Metabolic Diversity and Therapeutic Potential of Holarrhena pubescens: An Important Ethnomedicinal Plant
Abstract
:1. Introduction
1.1. Geographical Distribution
1.2. Morphological Description
2. Phytoconstituents
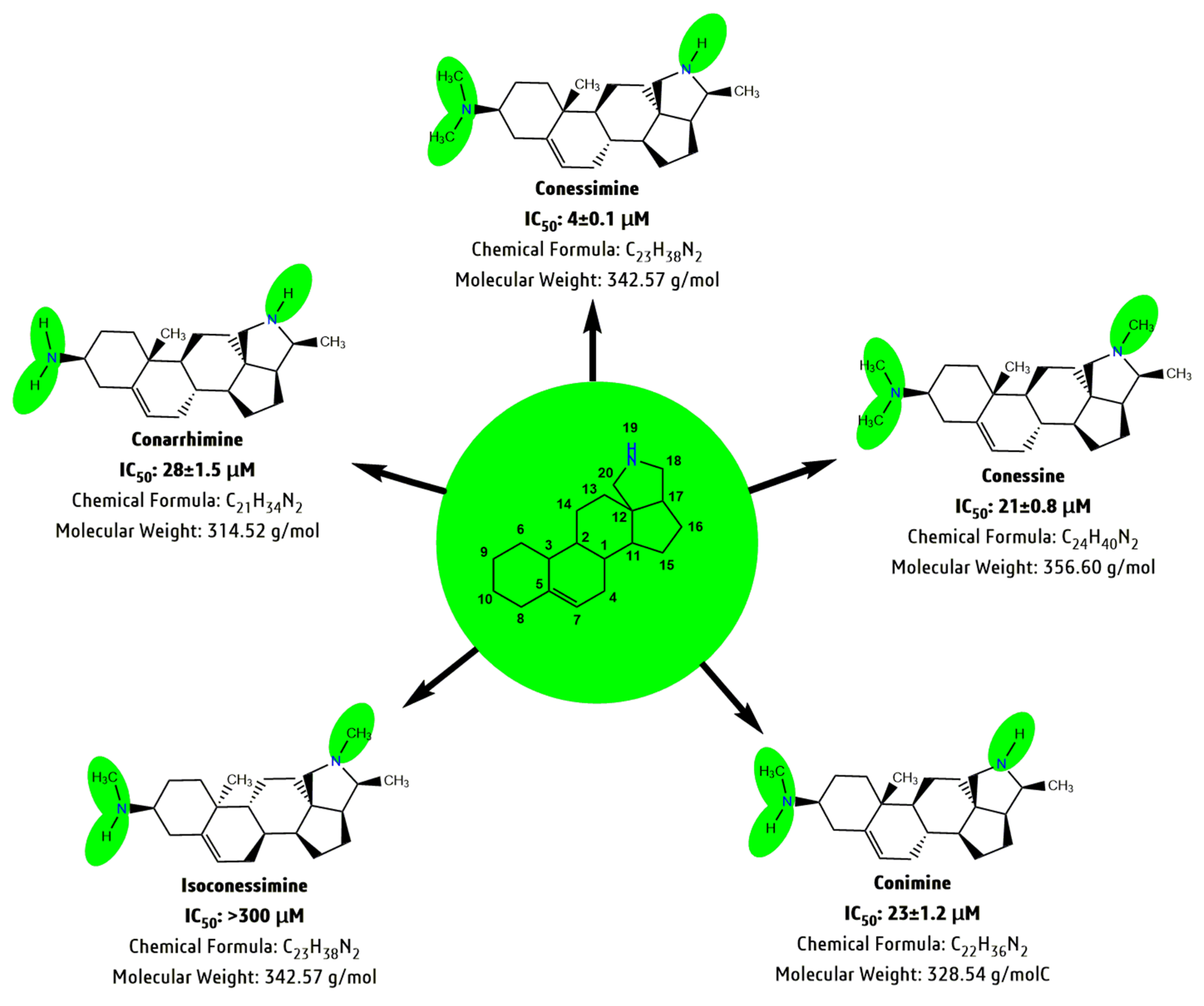
- Steroidal alkaloids: conarrhimine, conessine, holantosines a, b, c, d, e and f, holarrhessimine, holarrhidine, holarrhine, holonamine, hydroxyconessine, kurchiline, kurchine, kurchiphylline, norconessine, n,n,n′,n′tetramethylholarrhenine, holacin, kurchinine, conamine, holadysine, 12-hydroxyconessimine, holarrhimine, holadysamine, conessimine, isoconessimine, holarosine a, conessidine, kurchiphyllamine, 7α-hydroxyconessine [26,27].
- Uncharacterized alkaloid: lettocine [28].
- Sterols: sitosta-5,23-dien-3β-ol, stigmasterol [31].
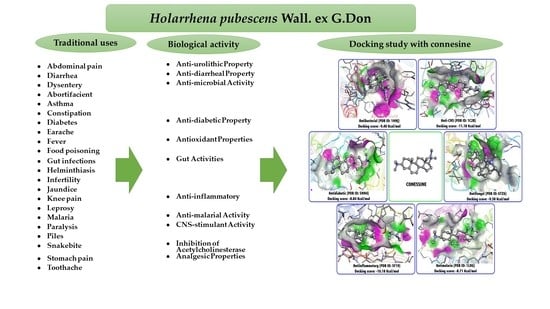
3. Traditional Uses
3.1. Bark
3.2. Leaf
- In Ayurveda, H. pubescens leaves are not reported to have medicinal value.
- In Unani medicine, leaves are used as aphrodisiac, tonic, astringent and galactagogue, and are thus used for treating chronic bronchitis, urinary discharges, wounds, ulcers, as well as for muscles relaxation; they are also useful to regulate menstruation [72].
3.3. Roots
3.4. Flowers
3.5. Seeds
- In Unani medicine, seeds are used as carminative, aphrodisiac, astringent and lithotriptic [78].
- In Tibetan medicine, they are used as alexipharmic, antidiarrheal, cholagogue, and analgesic [79].
- In the indigenous Bangladesh system of medicine, they are used as astringent, anthelmintic, febrifuge, stomachic, anti-dysenteric and anti-diarrheal [80].
4. Pharmacology
4.1. Anti-diabetic Property
Mechanism of Action
4.2. Anti-Diarrheal Property
4.3. Anti-inflammatory and Analgesic Properties
4.4. Antioxidant/Free Radical Scavenging Properties
4.5. Anti-Urolithic Property
4.6. Diuretic Property
Gut Activities
4.7. Inhibition of Acetylcholinesterase and CNS-Stimulant Activity
4.8. Anti-Microbial Activity
4.8.1. Synergy and Mechanism of Action
4.8.2. Conessine is Major Compound Responsible for Antimicrobial Activity
4.9. Anti-malarial Activity
4.10. Stracture Activity Relationship (SAR) Study
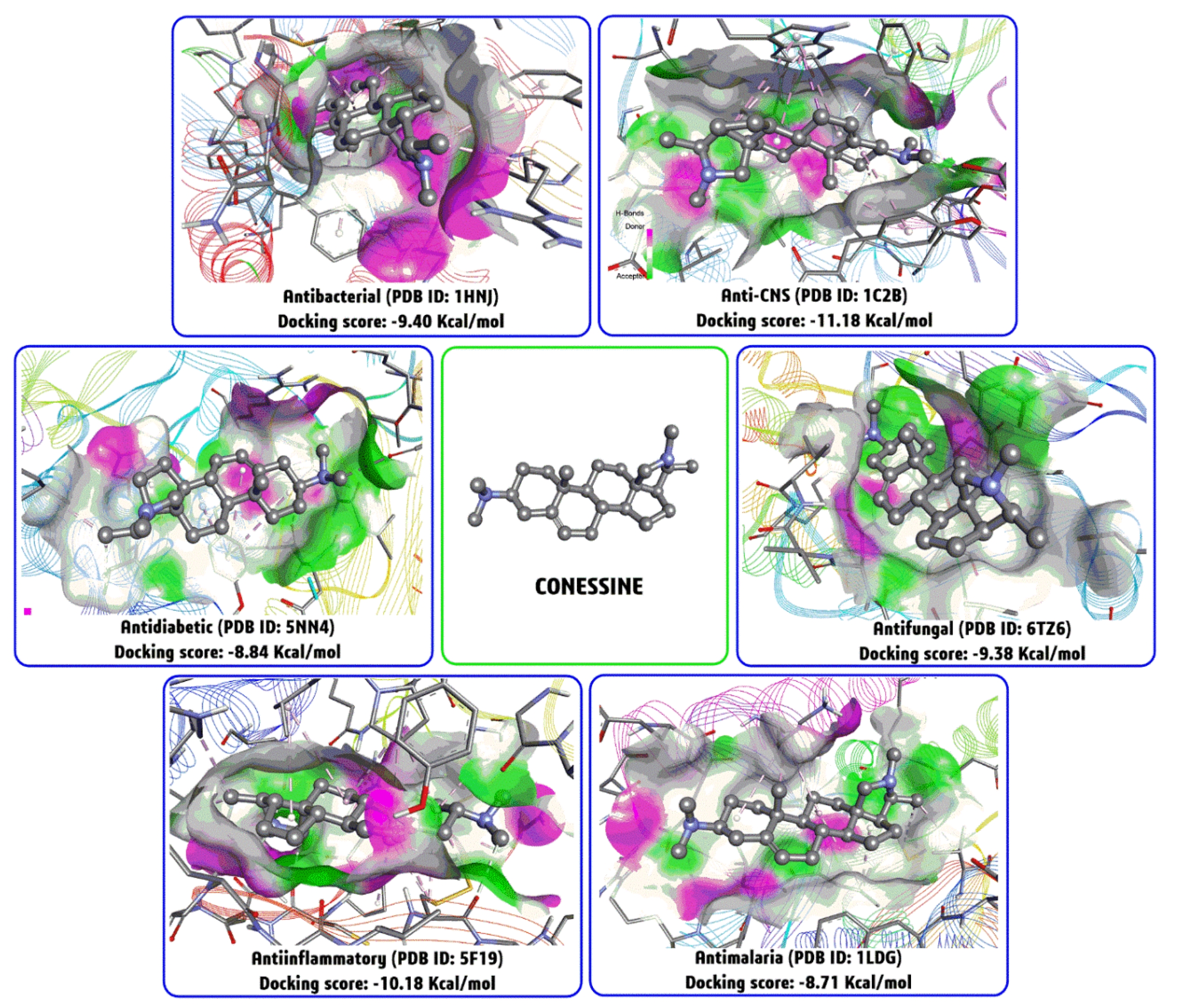
4.11. Molecular Docking Studies with Conessine
5. Safety and Toxicity Studies
6. Clinical Trials
7. The Way Forward
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamilton, A.C. Medicinal plants, Conservation, and livelihoods. Biodiv. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanic Gardens, Kew, Plants of the World online. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:79318-1 (accessed on 16 September 2020).
- Jamadagni, P.S.; Pawar, S.D.; Jamadagni, S.B.; Chougule, S.; Gaidhani, S.N.; Murthy, S.N. Review of Holarrhena antidysenterica (L.) Wall. ex A. DC.: Pharmacognostic, Pharmacological, and Toxicological Perspective. Pharmacogn. Rev. 2017, 11, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharat, K.; Pradhan, K.; Hemant, K.; Badola, K. Ethnomedicinal plant use by Lepcha tribe of Dzongu valley, bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J. Ethnobiol. Ethnomed. 2008, 4, 22–40. [Google Scholar]
- Panda, S.K. Ethnomedicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 2014, 151, 158–175. [Google Scholar] [CrossRef]
- Devi Prasad, A.G.; Shyma, T.B.; Raghavendra, M.P. Plants used by the tribes of for the treatment of digestive system disorders in Wayanad district, Kerala. J. Appl. Pharm. Sci. 2013, 3, 171–175. [Google Scholar]
- Manika, N. Identification of Alternative Plant Parts of Some Important Bark Drugs for Sustainable Harvesting. Ph.D. Thesis, University of Lucknow, Lucknow, India, 2014. [Google Scholar]
- Ali, K.M.; Chatterjee, K.; De, D.; Bera, T.K.; Ghosh, D. Efficacy of aqueous extract of seed of Holarrhena antidysenterica for the management of diabetes in experimental model rat: A correlative study with antihyperlipidemic activity. Int. J. Appl. Res. Nat. Prod. 2009, 2, 13–21. [Google Scholar]
- Ali, K.M.; Chatterjee, K.; De, D.; Jana, K.; Bera, T.K.; Ghosh, D. Inhibitory effect of hydro-methanolic extract of seed of Holarrhena antidysenterica on alpha-glucosidase activity and postprandial blood glucose level in normoglycemic rat. J. Ethnopharmacol. 2011, 135, 194–196. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I. Antibacterial properties of traditionally used Indian medicinal plants. Exp. Clin. Pharmacol. 2007, 29, 79–92. [Google Scholar] [CrossRef]
- Husain, N.; Trak, T.H.; Chauhan, D. Floristic diversity of Jammu and Kashmir, India, especially in context to skin care. Int. J. Adv. Sci. Technol. 2020, 29, 2358–2386. [Google Scholar]
- Diallo, A.; Traore, M.S.; Keita, S.M.; Balde, M.A.; Keita, A.; Camara, M.; Van Miert, S.; Pieters, L.; Balde, A.M. Management of diabetes in Guinean Traditional Medicine: An ethnobotanical investigation in the Coastal Lowlands. J. Ethnopharmacol. 2012, 144, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.D.; Pandey, A.K. Ethnomedicobiology of Similipal Biosphere Reserve, Orissa. In Advances in Ethnobotany; Das, A.P., Pandey, A.K., Eds.; Bishen Singh Mahendra Pal Singh: Dehera Dun, India, 2007; pp. 247–252. [Google Scholar]
- Bikram, K.; Mallik, R.; Panda, T.; Rabindra, N. Traditional herbal practices by the ethnic people of Kalahandi District of Odisha, India. Asian Pac. J. Trop. Biomed. 2012, 2, S988–S994. [Google Scholar]
- Dey, A.; Jitendra, N.D. Ethnobotanical survey of Purulia district, West Bengal, India for medicinal plants used against gastrointestinal disorders. J. Ethnopharmacol. 2012, 143, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bari, M.A.; Roy, M.; Bhadra, S.K. Inherited folk pharmaceutical knowledge of tribal people in the Chittagong Hill tracts, Bangladesh. Indian J. Tradit. Knowl. 2010, 9, 77–89. [Google Scholar]
- Chopra, R.N.; Chopra, I.C.; Handa, K.L.; Kapur, I.D. Chopras Indigenous Drugs of India; Academic Press: New Delhi, India, 1982; p. 342. [Google Scholar]
- Rout, S.D.; Panda, T.; Mishra, N. Ethno-medicinal plants used to cure different diseases by tribals of Mayurbhanj district of North Orissa. Stud. Ethno Med. 2009, 3, 27–32. [Google Scholar] [CrossRef]
- Jena, M.; Sahoo, S.; Sahu, R.K. Some ethno medicinal plants for the treatment of common health problems in Mayurbhanj District, Orissa, India. N. Y. Sci. J. 2011, 4, 87–92. [Google Scholar]
- Gaur, R.D.; Sharma, J.; Painuli, R.M. Plants used in traditional healthcare of livestock by Gujjar community of Sub-Himalayan tracts, Uttarakhand, India. Indian J. Nat. Prod. Resour. 2010, 1, 243–248. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research: New Delhi, India, 1956. [Google Scholar]
- Ghani, A. Medicinal Plants of Bangladesh: Chemical Constituents and Uses, 1st ed.; Asiatic Society of Bangladesh: Dacca, Bangladesh, 1998; pp. 195–196. [Google Scholar]
- Dua, V.K.; Gaurav, V.; Bikram, S.; Aswathy, R.; Upma, B.; Dayal, D.; Gupta, N.C.; Sandeep, K.; Ayushi, R. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar. J. 2013, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Maroyi, A. Holarrhena pubescens Wall. ex G. Don. In Plant Resources of Tropical Africa 11, Medicinal Plants 1; Schmelzer, G.H., Gurib-Fakim, A., Eds.; PROTA Foundation: Wageningen, The Netherlands, 2008; pp. 332–335. [Google Scholar]
- Begum, S.; Usmani, S.B.; Siddiqui, B.S.; Siddiqui, S. Alkaloidal constituents of the bark of Holarrhena antidysenterica. Heterocycles 1993, 36, 717–723. [Google Scholar]
- Alauddin, M.; Martin-Smith, M. Biological activity in steroids possessing nitrogen atoms. J. Pharm. Pharmacol. 1962, 14, 469–495. [Google Scholar] [CrossRef]
- Daniel, M. Medicinal Plants: Chemistry and Properties; Science: Enfield, NH, USA, 2006. [Google Scholar]
- Stephenson, R.P. The pharmacological properties of conessine, isoconessine and neoconessine. Br. J. Pharmacol. 1948, 3, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Singh, B.; Bhandari, P.; Gupta, A.P.; Kaul, V.K. Steroidal alkaloids from Holarrhena antidysenterica (L.) WALL. Chem. Pharma. Bull. 2007, 55, 912–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, B.S.; Usmani, S.B.; Ali, S.T.; Begum, S.; Rizwani, G.H. Further constituents from the bark of H. pubescens. Phytochemistry 2001, 58, 1199–1204. [Google Scholar] [CrossRef]
- Singh, A.K.; Raghubanshi, A.S. Medical ethnobotany of the tribals of Sonaghati of Sonbhadra district, Uttar Pradesh, India. J. Ethnopharmacol. 2002, 81, 31–41. [Google Scholar] [CrossRef]
- Kadir, M.F.; Sayeed, M.S.B.; Mia, M.M.K. Ethnopharmacological survey of medicinal plants used by traditional healers in Bangladesh for gastrointestinal disorders. J. Ethnopharmacol. 2013, 147, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Anantha Narayana, D.B. The Ayurvedic Pharmacopoeia of India: Part II. A Good Beginning. (Formulations). Curr. Sci. 2008, 94, 1086–1087. [Google Scholar]
- Rudolf, H. Hagers Handbuch der Pharmazeutischen Praxis; Springer: New York, NY, USA, 1976; pp. 92–95. [Google Scholar]
- Marius, H.; Yetein, A.N.; Houessou, L.G.; Toussaint, O.; Lougbe´gnon, A.C.; BriceTente, O.K. Ethnobotanical study of medicinal plants used for the treatment of malaria in plateau of Allada, Benin (West Africa). J. Ethnopharmacol. 2013, 146, 154–163. [Google Scholar]
- Ghimire, K.A.; Bastakoti, R.R. Ethnomedicinal knowledge and healthcare practices among the Tharus of Nawalparasi district in central Nepal. For. Ecol. Manag. 2009, 257, 2066–2072. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Gericke, N. People’s Plants. A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000. [Google Scholar]
- Steenkamp, V. Traditional herbal remedies used by South African women for gynecological complaints. J. Ethnopharmacol. 2003, 86, 97–108. [Google Scholar] [CrossRef]
- Rout, S.D.; Panda, S.K. Ethnomedicinal plant resources of Mayurbhanj district, Orissa. Indian J. Trad. Knowl. 2010, 9, 68–72. [Google Scholar]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.H.; Dicato, M.; Diederich, M. Traditional West African Pharmacopeia, Plants and derived. Biochem. Pharmacol. 2012, 84, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Koudouvoa, K.; Karoua, D.S.; Kokoua, K.; Essiena, K.; Aklikokoua, K.; Glithob, I.A.; Simporec, J.; Sanogod, R.; De Souzaa, C.; Gbeassora, M. An ethnobotanical study of antimalarial plants in Togo Maritime Region. J. Ethnopharmacol. 2011, 134, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ngarivhume, T.; Charlotte, I.E.A.; Klooster, C.; Joop, T.V.M.; Dejong, C.; Jan, H.V. Westhuizen medicinal plants used by traditional healers for the treatment of malaria in the Chipinge District in Zimbabwe. J. Ethnopharmacol. 2015, 159, 224–237. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, M.; Mavi, S.; Drummond, R.B.; Ndemera, B. The Traditional Medical Practitioner in Zimbabwe: His Principles of Practice and Pharmacopoeia; Mambo Press: Gweru, Zimbabwe, 1985. [Google Scholar]
- Chhabra, S.C.; Mahunnah, R.L.A.; Mshiu, E.N. Plants used in traditional medicine in Eastern Tanzania. I. Pteridophytes and Angiosperms (Acanthaceae to Canellaceae). J. Ethnopharmacol. 1897, 21, 253–277. [Google Scholar] [CrossRef]
- Bruschi, P.; Morganti, M.; Mancini, M.M.A. Signorini traditional healers and laypeople: A qualitative and quantitative approach to local knowledge on medicinal plants in Muda (Mozambique). J. Ethnopharmacol. 2011, 138, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Olorunnisola, O.S.; Adetutu, A.; Afolayan, A.J.A. An inventory of plants commonly used in the treatment of some disease conditions in Ogbomoso, South West, Nigeria. J. Ethnopharmacol. 2014, 161, 60–68. [Google Scholar] [CrossRef]
- Sheng, P.J. Preliminary study of ethnobotany in Xishuang Banna, People’s Republic of China. J. Pharmacol. 1985, 13, 121–137. [Google Scholar]
- Khuankaew, S.; Srithi, K.; Tiansawat, P.; Jampeetong, A.; Inta, A.; Wang, P.P.W. Ethnobotanical study of medicinal plants used by tai yai in Northern Thailand. J. Pharmacol. 2014, 151, 829–838. [Google Scholar] [CrossRef]
- Jayaswal, S.B. Amoebicidal activity of steroidal alkaloids of Wrightia tomentosa in vitro. Indian J. Pharm. 1976, 38, 112–113. [Google Scholar]
- Juyal, P.; Ghildiyal, M. Medicinal phytodiversity of Bhabar Tract of Garhwal Himalaya. J. Med. Plant. Stud. 2013, 1, 43–57. [Google Scholar]
- Girach, R.D.; Singh, S.; Brahmam, M.; Misra, M.K. Traditional treatment of skin diseases in Bhadrak District, Orissa. J. Econ. Taxon. Botany 1999, 27, 754–760. [Google Scholar]
- Kumar, M.; Bussmann, R.W.; Mukesh, J.; Kumar, P. Ethnomedicinal uses of plants close to rural habitation in Garhwal Himalaya, India. J. Med. Plant Res. 2011, 11, 2252–2260. [Google Scholar]
- Haerdi, F. Die Eingeborenen-Heilpflanzen Des Ulanga-Distriktes Tanganjikas (Ostafrika). Acta Trop. 1964, 8, L-278. [Google Scholar]
- Kapur, S.K. Traditionally important medicinal plants of Dudu valley-Jammu. J. Econ. Taxon. Botany 1991, 15, 1–10. [Google Scholar]
- Priya, V.K.; Gopalan, R. Ethnomedicinal studies in selected medicinal plants of Dhoni Forest, Western Ghats, Kerala. Asian J. Pharm. Clin. Res. 2014, 7, 3–6. [Google Scholar]
- Bhat, P.; Hegde, G.; Ganesh, R. ethnomedicinal practices in different communities of Uttara Kannada district of Karnataka for treatment of wounds. J. Pharmacol. 2012, 143, 501–514. [Google Scholar] [CrossRef]
- Mahato, S.; Mehta, A.; Roy, S. Studies on antibacterial effects of bark, seed and callus extracts of Holarrhena antidysenterica Wall. Bioscan 2013, 8, 717–721. [Google Scholar]
- Guha Bakshi, D.N.; Sensarma, P.; Pal, D.C. A Lexicon of Medicinal Plants in India; Naya Prokash Publishers: Calcutta, India, 2001; Volume II, pp. 356–358. [Google Scholar]
- Panda, S.K.; Bastia, A.K.; Dutta, S.K. Antidiarrheal activity of Terminalia arjuna Roxb from India. J. Biol. Act. Prod. Nat. 2011, 1, 236–247. [Google Scholar]
- Hossan, M.S.; Hanif, A.; Agarwala, B.; Sarwar, M.S.; Karim, M.; Taufiq-Urrahman, M.; Jahan, R.; Rahmatullah, M. Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot. Res. Appl. 2010, 8, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Rahmatullah, M.; Azam, M.N.K.; Malek, I.; Nasrin, D.; Jamal, F.; Rahman, M.A.; Khatun, Z.; Jahan, S.; Seraj, S.; Jahan, R. An ethnomedicinal survey among the Marakh sect of the garo tribe of Mymensingh district, Bangladesh. Int. J. PharmTech Res. 2012, 4, 141–149. [Google Scholar]
- Rahmatullah, M.; Ayman, U.; Akter, F.; Sarker, M.; Sifa, R.; Sarker, B.; Chyti, H.N.; Jahan, F.I.; Chowdhury, M.H.; Chowdhury, S.A. Medicinal formulations of a Kanda tribal healer—A tribe on the verge of disappearance in Bangladesh. Afr. J. Trad. Complement Alt. Med. 2013, 10, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumpa, S.I.; Hossain, M.I.; Ishika, T. Ethnomedicinal uses of herbs by indigenous medicine practitioners of Jhenaidah District, Bangladesh. J. Pharmacogn. Phytochem. 2014, 3, 23–33. [Google Scholar]
- Uddin, M.Z.; Hassan, M.A.; Sultana, M. Ethnobotanical survey of medicinal plants in Phulbari Upazila of Dinajpur District, Bangladesh. Bangladesh J. Plant Taxon. 2006, 13, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Zerin, Z.; Harun-ar-Rashid, M.; Islam, A.; Khatun, Z.; Rahmatullah, M. Medicinal plants and formulations of a community of the Tonchongya tribe in Bandarban District of Bangladesh. Am. Eur. J. Sustain. Agric. 2012, 6, 292–298. [Google Scholar]
- Shaheen, H.D.; Potter, M.F.; Qaseem, A.; Qureshi, R. Heliotropium pakistanicum sp. nov. (Boraginaceae) from Pakistan. Planta Daninha 2019, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Qaseem, M.; Qureshi, R.; Amjad, M.S.; Ahmed, W.; Masood, A.; Shaheen, H. Ethnobotanical evaluation of indigenous flora from the communities of Rajh mehal and Goi union councils of district Kotli, Azad Jammu Kashmir, Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 2799–2829. [Google Scholar] [CrossRef]
- Qureshi, R. Medicinal Uses of Trees and Shrubs by the Inhabitants of Nara Desert, Pakistan. In Plant and Human Health; Volume 1: Ethnobotany and Physiology; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing Ag, Springer Nature: Cham, Switzerland, 2018; pp. 391–407. [Google Scholar]
- Khan, A.M.; Qureshi, R.; Saqib, Z. Multivariate analyses of the vegetation of the western Himalayan forests of Muzaffarabad District, Azad Jammu and Kashmir, Pakistan. Ecol. Indic. 2019, 104, 723–736. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Jahan, R.; Hossan, M.S.; Seraj, S.; Rahman, M.M.; Chowdhury, A.R.; Begum, R.; Nasrin, D.; Khatun, Z.; Hossain, M.S.; et al. A Comparative analysis of medicinal plants used by three tribes of Chittagong Hill tracts region, Bangladesh to treat leucorrhea. Adv. Nat. Appl. Sci. 2010, 2, 148–152. [Google Scholar]
- Dubey, D.; Padhy, R.N. Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethnomedicinal plants used by an aborigine of India. Asian Pac. J. Trop. Dis. 2012, 2, 273–281. [Google Scholar] [CrossRef]
- Rajakumar, N.; Shivanna, M.B. Ethno-medicinal application of plants in the eastern region of Shimoga District, Karnataka, India. J. Pharmacol. 2009, 126, 64–73. [Google Scholar] [CrossRef]
- Khaleel, S.B.; Sudarsanam, G. Traditional use of plants against snakebite in Sugali tribes of Yerramalais of Kurnool District, Andhra Pradesh, India. Asian Pac. J. Trop. Biomed. 2012, 2, 575–579. [Google Scholar]
- Jain, S.P.; Puri, H.S. Ethnomedicinal plants of Jaunsar-Bawar Hills, Uttar Pradesh, India. J. Pharmacol. 1984, 12, 213–222. [Google Scholar] [CrossRef]
- Painuli, R.M.; Maheswari, J.K. Medicinal plants used by tribals of Panchmahals District, Gujarat. Ancient Sci. Life 1994, 3, 253–258. [Google Scholar]
- Harpreet, B.; Sharma, Y.A.; Manhas, R.K.; Kumar, K. Ethnomedicinal plants used by the villagers of District Udhampur, J&K, India. J. Pharmacol. 2014, 151, 1005–1018. [Google Scholar]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef]
- Mahishi, P.; Srinivasa, B.H.; Shivanna, M.B. Medicinal plant wealth of local communities in some villages in Shimoga District of Karnataka, India. J. Pharmacol. 2005, 98, 307–312. [Google Scholar] [CrossRef]
- Gangwar, K.K.; Deepali, G.R.; Gangwar, R.S. Ethnomedicinal plant diversity in Kumaun Himalaya of Uttarakhand, India. Nat. Sci. 2010, 8, 66–78. [Google Scholar]
- Kabir, A.K.L.; Begum, M.M.; Islam, T. Study of Bioactivities of Holarrhena pubescens growing in Bangladesh. Dhaka Univ. J. Pharm. Sci. 2018, 17, 131–137. [Google Scholar] [CrossRef]
- Rajakumar, N.; Shivanna, M.B. Traditional veterinary healthcare practices in Shimoga District of Karnataka, India. Indian J. Trad. Knowl. 2012, 11, 283–287. [Google Scholar]
- Sanjib, S.; Shil, N.; Choudhury, M.S.; Das, S. Indigenous knowledge of medicinal plants used by the Reang tribe of Tripura state of India. J. Pharmacol. 2014, 152, 135–141. [Google Scholar]
- Tarafdar, R.G.; Nath, S.; Talukdar, A.D.; Manabendra, D.C. Antidiabetic plants used among the ethnic communities of Unakoti District of Tripura, India. J. Pharmacol. 2015, 160, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R.; De, B.; Devanna, N. An ethnobotanical survey of medicinal plants used by ethnic people in west and south District of Tripura, India. J. For. Res. 2011, 22, 417–426. [Google Scholar] [CrossRef]
- Shukla, A.N.; Srivastava, S.; Rawat, A.K.S. A survey of traditional medicinal plants of Uttar Pradesh (India)-used in treatment of infectious diseases. Nat. Sci. 2013, 11, 24–36. [Google Scholar]
- Tripathi, S.; Mondal, A.K.; Verma, K. Rare ethno medicinal plants of South West Bengal, India with their different medicinal uses: Needs conservation. Int. J. Life Sci. Pharm. Res. 2013, 2, 114–122. [Google Scholar]
- Sikarwar, R.L.S.; Pathak, B.; Jaiswal, A. Some unique ethnomedicinal perceptions of tribal communities of Chitrakoot, Madhya Pradesh. Indian J. Trad. Knowl. 2008, 7, 613–617. [Google Scholar]
- Kosalge, S.B.; Fursule, R.A. Investigation of ethnomedicinal claims of some plants used by tribals of Satpuda Hills in India. J. Pharmacol. 2009, 121, 456–461. [Google Scholar] [CrossRef]
- Sen, S.K.; Pattnaik, M.R.; Behera, L.M. Traditional use of herbal medicines against rheumatism by the tribals of Bargarh District in Odisha (India). Life Sci. Leaflets 2014, 51, 59–68. [Google Scholar]
- Mallick, S.N.; Ram, J.P.; Parida, N. Study of ethnomedicinal values of some shrubs in Rourkela steel city and its surroundings, Sundargarh, Odisha. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 123–130. [Google Scholar]
- Rout, S.; Panda, S.P.; Patra, H.K. Ethnomedicinal studies on Bondo tribe of Malkangiri District, Odisha, India. Int. J. Biodiv. Conserv. 2014, 6, 326–332. [Google Scholar]
- Panda, S.K.; Patro, N.; Sahoo, G.; Bastia, A.K.; Dutta, S.K. Anti-diarrheal activity of medicinal plants of Similipal Biosphere Reserve, Odisha, India. Int. J. Med. Aromat. Plants 2012, 1, 123–134. [Google Scholar]
- Panda, S.K.; Bastia, A.K.; Sahoo, G. Process characteristics and nutritional evaluation of handia—A cereal based ethnic fermented food from Odisha. Indian J. Trad. Knowl. 2014, 13, 149–156. [Google Scholar]
- Panda, S.K.; Rout, S.D.; Mishra, N.; Panda, T. Phytotherapy and traditional knowledge of tribal communities of Mayurbhanj district, Orissa, India. J. Pharmacog. Phytother. 2011, 3, 101–113. [Google Scholar]
- Panda, S.K.; Padhi, L.; Leyssen, P.; Liu, M.; Neyts, J.; Luyten, W. Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the Similipal Biosphere Reserve, Odisha, India. Front. Pharmacol. 2017, 8, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padal, S.B.; Viyayakumar, Y. Traditional knowledge of Valmiki tribes of G. Madugula Mandalam, Visakhapatnam District, Andhra Pradesh. Int. J. Innov. Res. Dev. 2013, 2, 723–738. [Google Scholar]
- Manjula, R.R.; Koteswara Rao, J.; Seetharami Reddi, T.V.V. Ethnomedicinal plants used to cure jaundice in Khammam district of Andhra Pradesh, India. J. Phytol. 2011, 3, 33–35. [Google Scholar]
- Sandhya Sri, B.; Padal, S.B.; Ramakrishna, B. New traditional phytotherapy for gynecological disorders among the tribes of Visakhapatnam District, Andhra Pradesh, India. BMR Phytomed. 2014, 2, 1–7. [Google Scholar]
- Rajakumar, N.; Shivanna, M.B. Traditional herbal medicinal knowledge in Sagar Taluk of Shimoga District, Karnataka, India. Indian J. Nat. Prod. Res. 2010, 1, 102–108. [Google Scholar]
- Shivanna, M.B.; Rajakumar, N. Traditional medico-botanical knowledge of local communities in Hosanagara Taluk of Shimoga District in Karnataka, India. J. Herbs Spices Med. Plant. 2011, 17, 291–317. [Google Scholar] [CrossRef]
- Shivanna, M.B.; Rajakumar, N. Ethno-medico-botanical knowledge of rural folk in Bhadravathi taluk of Shimoga district, Karnataka. Indian J. Trad. Knowl. 2010, 9, 158–162. [Google Scholar]
- Bhandary, M.J.; Chandrashekar, K.R.; Kaveriappa, K.M. Medical ethnobotany of the Siddis of Uttara Kannada district, Karnataka, India. J. Pharmacol. 1995, 47, 149–158. [Google Scholar] [CrossRef]
- Sharma, J.; Gairola, S.; Sharma, Y.P.; Gaur, R.D. Ethnomedicinal plants used to treat skin diseases by Tharu community of District Udham Singh Nagar, Uttarakhand, India. J. Pharmacol. 2014, 158, 140–206. [Google Scholar] [CrossRef] [PubMed]
- Gairola, S.; Sharma, J.; Gaur, R.D.; Siddiqi, T.O.; Painuli, R.M. Plants used for treatment of dysentery and diarrhea by the Bhoxa community of district Dehradun, Uttarakhand, India. J. Pharmacol. 2013, 150, 989–1006. [Google Scholar]
- Sharma, J.; Gairola, S.; Gaur, R.D.; Painuli, R.M. The treatment of jaundice with medicinal plants in indigenous communities of the Sub-Himalayan region of Uttarakhand, India. J. Pharmacol. 2012, 143, 262–291. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Uprety, Y.; Burlakoti, C.; Chowdhary, C.L.; Bussmann, R.W. Indigenous use and ethnopharmacology of medicinal plants in Far-west Nepal. Ethnobot. Res. Appl. 2009, 7, 005–028. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Y.; Manral, M.S.; Kathait, V.; Prasar, B.; Kumar, R.; Sahu, R.K. Computation of in vivo antidiabetic activity of Holarrhena antidysenterica seeds extracts in Streptozotocin-induced diabetic rats. Iranian J. Pharmacol. Ther. 2016, 14, 22–27. [Google Scholar] [CrossRef]
- Keshri, U. Antidiabetic efficacy of ethanolic extract of Holarrhena antidysenterica seeds in streptozotocin induced diabetic rats and influence on certain biochemical parameters. J. Drug Deliv. Ther. 2012, 2, 159–162. [Google Scholar] [CrossRef]
- Bandawane, D.D.; Bibave, K.H.; Jaydeokar, A.V.; Patil, U.S.; Hivrale, M.G. Antihyperglycemic and antihyperlipidemic effects of methanolic extract of Holarrhena antidysenterica bark in alloxan induced diabetes mellitus in rats. Pharmacologia 2013, 4, 95–106. [Google Scholar] [CrossRef]
- Hegde, K.; Jaisal, K.K. Anti-diabetic potential of ethanolic extract of Holarrhena antidysenterica Linn leaves. Int. J. Pharm. Res. 2014, 5, 429–435. [Google Scholar]
- Bibave, K.H.; Bandawane, D.D.; Patil, U.S. Evaluation of in vitro antioxidant and in vivo antihyperglycemic effects of Holarrhena antidysenterica bark. J. Pharm. Res. 2012, 5, 5076–5080. [Google Scholar]
- Bhusal, A.; Jamarkattel, N.; Shrestha, A.; Lamsal, N.K.; Shakya, S.; Rajbhandari, S. Evaluation of antioxidative and antidiabetic activity of bark of H. pubescens wall. J. Clin. Diagn. Res. 2014, 8, HC05–HC08. [Google Scholar] [CrossRef]
- Sharma, D.K.; Gupta, V.K.; Kumar, S.; Joshi, V.; Mandal, R.S.; Prakash, A.G.; Singh, M. Evaluation of antidiarrheal activity of ethanolic extract of Holarrhena antidysenterica seeds in rats. Vet. World 2015, 8, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Daswani, P.G.; Birdi, T.J.; Antarkar, D.S.; Antia, N.H. Investigation of antidiarrhoeal activity of Holarrhena antidysentrica. Int. J. Pharm. Res. 2012, 64, 164–167. [Google Scholar]
- Saha, S.; Subrahmanyam, E.V.S. Evaluation of anti-inflammatory activity of ethanolic extract of seeds of (Holarrhena pubescens Buch.- Ham.) wall. Int. J. Pharm. Pharm. Sci. 2013, 5, 915–919. [Google Scholar]
- Khan, A.; Khan, S.R.; Gilani, A.H. Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol. Res. 2012, 40, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, D.; Shilpa, P.N.; Devaraj, S.N. Antibacterial and antidiarrhoeal effects of alkaloids of Holarrhena antidysenterica WALL. Indian J. Exp. Biol. 2004, 42, 589–594. [Google Scholar] [PubMed]
- Srivastava, N.; Saxena, V. Antibacterial activity of Kutaj (Holarrhena antidysenterica L.) in childhood diarrhoea—In vitro study. Pharm. Innov. J. 2015, 4, 97–99. [Google Scholar]
- Darji, V.C.; Deshpande, S.; Bariya, A.H. Effects of methanolic extract of Holarrhena antidysenterica bark against experimentally induced inflammatory bowel disease in rats. Int. Res. J. Pharm. 2012, 3, 152–154. [Google Scholar]
- Haque, A.; Islam, A.U. Evaluation of analgesic and central nervous system depressant effects of Holarrhena antidysenterica stem on Swiss albino mice model. Bangladesh Pharm. J. 2017, 20, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Ganapathy, S.; Ramachandra, Y.L.; Padmalatha, R. Anti-inflammatory and analgesic activities of Holarrhena antidysenterica Wall. leaf extract in experimental animal models. Int. J. Biomed. Pharm. Sci. 2011, 4, 101–103. [Google Scholar]
- Bhuiyan, M.; Bhuiya, N.; Hasan, M.N.; Nahar, U.J. In vivo and in silico evaluation of antinociceptive activities of seed extract from the Holarrhena antidysenterica plant. Heliyon 2020, 6, e03962. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.P.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Ganapathy, S.; Ramachandra, Y.L.; Padmalatha, R. In vitro antioxidant activity of Holarrhena antidysenterica Wall. methanolic leaf extract. J. Basic Clin. Pharm. 2011, 2, 175–178. [Google Scholar]
- Zahin, M.; Farrukh, A.; Iqbal, A. The in vitro antioxidant activity and total phenolic content of four Indian medicinal plants. Int. J. Pharm. Pharm. Sci. 2009, 1, 88–95. [Google Scholar]
- Khan, A.; Bashir, S.; Gilani, A.H. An in vivo study on the diuretic activity of Holarrhena antidysenterica. Afr. J. Pharm. Pharmacol. 2012, 6, 454. [Google Scholar]
- Gilani, A.H.; Khan, A.; Khan, A.U.; Bashir, S.; Rehman, N.U.; Mandukhail, S.U. Pharmacological basis for the medicinal use of Holarrhena antidysenterica in gut motility disorders. Pharm. Biol. 2010, 48, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.D.; Duan, D.Z.; Xue, W.W.; Yao, X.J.; Li, S. Steroidal alkaloids from Holarrhena antidysenterica as acetylcholinesterase inhibitors and the investigation for structure activity relationships. Life Sci. 2012, 90, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Madat, D.; Chauhan, K. Evaluation of central nervous system activity of Holarrhena antidysenterica Linn Apocynaceae bark. J. Pharm. Res. 2011, 4, 1760–1761. [Google Scholar]
- Siddiqui, B.S.; Ali, S.T.; Rizwani, G.H.; Begum, S.; Tauseef, S.; Ahmad, A. Antimicrobial activity of the methanolic bark extract of H. pubescens (Buch. Ham), its fractions and the pure compound conessine. Nat. Prod. Res. 2012, 26, 987–992. [Google Scholar] [CrossRef]
- Somanadhan, B.; Varughese, G.; Palpu, P. An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J. Pharmacol. 1999, 65, 103–112. [Google Scholar] [CrossRef]
- Raman, M.D.; Sultana, N.; Anwar, A. In vitro antimicrobial activity of Holarrifine 24-ol isolated from the stem bark of Holarrhena antidysenterica. Int. J. Agric. Biol. 2004, 6, 698–700. [Google Scholar]
- Phatthalung, P.N.; Chusri, S.; Voravuthikunchai, S.P. Thai ethnomedicinal plants as resistant modifying agents for combating Acinetobacter baumannii infections. BMC Complement. Alt. Med. 2012, 12, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chusri, S.; Na-Phatthalung, P.; Siriyong, T.; Paosen, S.; Voravuthikunchai, S.P. Holarrhena antidysenterica as a resistance modifying agent against Acinetobacter baumannii: Its effects on bacterial outer membrane permeability and efflux pumps. Microbiol. Res. 2014, 169, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Bastia, A.K. Antimicrobial efficacy of potential plants used in the indigenous preparation of traditional rice beverage “Handia”. Int. J. Phytomed. 2014, 6, 23–28. [Google Scholar]
- Siriyong, T.; Voravuthikunchai, S.P.; Coote, P.J. Steroidal alkaloids and conessine from the medicinal plant Holarrhena antidysenterica restore antibiotic efficacy in a Galleria mellonella model of multidrug-resistant Pseudomonas aeruginosa infection. BMC Complement. Alt. Med. 2018, 18, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriyong, T.; Chusri, S.; Srimanote, P.; Tipmanee, V.; Voravuthikunchai, S.P. Holarrhena antidysenterica extract and its steroidal alkaloid, conessine, as resistance-modifying agents against extensively drug-resistant Acinetobacter baumannii. Microb. Drug Resist. 2016, 22, 273–282. [Google Scholar] [CrossRef]
- Aqil, F.; Zahin, M.; Ahmad, I. Antimutagenic activity of methanolic extracts of four Ayurvedic medicinal plants. Indian J. Exp. Biol. 2008, 46, 668–672. [Google Scholar]
- Verma, G.; Dua, V.K.; Agarwal, D.D.; Atul, P.K. Anti-malarial activity of Holarrhena antidysenterica and Viola canescens, plants traditionally used against malaria in the Garhwal region of north-west Himalaya. Malar. J. 2011, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Nondo, R.; Moshi, M.; Paul, M.P.; Machumi, F.; Kidukuli, A.; Heydenreich, M.; Zofou, D. Anti-plasmodial activity of Norcaesalpin D and extracts of four medicinal plants used traditionally for treatment of malaria. BMC Complement. Alt. Med. 2017, 17, 167. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, H.T.; Nordskjold, J.B.; Smitt, U.W.; Nyman, U.; Palpu, P.; Joshi, P.; Varughese, G. In vitro screening of Indian medicinal plants for anti-plasmodial activity. J. Pharmacol. 2001, 74, 195–204. [Google Scholar]
- Itoh, H.; Inoue, M. Comprehensive Structure-Activity Relationship studies of macrocyclic natural products enabled by their total syntheses. Chem. Rev. 2019, 119, 10002–10031. [Google Scholar] [CrossRef]
- Ҫiçek, S.S. Structure-Dependent Activity of plant-derived sweeteners. Molecules 2020, 25, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheenpracha, S.; Jitonnom, J.; Komek, M.; Ritthiwigrom, T.; Laphookhieo, S. Acetylcholinesterase inhibitory activity and molecular docking study of steroidal alkaloids from Holarrhena pubescens barks. Steroids 2016, 108, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, M.; Bennani, Y.L.; Cowart, M.D.; Hancock, A.A.; Miller, T.R.; Witte, D.G.; Browman, K.E.; Krueger, K.M.; Marsh, K.C.; et al. The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. J. Med. Chem. 2008, 51, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N. Development of antibacterial conjugates using sulfamethoxazole with monocyclic terpenes: A systematic medicinal chemistry based computational approach. Comput. Methods Programs Biomed. 2017, 140, 185–194. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Dehury, B.; Das, M.; Vedithi, S.C.; Padhy, R.N. Computer-aided synthesis of dapsone-phytochemical conjugates against dapsone-resistant Mycobacterium leprae. Sci. Rep. 2020, 10, 6839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, V.K.; Maiti, A.; Gupta, S.S.; Shukla, I.; Rao, C.V. Effect of the standardized extract of Holarrhena antidysenterica seeds against Streptozotocin-induced diabetes in rats. Int. J. Pharm. Sci. Rev. Res. 2015, 4, 1–6. [Google Scholar]
- Singh, R.K. Pre-clinical toxicity studies of Holarrhena antidysenterica stem bark in mice and rats. World J. Pharm. Pharm. Sci. 2018, 7, 912–922. [Google Scholar]
- Kumar, S.; Yadav, A. Comparative study of hypoglycemic effect of Holarrhena antidysenterica seeds and glibenclamide in experimentally induced diabetes mellitus in albino rats. Biomed. Pharm. J. 2015, 8, 477–483. [Google Scholar] [CrossRef]
- Permpipat, U.; Chavalittumrong, P.; Attawish, A.; Chuntapet, P. Toxicity study of Holarrhena antidysenterica Wall. Bark. Bull. Dep. Med. Sci. 2012, 40, 145–157. [Google Scholar]
- Chopra, R.N.; De, N. The failure of the alkaloids of Holarrhena antidysenterica (kurchi) in the treatment of amoebic hepatitis. Indian Med. Gaz. 1930, 65, 391. [Google Scholar]
- Singh, K.P. Clinical studies on amoebiasis and giardiasis evaluating the efficacy of kutaja (Holarrhena antidysenterica) in Entamoeba histolytica cyst passers. Anc. Sci. Life 1986, 5, 228–231. [Google Scholar] [PubMed]
- Prakash, P.; Pralhad, P.; Nishikant, J. Efficacy of an indigenous formulation in patients with bleeding piles: A preliminary clinical study. Fitoterapia 2000, 71, 41–45. [Google Scholar]
- Atanu, P.; Sharma, P.P.; Mukherjee, P.K. A clinical study of (Holarrhena antidysentrica Wall) on Shonistarsha. AYU 2009, 30, 369–372. [Google Scholar]
- Kadam, A.; Prasad, B.S.; Bagadia, D.; Hiremath, V.R. Effect of Ayurvedic herbs on control of plaque and gingivitis: A randomized controlled trial. AYU 2011, 32, 532–535. [Google Scholar] [PubMed]
- Patel, M.V.; Patel, K.B.; Gupta, S.N. Effects of Ayurvedic treatment on forty-three patients of ulcerative colitis. AYU 2010, 31, 478–481. [Google Scholar] [CrossRef]
- Patel, K.; Dei, L.; Donga, S.; Nalini, A. Effect of Shatapushpa Taila Matra Basti and Pathadi Kwatha on poly cystic ovarian disease. AYU 2012, 33, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Johari, S.; Gandhi, T. A randomized single blind parallel group study comparing monoherbal formulation containing Holarrhena antidysenterica extract with mesalamine in chronic ulcerative colitis patients. Anc. Sci. Life 2016, 36, 19–27. [Google Scholar] [CrossRef]
- Kumari, V.; Singh, B.M.; Kumar, A.; Singh, G. Assessment of effect of Kutaja (Holarrhena antidysenterica Wall) in different Doshika Atisara in infants: A clinical study. J. Res. Ed. Indian Med. 1982, 1, 1–6. [Google Scholar]
- Mundhe, N.B.; Tamoli, S.M.; Pande, S.P.; Kulkarni, S.A.; Patil, V.G.; Mahadik, S.B. An open-label, prospective, multicenter, clinical study to evaluate efficacy of Ayuartis capsules in patients suffering from osteoarthritis of the knee(s). AYU 2019, 40, 16–22. [Google Scholar]



| Disease | Medicinal Property | Reference |
|---|---|---|
| Intestinal parasites | Anthelmintic for Guinea worm, roundworm, tapeworm, thread worm, other internal worms | [5] |
| Animal bites | Antidote for snake bite, scorpion sting, insect bite, dog bite | [6] |
| Indigestion | Appetizer, stomachic | [7] |
| Blood-related ailments | Anemia, blood infection, blood purifier, hemorrhage, nose bleeding, hypertension | [8] |
| Body pain | Analgesic for backache, body ache, headache, knee pain and rheumatic arthritis | [9] |
| Brain-related disorders | Improves depression and other nervous disorders, acts as memory enhancer | [10] |
| Cold and throat-related ailments | Expectorant for cold, cough, throat infection | [9,10] |
| Dental or oral ailments | Analgesic for toothache | [11] |
| Dermatological problems | Activity against abscess, acne, boils, bruises, dermatitis, leukoderma, pimples, ringworm, scabies, skin allergies, warts | [12] |
| Diabetes | Regulates blood sugar | [6,13] |
| Fever | Antipyretic, febrifuge for intermittent fever, pyrexia | [12] |
| Gastrointestinal disorders | Active against (hyper)acidity, intestinal ulcers, stomachache, dyspepsia, flatulence, cholera, diarrhea, dysentery, food poisoning, gastroenteritis, colic complaints, constipation | [7] |
| General health | Muscle strength, obesity, tonic | [14,15] |
| Gynecological disorders | Easy delivery, leucorrhea, toning up vaginal tissues after delivery | [16,17] |
| Joint- and muscle-related ailments | Active against arthritis, rheumatism | [18,19] |
| Liver complaints | Useful for bilious disorders, bile infection, jaundice | [20,21] |
| Piles | Active against piles, fissures, fistula, hemorrhoids | [22,23] |
| Respiratory disorders | Active against asthma, bronchitis | [24,25] |
| Urogenital disorders | Controls urination, cystitis, diuretic, dysuria, urinary problem, urinary tract infection, urine tract burning sensation | [25] |
| Geographic Location | Condition Treated | Plant Part Used | Method(s) of Preparation | Dosage Forms, and Method(s) of Administration | Reference |
|---|---|---|---|---|---|
| East Africa | Fever | Leaves, roots | Decoction | Bath is taken | [12] |
| Malaria | Roots | Decoction | Taken in the form of drink twice daily | [21] | |
| Southern Africa | Constipation, abdominal pains | Root | Infusion | Drink | [38] |
| Infertility/amenorrhea | Root | Decoction | Drink | [39] | |
| Toothache | Stem, bark | Decoction | Gargle | [40] | |
| Snakebite | Root | Boiled in milk | Applied externally | ||
| West Africa | Stomach pains | Leaves | Maceration | Drink | [40,41] |
| Togo Maritime region | Malaria | Leaves, roots | Decoction | Oral administration | [42] |
| Zimbabwe | Abortifacient/venereal diseases | Root | Infusions | Oral administration | [43] |
| Malaria | Root | Decoction | Oral administration | [34,44] | |
| Tanzania | Abdominal pain | Roots | Decoction | Taken in the form of drink on empty stomach | [45] |
| Mozambique | Stomachache/vomiting | Leaves, roots | Maceration | Oral administration | [46] |
| Earache | All parts | Maceration | Directly applied in the form of ear drops | ||
| Guinea | Diabetes | Whole plant | Not stated | Not stated | [13] |
| South West Nigeria | Inflammatory diseases | Leaves | Infusion | Oral administration | [47] |
| Republic of China | Diarrhea, dysentery | Bark | Decoction | Oral administration | [48] |
| Northern Thailand | Diarrhea and weight loss | Stem, bark | Boiled | Oral administration | [49] |
| India | Low fever | Seeds | Powder | Oral administration, 2–3 g mixed in one glass of water | [50,51] |
| Knee pain | Bark | Decoction | Oral administration, mixed with about 100 g of jaggery | [52,53] | |
| Leprosy | Seeds | Decoction | Oral administration | [54,55] | |
| Snakebite | Roots | Paste | Directly applied to bite wound | [56] | |
| Dysentery | Bark, leaves | Powder | Taken with water | [57,58] | |
| Amoebic dysentery | Bark | Powder | Oral administration | [59,60] | |
| Nepal | Paralysis | Bark, root | Powder | One spoonful powder or paste from a mixture of (5 g H. pubescens root, 5 g Terminalia alata bark, 2 g Cissampelos pareira root, 5 g H. pubescens bark, 2 g Psidum guajava bark, 1 g Allium sativum bulb and 2 g Trachyspermum ammi seeds), given once a day | [37] |
| Backache, high fever | Bark | Infusion | Oral administration | [4] | |
| Bangladesh | Bloody dysentery | Bark | Boil | 1 cupful bark of H. pubescens is boiled with 4 cups of water to make 1 cup. A 1.5 mL solution with trace amount of honey is licked 3–4 times daily till cure | [61] |
| Stomach pain, food poisoning | Bark | Maceration | A red-hot iron rod is dipped in the juice, and the juice is taken while still warm | [62] | |
| Bark | Mixed with bark of Cinnamomum camphora and chewed. | [63] | |||
| Jaundice | Leaves | Macerated juice | Juice obtained from leaves of Cajanus cajan and H. pubescens are mixed with powdered seeds of Plantago ovata and taken (one glassful) in the morning on an empty stomach for one month | [64] | |
| Helminthiasis | Seeds | Powder | Taken with cold water every morning | [65] | |
| Piles | Bark | Powder | Mixed with honey and taken orally | ||
| Abdominal pain, diarrhea | Bark | Juice | A ½ cup is taken 2–3 times orally | [66] | |
| Asthma | Root | Juice | Taken 4–5 times daily for a week | ||
| Abdominal pain | Bark/leaf | Juice | 2–3 spoons along with honey on empty stomach | ||
| Pakistan | Diabetes | Root | Powder | Salacia reticulate, Annona squamosa and H. pubescens roots were Ground with lime and taken orally | [67] |
| Malaria | Root | Decoction | Oral administration | [68] | |
| Diarrhea | Bark | Decoction | Oral administration | [69] | |
| Gut infections | Leaves | Juice | Taken daily | [70,71] |
| State/Province, Tribe(s) | Disease/Indication | Dosage Forms, and Method(s) of Administration | References |
|---|---|---|---|
| Tripura state, reang tribes | Dog bite | Pills prepared from bark | [83] |
| Unakoti district | Antidiabetic | - | [84] |
| West and south district of Tripura | Dysentery, fever, cold and piles | - | [85] |
| Uttar Pradesh state | Dysentery | Bark decoction | [86] |
| Diarrhea | |||
| Sonaghati of Sonbhadra district | Stimulate discharge of urine and to remove constipation | 10–20 g of root paste is taken orally with water | [32] |
| Jaunsar-bawar hills | Dysentery and stomachache | Dry stem bark mixed with dried ginger and black pepper are powdered and made into pills with butter oil, 2–3 of these pills (pea size) are administered daily | [75] |
| West Bengal state | Blood dysentery, piles, leprosy, headache | Bark | [87] |
| Diabetes, intestinal worms; roots to stop bleeding from nose | Seeds | ||
| Dropsy | The dried bark is rubbed over the body | ||
| Madhya Pradesh state, tribal communities of chitrakoot region | Arthritis and diarrhea in cattle | Leaf decoction twice a day | [88,89] |
| Odisha state | Rheumatism | Root bark | [90] |
| Tribals of Bargarh district | Rheumatism | 10 g of root bark is boiled in water (400 mL) and the prepared decoction (100 mL) is taken 1–2 times daily on empty stomach | |
| Sundargarh district | Boils, cut, abscess and wounds | Root paste | [91] |
| Bondo tribe of Malkangiri district | Rheumatic pain | Two to three leaves are attached with the latex of the same plant and fomented externally over backbone | [92] |
| Dysentery | Root powder | ||
| Tribals of Similipal | Malaria and dysentery | Stem bark | [93] |
| Dysentery | Stem bark infusion with honey in a ratio of 3:1 is taken once a day on empty stomach | [94] | |
| Dysentery | From bark of H. pubescens, Terminalia arjuna and Pterocarpus marsupium (in equal ratio) pill is prepared. One pill is taken orally on empty stomach for three days | [95] | |
| Tribes of Mayurbhanj district | Stomach pain and blood dysentery | [94] | |
| Headache | Decoction of roots with garlic and mustard is made into paste and applied externally as an ointment | [95] | |
| Skin infection, jaundice | Leaf paste | [96] | |
| Bhadrak district | Deep cuts | Bark and latex | [52] |
| Kalahandi district | Dysentery | Stem bark of Careya arborea and H. pubescens with water | [33] |
| Andhra Pradesh state, visakhapatnam district | Nerve disorder | Spoonful of shade-dried stem bark powder was taken orally with glass of water daily | [97] |
| Khammam district | Post-partum problems | 15 g of root is ground with 20 mL country liquor of rice. Five spoons of this were taken immediately after delivery followed by 2 g of Ferula asafoetida rhizome powder | [98] |
| Visakhapatnam district | Fever | Decoction prepared by adding 100–400 mL water with leaves of H. pubescens and root of Andographis paniculata, given twice a day | [99] |
| Karnataka state, Hosanagara taluk of Shimoga district | Cancer | One handful of roots ground in cow’s buttermilk and given orally, twice daily for one month | [100] |
| Stomachache | Roots crushed in water and juice is taken orally, twice daily for 1–2 days | [101] | |
| Tribes of the Shimoga district | Ringworm and poor milk production | Bark | [102] |
| Uttara kannada | Ulcer in intestine | Used a mixture of plants viz. Syzygium cumini (bark); Holarrhena pubescens (bark), Madhuca indica (leaves and bark), Careya arborea (bark), Elaegnus conferta (bark), Myristica fragrans (fruit), Syzygium aromaticum (flower bud), Piper nigrum (fruit), Trachyspermum ammi (fruit), Zingiber officinale (rhizome), Cuminum cyminum (fruit) in decoction form | [103] |
| Uttarakhand state, Tharu community of district Udham Singh nagar | Chronic dysentery | Paste made with flower and cow’s milk taken orally, for 4 days | [104,105,106] |
| Theni district (Western ghats) | Dysentery | Decoction made from the root bark is taken orally twice a day for two days | [107] |
| Gujrat, Rajstan and Kerala state | Dropsy and swelling | Bark extracts from Bombax ceiba, Hymenodictyon excelsium, Azadirachta indica and H. pubescens made by crushing is given with water in morning and evening for 5 days | [56] |
| Snakebite | The crushed root is given with ghee |
| Biological Activity | Parts | Extract/Compound | Effective Concentration/Dose | Study Model | References |
|---|---|---|---|---|---|
| Antihyperglycemic | Seeds | Aqueous and petroleum ether extract | 250 mg/kg BW | Rats | [109] |
| Seeds | Methanol extract | 300 mg/kg BW in rats | Rats | [110] | |
| Seeds | Ethanolic extract | 300 mg/kg and 600 mg/kg | Rats | [110] | |
| Leaves | Ethanolic extract | 400 mg/kg BW | Rats | [112] | |
| Anti-diarrheal | Seeds | Ethanolic extract | 200 and 400 mg/kg | Rats | [114] |
| Seeds | Alkaloids | 200–800 mg/kg | Rats | [115] | |
| Anti-inflammatory | Not stated | Not stated | 400 mg/kg | Rats | [116] |
| Diuretic | Seeds | Aqueous | 30–100 mg/kg | Rats | [117] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahara, K.; Panda, S.K.; Swain, S.S.; Luyten, W. Metabolic Diversity and Therapeutic Potential of Holarrhena pubescens: An Important Ethnomedicinal Plant. Biomolecules 2020, 10, 1341. https://doi.org/10.3390/biom10091341
Zahara K, Panda SK, Swain SS, Luyten W. Metabolic Diversity and Therapeutic Potential of Holarrhena pubescens: An Important Ethnomedicinal Plant. Biomolecules. 2020; 10(9):1341. https://doi.org/10.3390/biom10091341
Chicago/Turabian StyleZahara, Kulsoom, Sujogya Kumar Panda, Shasank Sekhar Swain, and Walter Luyten. 2020. "Metabolic Diversity and Therapeutic Potential of Holarrhena pubescens: An Important Ethnomedicinal Plant" Biomolecules 10, no. 9: 1341. https://doi.org/10.3390/biom10091341
APA StyleZahara, K., Panda, S. K., Swain, S. S., & Luyten, W. (2020). Metabolic Diversity and Therapeutic Potential of Holarrhena pubescens: An Important Ethnomedicinal Plant. Biomolecules, 10(9), 1341. https://doi.org/10.3390/biom10091341