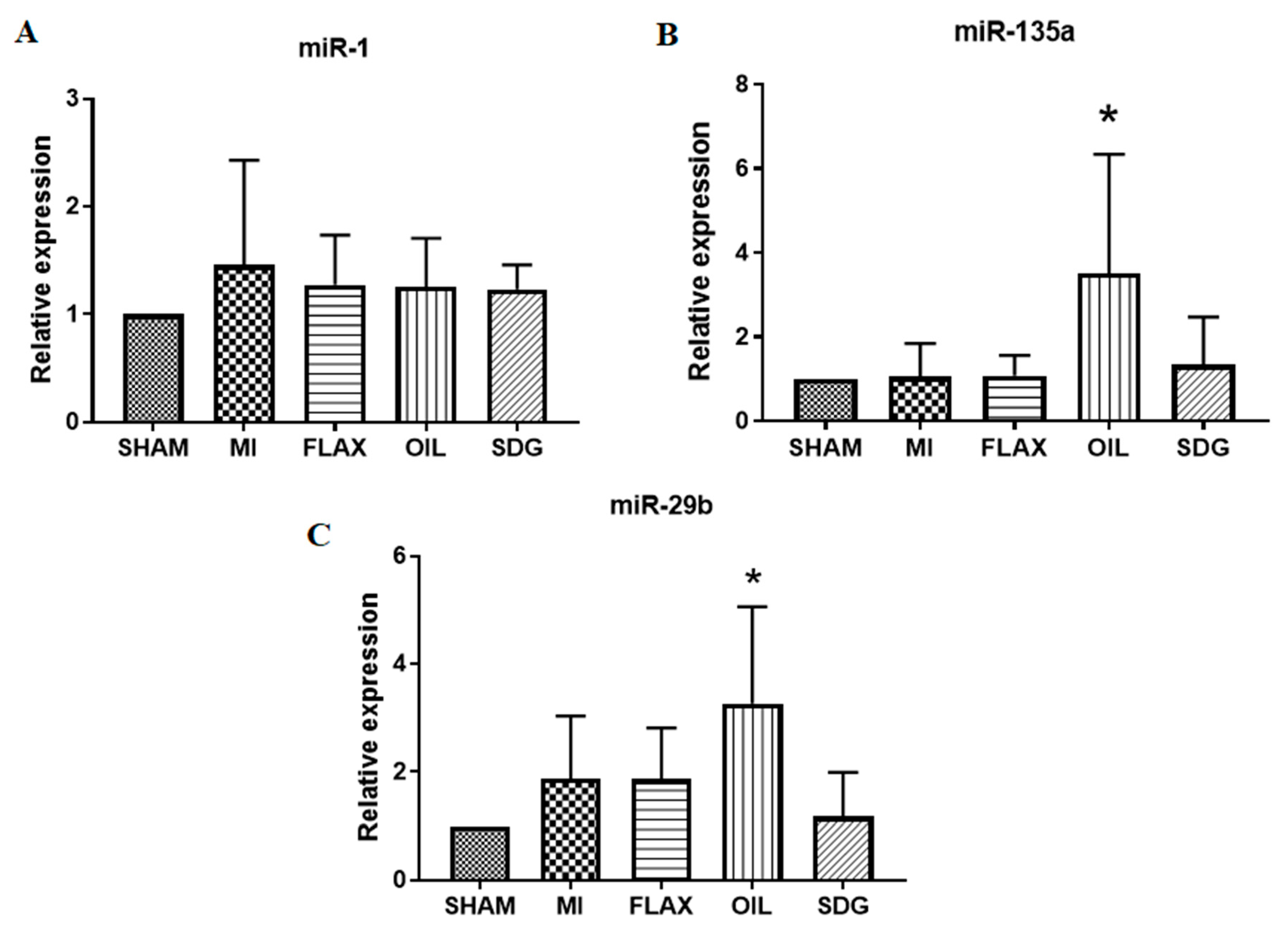
3.3. The Expression of miR-133a Is Sensitive to the Flax Oil Treatment
3.4. Upregulation of miR-135a and -29b by the Flax Oil Supplementation
3.5. The Effect of Dietary Flaxseed and Its Components on the Expression of NCX
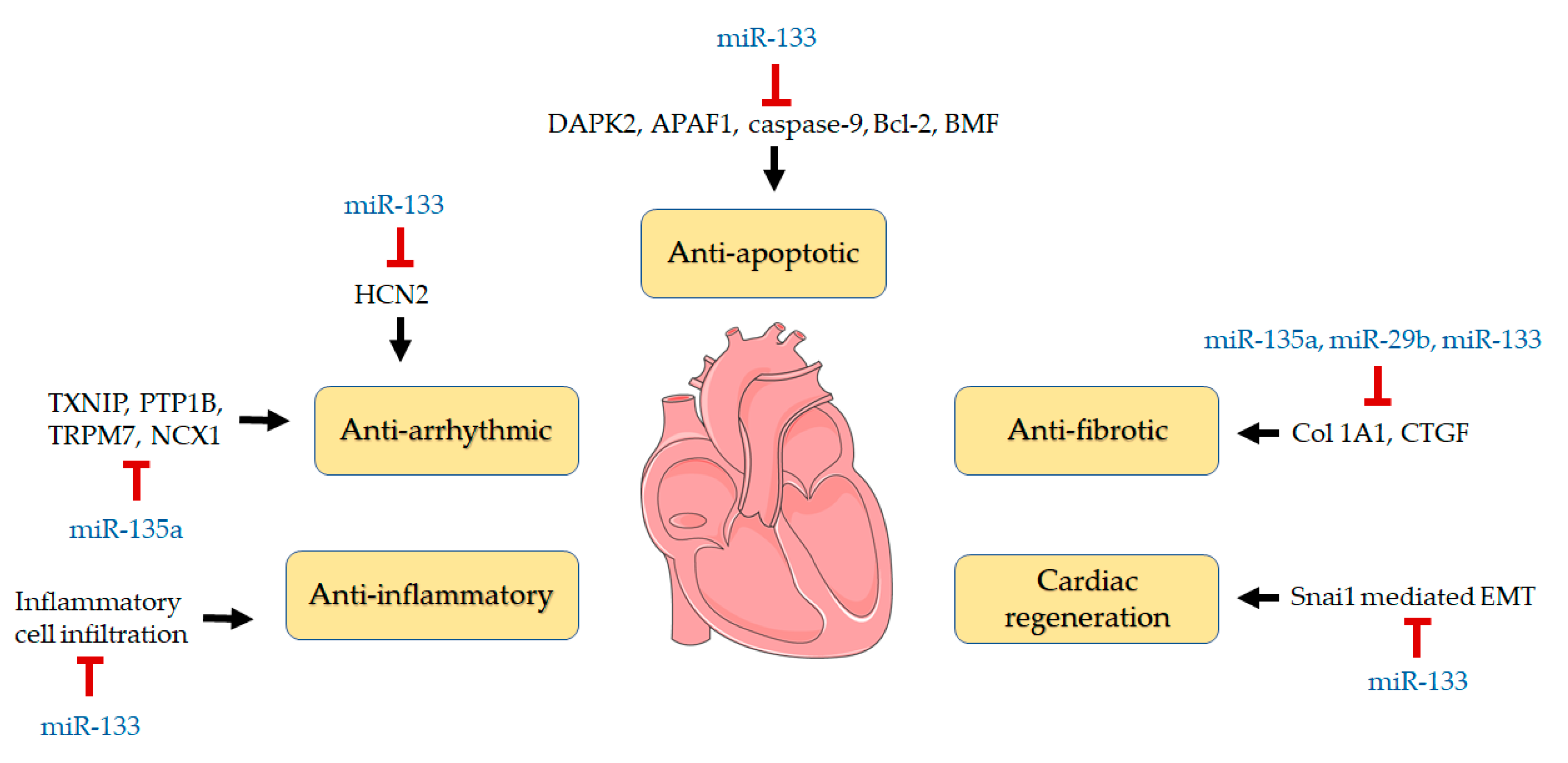
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Sun, Y. Myocardial repair/remodelling following infarction: Roles of local factors. Cardiovasc. Res. 2009, 81, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Parikh, M.; Slezak, J.; Pierce, G.N. The influence of diet on micrornas that impact cardiovascular disease. Molecules 2019, 24, 1509. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Thum, T. Micrornas in myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. Microrna in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Dong, D.-L.; Yang, B.-F. Role of micrornas in cardiac hypertrophy, myocardial fibrosis and heart failure. Acta Pharm. Sin. B 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Colpaert, R.M.W.; Calore, M. Micrornas in cardiac diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Z.; Chen, X.; Xue, H. Microrna-135a protects against myocardial ischemia-reperfusion injury in rats by targeting protein tyrosine phosphatase 1b. J. Cell. Biochem. 2019, 120, 10421–10433. [Google Scholar] [CrossRef]
- Kim, G.H. Microrna regulation of cardiac conduction and arrhythmias. Transl. Res. 2013, 161, 381–392. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific mirna in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. Microrna-133a and myocardial infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Bostjancic, E.; Zidar, N.; Stajner, D.; Glavac, D. Microrna mir-1 is up-regulated in remote myocardium in patients with myocardial infarction. Folia Biol. (Praha) 2010, 56, 27–31. [Google Scholar] [PubMed]
- Boštjančič, E.; Brandner, T.; Zidar, N.; Glavač, D.; Štajer, D. Down-regulation of mir-133a/b in patients with myocardial infarction correlates with the presence of ventricular fibrillation. Biomed. Pharmacother. 2018, 99, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. Mir-133 promotes cardiac reprogramming by directly repressing snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of micrornas after myocardial infarction reveals a role of mir-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Zhu, J.-N.; Chen, R.; Fu, Y.-H.; Lin, Q.-X.; Huang, S.; Guo, L.-L.; Zhang, M.-Z.; Deng, C.-Y.; Zou, X.; Zhong, S.-L.; et al. Smad3 inactivation and mir-29b upregulation mediate the effect of carvedilol on attenuating the acute myocardium infarction-induced myocardial fibrosis in rat. PLoS ONE 2013, 8, e75557. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.R.; Wei, L.H.; Chung, A.C.; Yu, C.M.; Lan, H.Y. Mir-29b as a therapeutic agent for angiotensin ii-induced cardiac fibrosis by targeting tgf-β/smad3 signaling. Mol. Ther. 2014, 22, 974–985. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Pan, Y.; Lu, C.; Xu, H.; Wang, X.; Liu, T.; Feng, K.; Tang, Y. Microrna-135a inhibits cardiac fibrosis induced by isoproterenol via trpm7 channel. Biomed. Pharmacother. 2018, 104, 252–260. [Google Scholar] [CrossRef]
- Duong, E.; Xiao, J.; Qi, X.Y.; Nattel, S. Microrna-135a regulates sodium-calcium exchanger gene expression and cardiac electrical activity. Heart Rhythm 2017, 14, 739–748. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Parikh, M.; Netticadan, T.; Pierce, G.N. Flaxseed: Its bioactive components and their cardiovascular benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H146–H159. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Raj, P.; Austria, J.A.; Yu, L.; Garg, B.; Netticadan, T.; Pierce, G.N. Dietary flaxseed protects against ventricular arrhythmias and left ventricular dilation after a myocardial infarction. J. Nutr. Biochem. 2019, 71, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Tuana, B.S.; Casieri, V.; Parikh, M.; Pierce, G.N. Importance of functional food compounds in cardioprotection through action on the epigenome. Eur. Heart J. 2019, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.M.; McCullough, R.S.; Edel, A.L.; Patenaude, A.; LaVallee, R.K.; Pierce, G.N. The α-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2220–H2226. [Google Scholar] [CrossRef]
- Thum, T.; Galuppo, P.; Wolf, C.; Fiedler, J.; Kneitz, S.; van Laake, L.W.; Doevendans, P.A.; Mummery, C.L.; Borlak, J.; Haverich, A.; et al. Micrornas in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation 2007, 116, 258–267. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. Microrna-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhang, X.J.; Li, Q.; Wang, K.; Wang, Y.; Jiao, J.Q.; Feng, C.; Teng, S.; Zhou, L.Y.; Gong, Y.; et al. Microrna-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting fadd. Circ. Res. 2015, 117, 352–363. [Google Scholar] [CrossRef]
- Zheng, Z.; Ge, Y.; Zhang, J.; Xue, M.; Li, Q.; Lin, D.; Ma, W. Pufa diets alter the microrna expression profiles in an inflammation rat model. Mol. Med. Rep. 2015, 11, 4149–4157. [Google Scholar] [CrossRef]
- Ma, H.; Chen, P.; Sang, C.; Huang, D.; Geng, Q.; Wang, L. Modulation of apoptosis-related micrornas following myocardial infarction in fat-1 transgenic mice vs wild-type mice. J. Cell. Mol. Med. 2018, 22, 5698–5707. [Google Scholar] [CrossRef]
- LeMay-Nedjelski, L.; Mason-Ennis, J.K.; Taibi, A.; Comelli, E.M.; Thompson, L.U. Omega-3 polyunsaturated fatty acids time-dependently reduce cell viability and oncogenic microrna-21 expression in estrogen receptor-positive breast cancer cells (mcf-7). Int. J. Mol. Sci. 2018, 19, 244. [Google Scholar] [CrossRef]
- Taibi, A.; Lin, Z.; Tsao, R.; Thompson, L.U.; Comelli, E.M. Effects of flaxseed and its components on mammary gland mirnome: Identification of potential biomarkers to prevent breast cancer development. Nutrients 2019, 11, 2656. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary flaxseed modulates the mirna profile in irradiated and non-irradiated murine lungs: A novel mechanism of tissue radioprotection by flaxseed. Cancer Biol. Ther. 2014, 15, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, H.; Tang, Q. Mir-133: A suppressor of cardiac remodeling? Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, Y.; Zhang, J.F.; Fu, W.M. Microrna-133 mediates cardiac diseases: Mechanisms and clinical implications. Exp. Cell Res. 2017, 354, 65–70. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte regeneration: A consensus statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The mir-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. CMLS 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Worke, L.J.; Barthold, J.E.; Seelbinder, B.; Novak, T.; Main, R.P.; Harbin, S.L.; Neu, C.P. Densification of type i collagen matrices as a model for cardiac fibrosis. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Castoldi, G.; Di Gioia, C.R.; Bombardi, C.; Catalucci, D.; Corradi, B.; Gualazzi, M.G.; Leopizzi, M.; Mancini, M.; Zerbini, G.; Condorelli, G.; et al. Mir-133a regulates collagen 1a1: Potential role of mir-133a in myocardial fibrosis in angiotensin ii-dependent hypertension. J. Cell. Physiol. 2012, 227, 850–856. [Google Scholar] [CrossRef]
- Quinn, F.R.; Currie, S.; Duncan, A.M.; Miller, S.; Sayeed, R.; Cobbe, S.M.; Smith, G.L. Myocardial infarction causes increased expression but decreased activity of the myocardial Na+-Ca2+ exchanger in the rabbit. J. Physiol. 2003, 553, 229–242. [Google Scholar] [CrossRef]
- Ander, B.P.; Hurtado, C.; Raposo, C.S.; Maddaford, T.G.; Deniset, J.F.; Hryshko, L.V.; Pierce, G.N.; Lukas, A. Differential sensitivities of the ncx1.1 and ncx1.3 isoforms of the Na+-Ca2+ exchanger to alpha-linolenic acid. Cardiovasc. Res. 2007, 73, 395–403. [Google Scholar] [CrossRef]
- Ander, B.P.; Weber, A.R.; Rampersad, P.P.; Gilchrist, J.S.C.; Pierce, G.N.; Lukas, A. Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic rabbits. J. Nutr. 2004, 134, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, B.J.; Reis, B.Z.; Duarte, G.B.S.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of micrornas and nutrition in modulating inflammation and chronic diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dalli, J.; Chiang, N.; Baron, R.M.; Quintana, C.; Serhan, C.N. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by microrna and proresolving mediators. Immunity 2013, 39, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Austria, J.A.; Richard, M.N.; Chahine, M.N.; Edel, A.L.; Malcolmson, L.J.; Dupasquier, C.M.; Pierce, G.N. Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J. Am. Coll. Nutr. 2008, 27, 214–221. [Google Scholar] [CrossRef]
- Sun, Y.; Weber, K.T. Infarct scar: A dynamic tissue. Cardiovasc. Res. 2000, 46, 250–256. [Google Scholar] [CrossRef]
- Shi, B.; Guo, Y.; Wang, J.; Gao, W. Altered expression of micrornas in the myocardium of rats with acute myocardial infarction. BMC Cardiovasc. Disord. 2010, 10, 11. [Google Scholar] [CrossRef]
- Port, J.D.; Walker, L.A.; Polk, J.; Nunley, K.; Buttrick, P.M.; Sucharov, C.C. Temporal expression of mirnas and mrnas in a mouse model of myocardial infarction. Physiol. Genom. 2011, 43, 1087–1095. [Google Scholar] [CrossRef]
- Montgomery, R.L.; Yu, G.; Latimer, P.A.; Stack, C.; Robinson, K.; Dalby, C.M.; Kaminski, N.; van Rooij, E. Microrna mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 2014, 6, 1347–1356. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiok, T.C.; Richards, A.M.; Wang, P. Microrna-101a suppresses fibrotic programming in isolated cardiac fibroblasts and in vivo fibrosis following trans-aortic constriction. J. Mol. Cell. Cardiol. 2018, 121, 266–276. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A.L.; LaVallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T.G.; Ramjiawan, B.; Aliani, M.; Guzman, R.; et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension 2013, 62, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Dupasquier, C.M.; Dibrov, E.; Kneesh, A.L.; Cheung, P.K.; Lee, K.G.; Alexander, H.K.; Yeganeh, B.K.; Moghadasian, M.H.; Pierce, G.N. Dietary flaxseed inhibits atherosclerosis in the ldl receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2394–H2402. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.A.; Deniset, J.F.; Austria, J.A.; LaValleé, R.K.; Maddaford, G.G.; Hedley, T.E.; Dibrov, E.; Pierce, G.N. Effects of dietary flaxseed on atherosclerotic plaque regression. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1743–H1751. [Google Scholar] [CrossRef] [PubMed]



| Fatty Acid | CONTROL | FLAX | OIL | SDG |
|---|---|---|---|---|
| C10:0 | t | t | t | t |
| C12:0 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 * |
| C14:0 | 0.49 ± 0.00 | 0.51 ± 0.01 | 0.50 ± 0.00 | 1.06 ± 0.00 * |
| C15:0 | 0.07 ± 0.00 * | 0.08 ± 0.00 * | 0.08 ± 0.00 * | 0.16 ± 0.00 * |
| C16:0 | 13.67 ± 0.02 * | 10.17 ± 0.02 * | 10.16 ± 0.02 * | 16.37 ± 0.01 * |
| C17:0 | 0.12 ± 0.00 | 0.12± 0.00 ‡ | 0.12 ± 0.00 * | 0.21 ± 0.00 * |
| C18:0 | 1.92 ± 0.00 | 3.00 ± 0.00 * | 4.10 ± 0.00 * | 2.37 ± 0.00 * |
| C20:0 | 0.32 ± 0.00 | 0.19 ± 0.00 * | 0.21 ± 0.00 * | 0.22 ± 0.00 * |
| C22:0 | 0.17 ± 0.00 | 0.18 ± 0.00 * | 0.19 ± 0.00 * | 0.22 ± 0.00 * |
| C24:0 | 0.19 ± 0.00 | 0.17 ± 0.00 ‡ | 0.15 ± 0.00 * | 0.21 ± 0.00 |
| Total SFA | 16.98 ± 0.02 | 14.43 ± 0.02 * | 15.51 ± 0.02 * | 20.82 ± 0.01 * |
| C14:1 | t | t | t | t |
| C16:1t | 0.05 ± 0.00 | 0.05 ± 0.00 ‡ | 0.04 ± 0.00 * | 0.07 ± 0.00 * |
| C16:1 | 0.68 ± 0.01 | 0.66 ± 0.01 | 0.60 ± 0.01 ‡ | 1.33 ± 0.02 * |
| C17:1 | 4.19 ± 0.03 | 4.16 ± 0.01 | 3.93 ± 0.02 * | 9.57 ± 0.06 * |
| C18:1 | 23.16 ± 0.01 | 19.53 ± 0.01 * | 18.43 ± 0.01 * | 15.15 ± 0.01 * |
| C18:1n7c | 0.81 ± 0.00 | 0.87 ± 0.00 * | 0.83 ± 0.00 ‡ | 1.19 ± 0.01 * |
| C20:1 | 0.39 ± 0.00 | 0.33 ± 0.00 * | 0.25 ± 0.01 * | 0.50 ± 0.01 * |
| C22:1 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 * | 0.05 ± 0.00 * |
| C24:1 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.10 ± 0.00 * |
| Total MUFA | 29.36 ± 0.03 | 25.66 ± 0.01 * | 24.16 ± 0.01 * | 27.95 ± 0.05 * |
| C18:2 | 49.82 ± 0.04 | 27.13 ± 0.03 * | 25.55 ± 0.06 * | 43.53 ± 0.03 * |
| C18:3n6 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.00 * |
| C20:2 | 0.04 ± 0.00 | 0.05 ± 0.00 ‡ | 0.04 ± 0.00 | 0.08 ± 0.00 * |
| C20:3n6 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| C20:4 | 0.06 ± 0.00 | 0.08 ± 0.00 * | 0.07 ± 0.00 * | 0.16 ± 0.00 * |
| C20:3n3 | 0.00 ± 0.00 | 0.01 ± 0.00 * | 0.01 ± 0.00 * | 0.00 ± 0.00 |
| C20:5 | 0.75 ± 0.00 | 0.77 ± 0.00 | 0.72 ± 0.00 | 1.65 ± 0.01 * |
| C22:2 | t | t | t | t |
| C22:4 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 * |
| C22:5n6 | 0.01 ± 0.00 | 0.02 ± 0.00 * | 0.01 ± 0.00 | 0.04 ± 0.00 * |
| C22:5n3 | 0.09 ± 0.00 | 0.10 ± 0.00 * | 0.09 ± 0.00 | 0.21 ± 0.00 * |
| C22:6n3 | 0.70 ± 0.00 | 0.75 ± 0.00 * | 0.70 ± 0.00 | 1.58 ± 0.01 * |
| Total PUFA | 53.66 ± 0.04 | 59.91 ± 0.02 * | 60.33 ± 0.02 * | 51.23 ± 0.04 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, M.; Kura, B.; O’Hara, K.A.; Dibrov, E.; Netticadan, T.; Slezak, J.; Pierce, G.N. Cardioprotective Effects of Dietary Flaxseed Post-Infarction Are Associated with Changes in MicroRNA Expression. Biomolecules 2020, 10, 1297. https://doi.org/10.3390/biom10091297
Parikh M, Kura B, O’Hara KA, Dibrov E, Netticadan T, Slezak J, Pierce GN. Cardioprotective Effects of Dietary Flaxseed Post-Infarction Are Associated with Changes in MicroRNA Expression. Biomolecules. 2020; 10(9):1297. https://doi.org/10.3390/biom10091297
Chicago/Turabian StyleParikh, Mihir, Branislav Kura, Kimberley A. O’Hara, Elena Dibrov, Thomas Netticadan, Jan Slezak, and Grant N. Pierce. 2020. "Cardioprotective Effects of Dietary Flaxseed Post-Infarction Are Associated with Changes in MicroRNA Expression" Biomolecules 10, no. 9: 1297. https://doi.org/10.3390/biom10091297
APA StyleParikh, M., Kura, B., O’Hara, K. A., Dibrov, E., Netticadan, T., Slezak, J., & Pierce, G. N. (2020). Cardioprotective Effects of Dietary Flaxseed Post-Infarction Are Associated with Changes in MicroRNA Expression. Biomolecules, 10(9), 1297. https://doi.org/10.3390/biom10091297