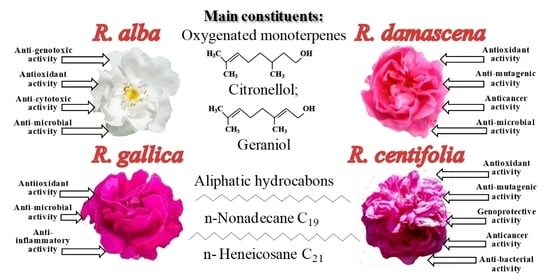
Rose Flowers—A Delicate Perfume or a Natural Healer?
Abstract
:1. Introduction
2. A Short Introduction on Habitat, Cultivation, and Comparative Morphological Characteristics of Rose Plants
2.1. Rosa Damascena Mill. Forma Trigintipetala Dieck
2.2. Rosa alba L.—Rosa damascena Mill. var. alba
2.3. Rosa gallica L.—Gallic or Red Oil-Bearing Rose
2.4. Rosa centifolia L.—A Rose with a Hundred Leaves
3. Composition of Rose Oils
4. Biological Activities of Rosa Species
4.1. Cytotoxic/Anticytotoxic, Mutagenic/Antimutagenic and Genotoxic/Anti-Genotoxic Potential
4.2. Antiviral Activity of Representatives of the Rose Family and Their Metabolites
4.3. Antimicrobial Activities
4.4. The Role of Rose Oils in the Treatment of Respiratory Tract Diseases
4.5. Free Radical Scavenger and Antioxidant Activity of Rose Extracts
4.6. Antineoplastic Activities
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AA | Total antioxidant activity |
| ABTS | 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid |
| AGS | Adenocarcinoma gastric cell line |
| AGT | Anti-genotoxicity |
| AMG | Anti-mutagenicity |
| AMR | Antimicrobial resistance |
| ATC | Anti-cytotoxicity |
| BHA | Butyl hydroxyanisole |
| BHT | Butyl hydroxy toluene |
| CMS | Chronic mild stress |
| DIZ | Diameter of inhibition zone |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EAC | Ehrlich ascites carcinoma cells |
| EPR | Electron paramagnet resonance |
| EtOH | Ethanol |
| FRAP | Ferric-reducing antioxidant power |
| GAE | Gallic acid equivalent |
| GC | Gas chromatograph |
| GT | Genotoxicity |
| HepG2 | Liver hepatocellular cells |
| IACG | Interagency coordinating group |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| IC50 | Inhibitory concentration 50 |
| LP | Lipid peroxidation |
| MBC | Minimal bactericidal concentrations |
| MCF7 | Breast cancer cell line |
| MDA | Malonaldehyde |
| MeOH | Methanol |
| MG | Mutagenicity |
| MIC | Minimal inhibitory concentrations |
| MKN45 | Gastric cancer cell line |
| MTT NCBI | Assay for cell viability as a function of redox potential HeLa tumor cell line available in Sequence Read Archive |
| N.d. | No published data |
| ORAC | Oxygen radical absorbance capacity |
| PCC | Protein carbonyl content |
| 7-AAD | 7-aminoactinomycin D |
| SNEDDS | A self-nanoemulsifying drug delivery system |
| SW742 | Colon cancer cell line |
| T/CT | Toxicity/cytotoxicity |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TRP | Total reducing power |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| •O2− | Superoxide anion radical |
| •OH | Hydroxyl radical |
References
- Baser, K.; Altintas, A.; Kurkcuoglu, M. Turkish rose: A review of the history, ethnobotany and modern uses of rose petals, rose oil, rose water and other rose products. HerbalGram 2012, 96, 40–53. [Google Scholar]
- Mahboubi, M. Rosa damascena as holy ancient herb with novel applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labban, L.; Thallaj, N. The medicinal and pharmacological properties of Damascene Rose (Rosa damascena): A review. Int. J. Herb. Med. 2020, 8, 33–37. [Google Scholar]
- Akram, M.; Riaz, M.; Munir, N.; Akhter, N.; Zafar, S.; Jabeen, F.; Ali Shariati, M.; Akhtar, N.; Riaz, Z.; Altaf, S.H.; et al. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: A literature review. J. Pharm. Pharm. 2020, 72, 161–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarenko, L.; Minkov, B.; Mustyatse, G.; Murin, A. Culture Oil-Bearing Rose; Pulisher Shtinitsa: Kishinev, Moldova, 1983. (In Russian) [Google Scholar]
- Antonelli, A.; Fabbri, C.; Giorgioni, M.E.; Bazzocchi, R. Characterization of 24 old (true) garden roses from their volatile compositions. J. Agric. Food Chem. 1997, 45, 4435–4439. [Google Scholar] [CrossRef]
- Gudin, S. Rose: Genetics and breeding. Plant. Breed. Rev. 2000, 17, 159–190. [Google Scholar]
- Gochev, V.; Dobreva, A.; Girova, T.; Stoyanova, A. Antimicrobial activity of essential oil from Rosa alba. Biotechnol. Biotechnol. Equip. 2010, 24, 512–515. [Google Scholar] [CrossRef] [Green Version]
- Guenther, E. Individual Essential Oils of the Plant Family. In The Essential Oils. Chapter I. Essential Oils of the Family Rosaceae; D. Van Nostrad Company, Inc.: New York, NY, USA, 1952; Volume 5. [Google Scholar]
- Ng, T.; He, J.; Niu, S.; Pi, Z.; Shao, W.; Liu, F.; Zhao, L. A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J. Pharm. Pharmacol. 2004, 56, 537–545. [Google Scholar] [CrossRef]
- Pal, P.K. Evaluation, genetic diversity, recent development of distillation method, challenges and opportunities of Rosa damascena: A review. J. Essent. Oil Bear. Plants 2013, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rusanov, K.; Kovacheva, N.; Dobreva, A.; Atanassov, I. Rosa x damascena Mill. (Rose). In Medicinal, Aromatic and Stimulant Plants; Springer: Cham, Switzerland, 2020; pp. 467–500. [Google Scholar]
- Younis, A.; Khan, M.; Ali, A.; Pervez, M. Performance of four Rosa species under Faisalabad agro-climatic conditions. Cad. Pesqui. J. 2006, 18, 8–15. [Google Scholar]
- Caissard, J.-C.; Bergougnoux, V.; Martin, M.; Mauriat, M.; Baudino, S. Chemical and histochemical analysis of ‘Quatre Saisons Blanc Mousseux’, a moss rose of the Rosa× damascena group. Ann. Bot. 2006, 97, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginova, A.; Tsvetkov, I.; Kondakova, V. Rosa damascena Mill.—An overview for evaluation of propagation methods. Bulg. J. Agric. Sci. 2012, 18, 545–556. [Google Scholar]
- Kovatcheva, N.; Zheljazkov, V.D.; Astatkie, T. Productivity, oil content, composition, and bioactivity of oil-bearing rose accessions. HortScience 2011, 46, 710–714. [Google Scholar] [CrossRef] [Green Version]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. Int. J. Devot. Pharmacol. Toxicol. Eval. Natl. Prod. Deriv. 2008, 22, 725–733. [Google Scholar] [CrossRef]
- Dossi, C.G.; Cadagan, C.; San Martín, M.; Espinosa, A.; González-Mañán, D.; Silva, D.; Mancilla, R.A.; Tapia, G.S. Effects of rosa mosqueta oil supplementation in lipogenic markers associated with prevention of liver steatosis. Food Funct. 2017, 8, 832–841. [Google Scholar] [CrossRef]
- González-Mañán, D.; D’Espessailles, A.; Dossi, C.G.; San Martin, M.; Mancilla, R.A.; Tapia, G.S. Rosa mosqueta oil prevents oxidative stress and inflammation through the upregulation of PPAR-α and NRF2 in C57BL/6J mice fed a high-fat diet. J. Nutr. 2017, 147, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Mileva, M.; Krumova, E.; Miteva-Staleva, J.; Kostadinova, N.; Dobreva, A.; Galabov, A.S. Chemical compounds, in vitr o antioxidant and antifungal activities of some plant essentia l oils belonging to Rosaceae family. Compt. Rend. Acad. Bulg. Sci. 2014, 67, 1363–1368. [Google Scholar]
- Mileva, M.; Kusovski, V.K.; Krastev, D.S.; Dobreva, A.M.; Galabov, A.S. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L. essential oil against some oral pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 11–20. [Google Scholar]
- Mohebitabar, S.; Shirazi, M.; Bioos, S.; Rahimi, R.; Malekshahi, F.; Nejatbakhsh, F. Therapeutic efficacy of rose oil: A comprehensive review of clinical evidence. Avicenna J. Phytomed. 2017, 7, 206. [Google Scholar]
- Gehrcke, M.; Giuliani, L.M.; Ferreira, L.M.; Barbieri, A.V.; Sari, M.H.M.; da Silveira, E.F.; Azambuja, J.H.; Nogueira, C.W.; Braganhol, E.; Cruz, L. Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules. Mater. Sci. Eng. C 2017, 74, 279–286. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Habibi, E.; Modanloo, M. Cytotoxic and genotoxic studies of essential oil from Rosa damascene Mill., Kashan, Iran. Med. Glas. 2017, 14, 152–157. [Google Scholar]
- Achuthan, C.; Babu, B.; Padikkala, J. Antioxidant and hepatoprotective effects of Rosa Damascena. Pharm. Biol. 2003, 41, 357–361. [Google Scholar] [CrossRef]
- Basim, E.; Basim, H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia 2003, 74, 394–396. [Google Scholar] [CrossRef]
- Özkan, G.; Sagdic, O.; Baydar, N.; Baydar, H. Note: Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Staikov, V.; Kalaijiev, I. Study of oil roses (Rosa damascena Mill.) originated from India, Morocco, Iran and Bulgaria. Plant Sci. 1980, 17, 58–68. [Google Scholar]
- Topalov, V. The Kazanluk Rose and Rose Production in Bulgaria; Izd. Khristo G. Danov.: Plovdiv, Bulgaria, 1978. [Google Scholar]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial cultivation of oil bearing rose and rose oil production in Bulgaria during 21st century, directions and challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef] [Green Version]
- Iwata, H.; Kato, T.; Ohno, S. Triparental origin of Damask roses. Gene 2000, 259, 53–59. [Google Scholar] [CrossRef]
- Grin Species of Rosa. Available online: www.ars-grin.gov (accessed on 30 December 2020).
- Degraf, K. Volatile Ester Formation in Roses. Identification of an Acetyl- 2002. Roses beyond the Beginnings of Time; Bulgarian National Association of Essential Oils, Perfumery and Cosmetics (BNAEOPC): Plovdiv, Bulgaria, 2003; Volume 75, pp. 4–5. (In Bulgarian) [Google Scholar]
- Nedkov, N.; Dobreva, A.; Kovacheva, N.; Bardarov, V.; Velcheva, A. Bulgarian rose oil of white oil-bearing rose. Bulg. J. Agric. Sci. 2009, 15, 318–322. [Google Scholar]
- Basu, S.; Zandi, P.; Cetzal-Ix, W.; Sengupta, R. The Genus Rosa: An Aristocrat from the Plant Family with Class, Color and Fragrance. Iranian Society of Environmentalists Newsletter. 2014, pp. 1–9. Available online: https://www.academia.edu/download/38410616/Basu_et_al_Roses_2015.pdf (accessed on 1 December 2020).
- Nasir, M.H.; Nadeem, R.; Akhtar, K.; Hanif, M.A.; Khalid, A.M. Efficacy of modified distillation sludge of rose (Rosa centifolia) petals for lead (II) and zinc (II) removal from aqueous solutions. J. Hazard. Mater. 2007, 147, 1006–1014. [Google Scholar] [CrossRef]
- ISO 9842:2003. International Organization for Standardization: ISO 9842:2003, Oil of Rose (Rosa × damascena Miller). Available online: https://www.iso.org/standard/28611.html (accessed on 30 December 2020).
- Baldermann, S.; Yang, Z.; Sakai, M.; Fleischmann, P.; Watanabe, N. Volatile constituents in the scent of roses. Floric. Ornam. Biotechnol. 2009, 3, 89–97. [Google Scholar]
- Kováts, E.S. Composition of essential oils. Part 7. Bulgarian oil of rose (Rosa damascena Mill). J. Chromatogr. 1987, 406, 185–222. [Google Scholar]
- Omata, A.; Yomogida, K.; Nakamura, S.; Ota, T.; Toyoda, T.; Amano, A.; Muraki, S. New sulphur components of rose oil. Flavour Fragr. J. 1991, 6, 149–152. [Google Scholar] [CrossRef]
- Dobreva, A.; Nedeva, D.; Mileva, M. Recent results of the yield and composition of rose oil from the oil-bearing roses, cultivated in Bulgaria. Bulg. Chem. Commun. 2021, in press. [Google Scholar]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of anti-inflammatory activity of Citrus latifolia Tanaka essential oil and limonene in experimental mouse models. Evid. Based Complement. Altern. Med. 2013, 2013, 859083. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Kamalakkannan, N.; Prince, P. Antidiabetic and anti-oxidant activity of Aegle marmelos extract in streptozotocin-induced diabetic rats. Pharm. Biol. 2004, 42, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Elisabetsky, E. In Advances in Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1. [Google Scholar]
- Sirilun, S.; Chaiyasut, C.H.; Sivamaruthi, B.S.; Peerajan, S.; Kumar, N.A.; Kesika, P.E. Phenethyl alcohol is an effective non-traditional preservative agent for cosmetic preparations. Asian J. Pharm. Clin Res. 2017, 10, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Umezu, T.; Ito, H.; Nagano, K.; Yamakoshi, M.; Oouchi, H.; Sakaniwa, M.; Morita, M. Anticonflict effects of rose oil and identification of its active constituents. Life Sci. 2002, 72, 91–102. [Google Scholar] [CrossRef]
- Nonato, F.R.; Santana, D.G.; de Melo, F.M.; dos Santos, G.G.L.; Brustolim, D.; Camargo, E.A.; de Sousa, D.P.; Soares, M.B.P.; Villarreal, C.F. Anti-inflammatory properties of rose oxide. Int. Immunopharmacol. 2012, 14, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Sadraei, H.; Asghari, G.; Emami, S. Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Res. Pharm. Sci. 2013, 8, 17. [Google Scholar] [PubMed]
- Abe, S.; Maruyama, N.; Hayama, K.; Ishibashi, H.; Inoue, S.; Oshima, H.; Yamaguchi, H. Suppression of tumor necrosis factor-alpha-induced neutrophil adherence responses by essential oils. Mediat. Inflamm. 2003, 12, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Nat. C 2007, 62, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.d.O.; Mendes, J.M.; Lima, I.O.; Mota, K.S.d.L.; Oliveira, W.A.d.; Lima, E.d.O. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Menezes, I.A.; Barreto, C.M.; Antoniolli, Â.R.; Santos, M.R.; de Sousa, D.P. Hypotensive activity of terpenes found in essential oils. Z. Nat. C 2010, 65, 562–566. [Google Scholar] [CrossRef]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Gloria, P.D. Cardiovascular effects of methyleugenol, a natural constituent of many plant essential oils, in normotensive rats. Life Sci. 2004, 74, 2401–2412. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, C.H.; Lee, H.S. Food protective effect of geraniol and its congeners against stored food mites. J. Food Prot. 2009, 72, 1468–1471. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol-a review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Bendre, R.S.; Rajput, J.D.; Bagul, S.D.; Karandikar, P.S. Outlooks on medicinal properties of eugenol and its synthetic derivatives. Nat. Prod. Chem Res. 2016, 4, 2. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Methyleugenol (CAS NO. 93-15-2) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2000, 491, 1–412.
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential anti-inflammatory and anti-cancer properties of farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolov, N. Essence de Roses et Autres Huiles Bulgares. 1976. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL7760004111 (accessed on 1 December 2020).
- Baser, K. Turkish rose oil. Perfum. Flavor. 1992, 17, 45. [Google Scholar]
- Dobreva, A.; Velcheva, A.; Bardarov, V.; Bardarov, K. Chemical composition of different genotypes oil-bearing roses. Bulg. J. Agric. Sci. 2013, 19, 1213–1218. [Google Scholar]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Flower phenotype variation, essential oil variation and genetic diversity among Rosa alba L. accessions used for rose oil production in Bulgaria. Sci. Hortic. 2013, 161, 76–80. [Google Scholar] [CrossRef]
- Neshev, G. Bulgarian Rose Oil-Pharmacological and Clinical Studies. Ph.D. Thesis, Medical University, Sofia, Bulgaria, 1990. [Google Scholar]
- Wittig, C.; Scheuer, C.; Parakenings, J.; Menger, M.D.; Laschke, M.W. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling. PLoS ONE 2015, 10, e0131946. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Gupta, A.; Kapahi, B.; Baleshwar; Thappa, R.; Suri, O. Chemical composition of rose water volatiles. J. Essent. Oil Res. 2005, 17, 265–267. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Kashami, D.A.; Rasooli, I.; Rezaee, M.B.; Owlia, P. Antioxidative properties and toxicity of white rose extract. Iran. J. Med. Aromat. Plants 2011, 5, 415–425. [Google Scholar]
- Meimandi, K.; Yaghoobi, M.M. Effects of aqueous and ethanolic extract of Rosa damascena Mill L. against human gastric cancer cells. J. Mol. Cell. Res. 2015, 28, 299–309. [Google Scholar]
- Zamiri-Akhlaghi, A.; Rakhshandeh, H.; Tayarani-Najaran, Z.; Mousavi, S.H. Study of cytotoxic properties of Rosa damascena extract in human cervix carcinoma cell line. Avicenna J. Phytomedicine 2011, 1, 74–77. [Google Scholar]
- Kalemba-Drożdż, M.; Cierniak, A. Antioxidant and genoprotective properties of extracts from edible flowers. J. Food Nutr. Res. 2019, 58, 42–50. [Google Scholar]
- Hagag, H.A.; Bazaid, S.A.; Abdel-Hameed, E.-S.S.; Salman, M. Cytogenetic, cytotoxic and GC–MS studies on concrete and absolute oils from Taif rose, Saudi Arabia. Cytotechnology 2014, 66, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef]
- Gateva, S.; Jovtchev, G.; Chanev, C.; Georgieva, A.; Stankov, A.; Dobreva, A.; Mileva, M. Assessment of anti-cytotoxic, anti-genotoxic and antioxidant potentials of Bulgarian Rosa alba L. essential Oil. Caryologia Int. J. Cytol. Cytosyst. Cytogenet. 2020. [Google Scholar] [CrossRef]
- Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Bakalova, R.; Aoki, I.; Mileva, M. Cytotoxic and genotoxic potential of Bulgarian Rosa alba L. essential oil—In vitro model study. Biotechnol. Biotechnol. Equip. 2018, 32, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Gautam, S.; Sharma, A. Identification of antimutagenic properties of anthocyanins and other polyphenols from rose (Rosa centifolia) petals and tea. J. Food Sci. 2013, 78, H948–H954. [Google Scholar] [CrossRef]
- EUROPEAN; MEDICINES; AGENCY. Assessment report on Rosa gallica L., Rosa centifolia L., Rosa damascena Mill., flos. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-rosa-centifolia-l-rosa-gallica-l-rosa-damascena-mill-flos_en.pdf (accessed on 30 December 2020).
- McCutcheon, A.; Roberts, T.; Gibbons, E.; Ellis, S.; Babiuk, L.; Hancock, R.; Towers, G. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995, 49, 101–110. [Google Scholar] [CrossRef]
- Rajbhandari, M.; Mentel, R.; Jha, P.; Chaudhary, R.; Bhattarai, S.; Gewali, M.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral activity of some plants used in Nepalese traditional medicine. Evid. Based Complement. Altern. Med. 2009, 6, 328279. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, S.C.; Choi, M.R.; Song, S.H.; Yoo, E.J.; Kim, S.H.; Miyashiro, H.; Hattori, M. Anti-HIV protease activity from rosa family plant extracts and rosamultin from Rosa rugosa. J. Med. Food 2005, 8, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Piacente, S.; Pizza, C.; Burke, A.; Khan, A.I.; Hay, A.J. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem. Biophys. Res. Commun. 1996, 229, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295. [Google Scholar] [PubMed]
- Gómez, L.A.; Stashenko, E.; Ocazionez, R.E. Comparative study on in vitro activities of citral, limonene and essential oils from Lippia citriodora and L. alba on yellow fever virus. Nat. Prod. Commun. 2013, 8, 1934578X1300800230. [Google Scholar] [CrossRef] [Green Version]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Mechanisms of antiviral action of plant antimicrobials against murine norovirus. Appl. Environ. Microbiol. 2014, 80, 4898–4910. [Google Scholar] [CrossRef] [Green Version]
- Vimalanathan, S.; Hudson, J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oils Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Orhan, İ.E.; Özçelik, B.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar]
- Mileva, M.; Nikolova, I.; Nikolova, N.; Mukova, L.; Georgieva, A.; Dobreva, A.; Galabov, A.S. Investigation of antioxidant and antiviral properties of geraniol. Acta Microbiol. Bulg. 2015, 31, 48–53. [Google Scholar]
- Khalifa, S.A.; Yosri, N.; El-Mallah, M.F.; Guo, R.G.Z.; Musharraf, S.G.; Du, M.; Khatib, A.; Xiao, J.; Saeed, A.; El-Seedi, H.H.; et al. Screening for natural and derived bio-active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic. Phytomedicine 2020, 153311. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative evaluation of antimicrobial activity and composition of rose oils from various geographic origins, in particular Bulgarian rose oil. Nat. Prod. Commun. 2008, 3, 1934578X0800300706. [Google Scholar] [CrossRef] [Green Version]
- Bayoub, K.; Baibai, T.; Mountassif, D.; Retmane, A.; Soukri, A. Antibacterial activities of the crude ethanol extracts of medicinal plants against Listeria monocytogenes and some other pathogenic strains. Afr. J. Biotechnol. 2010, 9, 4251–4258. [Google Scholar]
- Ali-Shtayeh, M.S.; Al-Assali, A.A.; Jamous, R.M. Antimicrobial activity of Palestinian medicinal plants against acne-inducing bacteria. Afr. J. Microbiol. Res. 2013, 7, 2560–2573. [Google Scholar]
- Gauniyal, P.; Teotia, U.S. Antimicrobial activity of sixteen medicinal plants against oral flora and its efficacy comparison with 2% chlorhexidine. Int. J. Multidiscip. Sci. Emerg. Res. 2015, 4, 2349–6037. [Google Scholar]
- Andoğan, B.C.; Baydar, H.; Kaya, S.; Demirci, M.; Özbaşar, D.; Mumcu, E. Antimicrobial activity and chemical composition of some essential oils. Arch. Pharm. Res. 2002, 25, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.H.; Nunes, N.S.; Resende, D.G.; Reis, Y.P. Verificação de atividade antimicrobiana de Extratos de Plantas Silvestres. Revista Eletrônica Biologia (REB) 2008, 1, 2–7, ISSN 1983-7682. [Google Scholar]
- Bocanegra-García, V.; del Rayo Camacho-Corona, M.; Ramírez-Cabrera, M.; Rivera, G.; Garza-González, E. The bioactivity of plant extracts against representative bacterial pathogens of the lower respiratory tract. BMC Res. Notes 2009, 2, 95. [Google Scholar] [CrossRef] [Green Version]
- Bonjar, S. Evaluation of antibacterial properties of some medicinal plants used in Iran. J. Ethnopharmacol. 2004, 94, 301–305. [Google Scholar] [CrossRef]
- Chalabian, F.; Monfared, A.; Larijani, K.; Saldouzi, S. Comparison of the essential oils of Chenopodium botrys L., Ferulago subvelutina Rech. F, Rosa gallica L. and antimicrobial activity of the oils against some microbes. Iran. J. Med. Aromat. Plants 2006, 22, 146–154. [Google Scholar]
- Haze, S.; Sakai, K.; Gozu, Y. Effects of fragrance inhalation on sympathetic activity in normal adults. Jpn. J. Pharmacol. 2002, 90, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köse, E.; Sarsılmaz, M.; Taş, U.; Kavaklı, A.; Türk, G.; Özlem Dabak, D.; Sapmaz, H.; Ögetürk, M. Rose oil inhalation protects against formaldehyde-induced testicular damage in rats. Andrologia 2012, 44, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongratanaworakit, T. Relaxing effect of rose oil on humans. Nat. Prod. Commun. 2009, 4, 1934578X0900400226. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.B.; Khorsan, R.; Vinjamury, S.P.; Der-Martirosian, C.; Kizhakkeveettil, A.; Anderson, T.M. Herbal treatments of asthma: A systematic review. J. Asthma 2007, 44, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant. Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, MS, USA, 2015. [Google Scholar]
- Yasa, N.; Masoumi, F.; Rankouhi Rouhani, S.E.; Haji, A.A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. Daru J. Pharm. Sci. 2009, 17, 175–180. [Google Scholar]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crop. Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Karamalakova, Y.D.; Adhikari, M.K.; Kovacheva, N.D.; Ivanov, V.A.; Nikolova, G.D.; Gadjeva, V.G. Rose oil isolated from oil-bearing Rosa damascena Mill. as a protectoragainst ionizing radiation-induced oxidative disorderst. Bulg. Chem. Commun. Spec. Issue C 2017, 50, 14–19. [Google Scholar]
- Georgieva, A.; Dobreva, A.; Tzvetanova, E.; Alexandrova, A.; Mileva, M. Comparative Study of Phytochemical Profiles and Antioxidant Properties of Hydrosols from Bulgarian Rosa Alba L. and Rosa Damascena Mill. J. Essent. Oil Bear. Plants 2019, 22, 1362–1371. [Google Scholar] [CrossRef]
- Nikolova, G.; Karamalakova, Y.; Kovacheva, N.; Stanev, S.; Zheleva, A.; Gadjeva, V. Protective effect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharmacol. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Senol, F.; Erdogan Orhan, I.; Kurkcuoglu, M.; Khan, M.T.H.; Altintas, A.; Sener, B.; Baser, K.H.C. A mechanistic investigation on anticholinesterase and antioxidant effects of rose (Rosa damascena Mill.). Food Res. Int. 2013, 53, 502–509. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Kozlu, S.; Yorgancıgil, E.; Uğuz, A.C.; Karakuş, K. Rose oil (from Rosa × damascena Mill.) vapor attenuates depression-induced oxidative toxicity in rat brain. J. Nat. Med. 2013, 67, 152–158. [Google Scholar] [CrossRef]
- Saleh, M.A.; Clark, S.; Woodard, B.; Deolu-Sobogun, S.A. Antioxidant and free radical scavenging activities of essential oils. Ethn. Dis. 2010, 20, 78. [Google Scholar]
- Selvan, S.; Gopi, H.; Natrajan, A.; Pandian, C.; Babu, M. Physical characteristics, chemical composition and fatty acid profile of ostrich eggs. Int. J. Sci. Environ. Technol. 2014, 3, 2242–2249. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Karamalakova, Y.D.; Nikolova, G.D.; Kovacheva, N.; Zheleva, A.M.; Gadjeva, V.G. Study of the radical-scavenging activities and radioprotective properties of Bulgarian essential rose oil from Rosa Damascena Mill. Bulg. Chem. Commun. 2019, 51, 101–107. [Google Scholar]
- Singh, H.P.; Mittal, S.; Kaur, S.; Batish, D.R.; Kohli, R.K. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J. Agric. Food Chem. 2009, 57, 6962–6966. [Google Scholar] [CrossRef]
- Jagdale, A.; Kamble, S.; Nalawade, M.; Arvindekar, A. Citronellol: A potential antioxidant and aldose reductase inhibitor from cymbopogon citratus. Int. J. Pharm. Pharm. Sci. 2015, 7, 203–209. [Google Scholar]
- Ren, G.; Xue, P.; Sun, X.; Zhao, G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complement. Altern. Med. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Farhath, M.S.; Vijaya, P.P.; Vimal, M. Antioxidant activity of geraniol, geranial acetate, gingerol and eugenol. Res. Pharm. 2013, 3. [Google Scholar]
- Prasad, S.N.; Muralidhara, M. Analysis of the antioxidant activity of geraniol employing various in-vitro models: Relevance to neurodegeneration in diabetic neuropathy. Asian J. Pharm. Clin. Res. 2017, 10, 101. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Idaomar, M. Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutat. Res. 2005, 585, 1–13. [Google Scholar] [CrossRef]
- Stan, M.S.; Chirila, L.; Popescu, A.; Radulescu, D.M.; Radulescu, D.E.; Dinischiotu, A. Essential oil microcapsules immobilized on textiles and certain induced effects. Materials 2019, 12, 2029. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.G.; Hildebrandt, L.A.; Elson, C.E. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J. Nutr. 1995, 125, 2763–2767. [Google Scholar]
- Ravizza, R.; Gariboldi, M.B.; Molteni, R.; Monti, E. Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol. Rep. 2008, 20, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandini, G.; Anil, M.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar]
- Nazari-Vanani, R.; Azarpira, N.; Heli, H. Development of self-nanoemulsifying drug delivery systems for oil extracts of Citrus aurantium L. blossoms and Rose damascena and evaluation of anticancer properties. J. Drug Deliv. Sci. Technol. 2018, 47, 330–336. [Google Scholar] [CrossRef]
- Artun, F.T.; Karagoz, A.; Ozcan, G.; Melikoglu, G.; Sezin, A.; Kultur, S.; Sutlupinar, N. In Vitro Anticancer and Cytotoxic Activities of Some Plant Extracts on HeLa and Vero Cell Lines. J. BUON 2016, 21, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yang, J.-X.; Lou, J.; Li, L.; Liu, G.-Y.; Hu, Q.-F.; Ye, Y.-Q.; Gao, X.-M. A New Isoprenylated Aurone from the Flowers of Rosa damascene and Its Cytotoxicities. Asian J. Chem. 2014, 26, 7122–7124. [Google Scholar] [CrossRef]
- Kalim, M.D.; Bhattacharyya, D.; Banerjee, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement. Altern. Med. 2010, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.F.; Wang, S.Y.; Wu, C.C.; Lin, W.Y.; Wu, T.Y.; Chang, F.R.; Wang, C.K. Cytotoxicity, anti-platelet aggregation assay and chemical components analysis of thirty-eight kinds of essential oils. J. Food Drug Anal. 2012, 20. [Google Scholar] [CrossRef]
- Heydarirad, G.; Keyhanmehr, A.S.; Mofid, B.; Nikfarjad, H.; Mosavat, S.H. Efficacy of aromatherapy with Rosa damascena in the improvement of sleep quality of cancer patients: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 35, 57–61. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Fallah, H.; Sharifi-Far, F.; Mirtajaddini, M. Alpha mannosidase inhibitory effect of some Iranian plant extracts. Int. J. Pharmacol. 2008, 4, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Antonyan, A.; Sharoyan, S.; Harutyunyan, H.; Movsisyan, N.; Sargisova, Y.; Stepanyan, H.; Mardanyan, S. Cytotoxicity of some edible plants toward Ehrlich Ascites carcinoma cells. Res. J. Med. Plant 2014, 8, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Thuncharoen, W.; Chulasiri, M.; Nilwarangkoon, S.; Nakamura, Y.; Watanapokasin, R. Apoptotic induction of skin cancer cell death by plant extracts. J. Med. Assoc. Thail. 2013, 96, S60–S64. [Google Scholar]
- Khatib, H.; Rezaei-Tavirani, M.; Keshel, S.H.; Azodi, M.Z.; Omidi, R.; Biglarian, M.; Sobhi, S. Flow cytometry analysis of rosa damascena effects on gastric cancer cell line (MKN45). Iran. J. Cancer Prev. 2013, 6. [Google Scholar]
- Rezaie-Tavirani, M.; Fayazfar, S.; Heydari-Keshel, S.; Rezaee, M.B.; Zamanian-Azodi, M.; Rezaei-Tavirani, M.; Khodarahmi, R. Effect of essential oil of Rosa Damascena on human colon cancer cell line SW742. Gastroenterol. Hepatol. Bed Bench 2013, 6, 25–31. [Google Scholar] [PubMed]
- Abdel-Hameed, E.S.S.; Bazaid, S.A.; Hagag, H.A. Chemical characterization of Rosa damascena Miller var. trigintipetala Dieck essential oil and its in vitro genotoxic and cytotoxic properties. J. Essent. Oil Res. 2016, 28, 121–129. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Bazaid, S.A.; Salman, M.S. Characterization of the phytochemical constituents of taif rose and its antioxidant and anticancer activities. BioMed Res. Int. 2013, 2013, 345465. [Google Scholar] [CrossRef] [Green Version]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [Green Version]

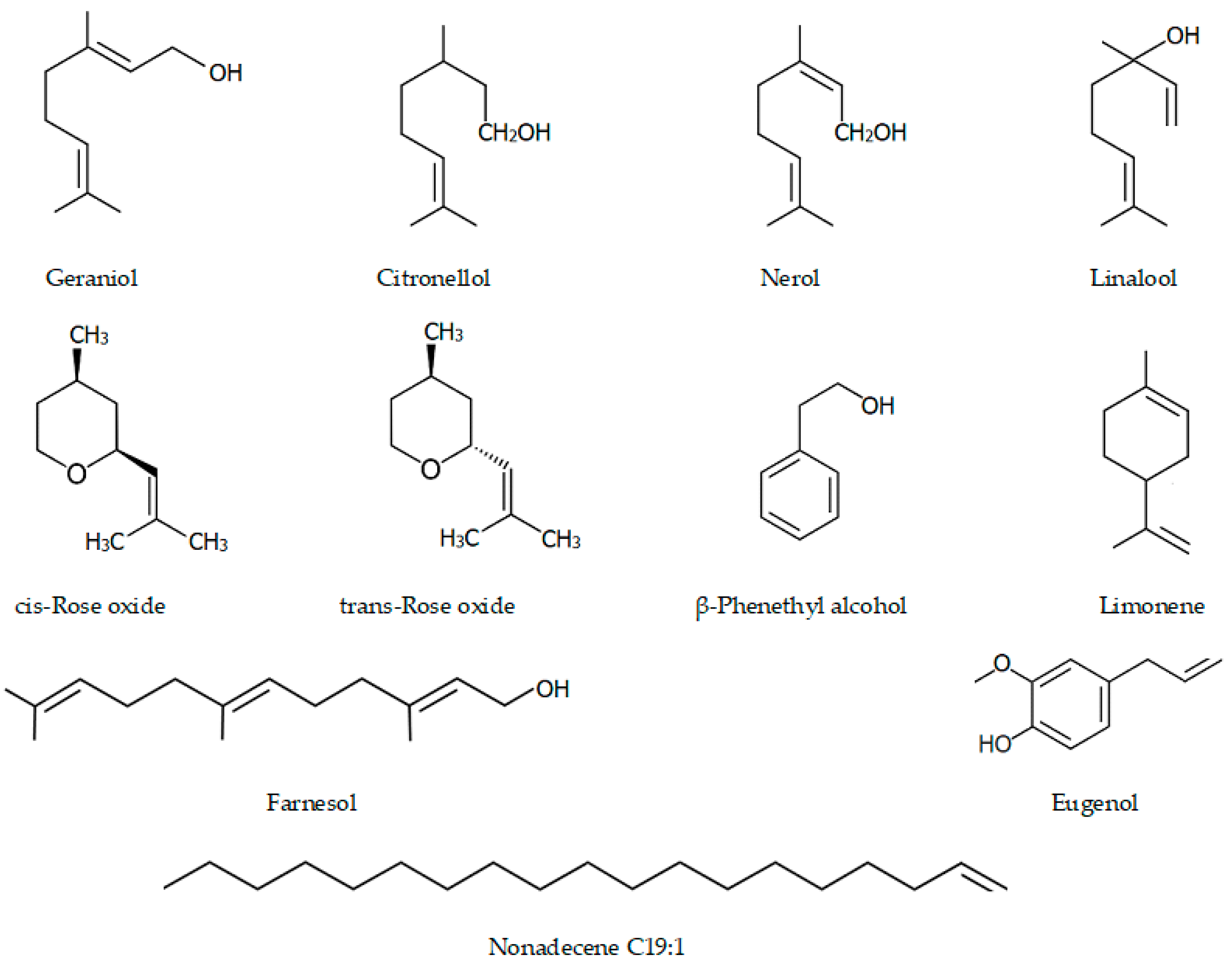
| № | Component | Roses and the biological activities of their major compounds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R. damasacena Mill. | Rosa alba L. | Rosa gallica L. | Rosa centifolia L. | ISO 9842:2003 | Biological Activities | ||||
| 1 | Ethanol | 0.80 | 0.15 | 0.01 | 0.00 | ≤2.0% | |||
| 2 | Limonene | 0.00 | 0.03 | 0.16 | 0.05 | Anti-inflammatory activity: inhibits pro-inflammatory mediators present in the inflammatory peritoneal exudate in zymosan-induced peritonitis; decreases ROS production, NF-κB activity, and eosinophil migration; has an antidiabetic effect on hyperlipidemia in mice | [42,43,44] | ||
| 3 | Linalool | 1.90 | 1.43 | 1.58 | 1.03 | Anticonvulsant, antiepileptic activity | [45] | ||
| 4 | β-Phenethyl alcohol | 0.40 | 0.25 | 0.36 | 0.09 | ≤3.5% | Wide spectrum antimicrobial: effective preservative agent for cosmetics; anti-anxiety-like effects | [46,47] | |
| 5 | Cis-Rose oxide | 0.22 | 0.08 | 0.05 | 0.07 | Anti-inflammatory properties: decreased the paw edema induced by carrageenan by the suppression of IL-1β production and leukocyte migration | [48] | ||
| 6 | Trans-Rose oxide | 0.13 | 0.06 | 0.00 | 0.04 | ||||
| 7 | Citronellol | 28.72 | 16.65 | 9.22 | 9.22 | 20.0–34.0% | Antispasmodic, anti-inflammatory, antibacterial, and antifungal: inhibits the mycelial growth, conidia germination, and fungal growth on nail fragments; active against C. jejuni, L. monocytogenes, and S. enterica | [49,50,51,52,53] | |
| 8 | Nerol | 4.80 | 10.10 | 4.72 | 4.36 | 0–12.0% | Systolic pressure falls and decreased heart rate | [54] | |
| 9 | Geraniol | 21.40 | 30.98 | 24.84 | 17.60 | 15.0–22.0% | Insecticidal, repellent, acaricidal activity, antibacterial, and antifungal | [55,56,57,58] | |
| 10 | Eugenol | 1.00 | 1.12 | 0.05 | 0.74 | Antiseptic, antibacterial effect against bacteria, pathogens, and harmful microorganisms | [59,60] | ||
| 11 | Methyl eugenol | 1.30 | 0.91 | 0.94 | 0.56 | Anesthetic in rodents; insect attractant | [61] | ||
| 12 | n-Heptadecane | 1.40 | 2.00 | 2.98 | 1.07 | 1.0–2.5% | |||
| 13 | Farnesol | 1.90 | 2.98 | 1.26 | 3.48 | Anti-inflammatory; anticancer properties | [62] | ||
| 14 | Nonadecene C19:1 | 2.30 | 4.35 | 1.25 | 2.28 | ||||
| 15 | n-Nonadecane C19 | 11.10 | 12.14 | 22.67 | 8.10 | 8.0–15.0% | |||
| 16 | n-Eicosane C20 | 1.00 | 1.01 | 1.06 | 0.55 | ||||
| 17 | n-Heneicosane C21 | 5.00 | 10.21 | 9.07 | 6.31 | 3.0–5.5% | |||
| 18 | n-Tricosane C23 | 1.30 | 0.81 | 2.58 | 5.90 | ||||
| 19 | n-Pentacosane C25 | 0.50 | 0.23 | 1.04 | 2.86 | ||||
| 20 | n-Heptacosane C27 | 0.40 | 0.10 | 0.55 | 1.79 | ||||
| Rosa Species | Type of Extract | Test Methods | Test Objects | Biological Activity | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T/CT | GT | MG | ATC | AGT | AMG | |||||
| R. alba L. (Bulgaria) | Essential oil | Chromosome aberration assay, | H. vulgare root tip meristems | − | − | + | + | [77,78] | ||
| Micronucleus assay, | + | |||||||||
| Comet assay | Human lymphocytes | + | ||||||||
| R. damascena white variety (Iran) | Aqueous and methanol extracts | Modified MTT assay, | HeLa cells line NCBI, | + | [71] | |||||
| Human lymphocytes, | − | |||||||||
| Hematology and clinical chemistry parameter analysis | Wistar rats | − | ||||||||
| R. damascena Mill. (Iran) | Essential oil | MTT assay, | Normal NIH3T3, | + | + | [24] | ||||
| Cancer cells A549, | + | + | ||||||||
| Micronucleus assay | Peripheral blood lymphocytes | + | + | |||||||
| R. damascena Mill. (Iran) | Ethanol extract | Modified MTT assay | HeLa cell line | + | [73] | |||||
| R. damascena Mill. | Aqueous and ethanol extract | MTT assay, BrdUassay, TUNEL assay | Human gastric cancer cell line AGS cells | + | [72] | |||||
| R. damascena trigintipetala Dieck (Saudi Arabia) | Concrete and absolute rose oil | Viability assay, | Human lymphocytes | − | − | + | [75] | |||
| Chromosome aberration assay, | ||||||||||
| Sulphorhodamine-B (SRB) assay | Cell lines HepG2 and MCF7 | + | ||||||||
| R. damascena and R. centifolia (Bulgaria) | Aqueous-alcoholic extracts | Double-staining fluorescence assay | Human lymphocytes | − | − | + | [74] | |||
| R. damascena Mill. | Essential oil | MTT assay | A549, PC-3, MCF-7 human tumor cell lines | + | [76] | |||||
| R. centifolia cultivars, “passion,” “pink noblesse”, and “sphinx” | Aqueous extract, Rose tea from petals | E. coli RNA polymerase B (rpoB)-based Rif S→Rif R (rifampicin sensitive to resistant) forward mutation assay | E. coli | + | [79] | |||||
| Rosa Species | Type of Extract | Test Methods | Observations (DIZ in mm, MIC and MBC in µg/mL, respectivley mg/mL−1 or in %) | References |
|---|---|---|---|---|
| R. damascena Mill. (Turkey) | 10% MeOH extracts from fresh flower1 and 10% MeOH extracts from spent flower2 | Agar diffusion method | A. hydrophila (218 mm), B. cereus (116 mm), E. feacalis (115 mm), E. coli (117 mm, 216 mm), M. smegmatis (115 mm, 221 mm), P. vulgaris (118 mm, 215 mm), P. aeruginosa (116 mm), P. fluorescens (215 mm), S. enteritidis (121 mm, 216 mm), S. typhimurium (117 mm), S. aureus (15 mm), Y. enterocolitica (115 mm) | [27] |
| R. damascena Mill. (Turkey) | 5% MeOH extracts from fresh flower1 and 5% MeOH extracts from spent flower2 | Agar diffusion method | A. hydrophila (215 mm), E. coli DM (116 mm), M. smegmatis (218 mm) | [27] |
| R. damascena Mill. (Bulgaria) | Essential oil | Disc diffusion and serial broth dilution methods | B. cereus (24 mm, MIC = MBC = 128 µg/mL), S. aureus (18 mm, MIC = MBC = 256 µg/mL), S. epidermidis (21 mm, MIC = MBC = 256 µg/mL), E. coli (17 mm, MIC = 512 µg/mL, MBC = 1024 µg/mL), C. albicans (17 mm, MIC = 1024 µg/mL, MBC = 2048 µg/mL), C. albicans (15 mm, MIC = 1024 µg/mL, MBC = 2048 µg/mL) | [93] |
| R. damascena Mill. (Turkey) | Essential oil | Disc diffusion method | B. cereus (22 mm), S. aureus (16 mm), S. epidermidis (20 mm), E. coli (16 mm), C. albicans (16 mm) | [93] |
| R. damascena Mill. (Morocco) | Essential oil | Disc diffusion method | B. cereus (20 mm), S. epidermidis (18 mm) | [93] |
| R. damascena Mill. (China) | Essential oil | Disc diffusion method | B. cereus (18 mm), S. epidermidis (17 mm) | [93] |
| R. damascena Mill. (Iran) | Essential oil | Serial broth dilution method | MIC = MBC = 1 µg/mL for S. aureus,S. agalactiae, S. sanguis, S. salivarius, E. coli, E. aerogenes, S. marcescens; MIC = 0.5 µg/mL and MBC = 1 µg/mL for S. saprophyticus, S. epidermidis, B. cereus, B. subtilis, S. dysenteriae, Shigella flexneri, C. albicans; MIC = 0.25 µg/mL and MBC = 0.5 µg/mL for S. pyogenes, MIC = 1 µg/mL and MBC = 2 µg/mL for E. faecalis, E. faecium, S. typhimurium, P. aeruginosa; MIC = 0.125 µg/mL and MBC = 0.25 µg/mL for K. pneumonia, P. vulgaris; MIC = 0.125 µg/mL and MBC = 1 µg/mL for A. flavus; MIC = MBC = 0.25 µg/mL for A. niger; MIC = 0.5 µg/mL and MBC = 2 µg/mL for A. parasiticus | [24] |
| R. alba L. (Bulgaria) | Essential oil | Disc diffusion and serial broth dilution methods | A. actinomycetemcomitans (MIC = 0.45 mg/mL−1), S. mutans (MIC = 0.82 mg/mL−1), E. faecalis (MIC = 1.36 mg/mL−1) | [21] |
| R. alba L. (Bulgaria) | Essential oil | Disc diffusion and serial broth dilution methods | B. cereus (14–15 mm, MIC = MBC = 0.05%), E. coli (MIC = MBC = 0.2%), S. aureus MIC = 0.41%, MBC = 0.82%), S. epidermidis (MIC = MBC = 0.1%), S. abony (MIC = MBC = 0.82%), C. albicans (MIC = MBC = 0.82%), C. tropicalis (MIC = 0.41%, MBC = 0.82%) | [8] |
| R. centifolia L. (Morocco) | 90% EtOH extract | Disc diffusion and serial broth dilution methods | L. monocytogenes ATCC 19117 (20 mm, MIC = 0.9 mg/mL) | [94] |
| R. centifolia L. (Palestine) | Crude EtOH extract | Disc diffusion method | P. acnes (19 mm), E. coli (31 mm), | [95] |
| R. centifolia L. (India) | EtOH extract | Agar diffusion method | S. mutans (15 mm), C. albicans (16 mm) | [96] |
| Rosa Species | Type of Extract | Observations | References |
|---|---|---|---|
| R. damascena Mill. from Gulbirlik Inc. (Isparta, Turkey) | Rose oil vapor of essential oil, obtained by water steam distillation | Inhibits lipid peroxidation (LP) induced by chronic mild stress (CMS) in the rat cerebral cortex homogenate; restores content of vitamin A, vitamin E, vitamin C, and b-carotene in brain cortex homogenate | [116] |
| R. damascena Mill. from Turkey | Essential oil by water steam distillation; Aromatic water (hydrosol) by water steam distillation; Methanol extract of fresh flowers and spent flowers | 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity; Fe2+metal-chelation activity; ferric-reducing antioxidant power DPPH radical scavenging activity; AOA by the formation of phosphomolybdenum complex | [27,115] |
| R. damascena Mill. from rose gardens of Kashan, city of Iran | Aqueous/methanol extract | DPPH radical scavenging activity; β-carotene bleaching effect; ferric-reducing antioxidant power | [71] |
| R. damascena Mill. from Iran, population of Guilan | Essential oil hydro-alcoholic extract | DPPH radical scavenging activity; LP inhibitory effects | [110] |
| R. damascena Mill. from Bulgaria, Turkey, and Egypt | Essential oil by water steam distillation | DPPH radical scavenging activity | [117] |
| R. centifolia L.from a local market near Jamia Hamdard | Ethanol (70%) extract of dried flowers | Dose-dependent acceptations of DPPH activity; antioxidant activity (AOA) by the formation of phosphomolybdenumcomplex; dose-dependent manner for scavenging superoxide anion radical ferrous iron chelating activity | [118] |
| R. gallica L. (Hungary) | Methanol extracts from dried fruit rosehips Water/ethanol 80/20 v/v, 20 °C Aqueous extract | Ferric-reducing antioxidant power (FRAP) activity in blood plasma; the activities are as follow: R. spinosissima >R. canina >R. rugosa >R. gallica Ethanol extracts of rosehips have higher phenolic content and antioxidant activity than water extracts | [119] |
| R. alba L. oil, R. damascena Mill. oil, and R. damascena Mill. oil from rose water (Bulgaria); R. gallica L. (Moldova) | Essential oil by water steam distillation | Superoxide anion radicals scavenging activity | [20] |
| R. alba L., R. damascena Mill. (Bulgaria) | Essential oil by water steam distillation Hydrosols by water steam distillation | DPPH radical-scavenging activity; Fe2+metal-chelation activity; inhibition of Fe2+-induced LP in egg liposomes; inhibition of Fe2+/asc. acid-nduced LP in egg liposomes; inhibition of hydroxyl radicals generation | [21,79,113] |
| R. damascena Mill. (Bulgaria) | Essential oil by water steam distillation | Inhybition of lipid peroxidaton in brain homogenate and the blood of mice with an experimental model of oxidative stress | [114] |
| R. damascena Mill. from International Flavors and Fragrances Inc. (New York). | Essential oil by water steam distillation | Inhibition of oxidation of hexanal to hexanoic acid in a dose-related manner; DPPH scavenging in a dose-dependent manner; inhibition of the formation ofmalonaldehyde (MDA) from squalene upon UVirradiation | [120] |
| R. damascena Mill. (Thrissur- Kerala, India). | Liophylisate from fresh juice of rose flower, eluted in silica gel column by petiole ether, chloroform, acetone, methanol, and water. | Acetone fraction: inhibits 50% superoxide radical production, hydroxyl radical generation, and inhibits Fe2+asc. acid induced lipid peroxidation in liver homogenate of mice, treated by CCl4 | [25] |
| R. damascena Mill. (fresh and spent flowers, and green leaves), Isparta, Turkey. | Hot extractions with methanol Cold extractions with methanol | Antiradical activity: DPPH, and FRAP methods | [111] |
| R. damascena Mill., Kazanlak, Bulgaria | Essential oil by water steam distillation | Inhibition of UV and γ- irradiation-induced oxygen/nitrogen free radicals; hydroxyl radical scavenging potential; examination by EPR assay, DPPH, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), Nitric oxide scavenging assay, and protection of liposomal lipids from soy lecithin and cholesterol; γ-irradiated and non-irradiated oils demonstrate an increase in scavenging abilities towards ABTS and NO in a concentration-dependent manner | [112,121] |
| Rosa Species | Type of Extract, Fraction, or Compound Investigated | Anticancer and Other Activities Supporting the Anticancer Therapy | References | Toxicological Data/Safety | References |
|---|---|---|---|---|---|
| R. damascena Mill. | Essential oil | – Lung cancer cell line A549 (IC50 = 36.4 µg/mL *); | [24] | – No toxic effects in doses up to 10 μg/mL | [24] |
| – Significant improvement of cancer patients’ sleep quality regarding sleep latency and duration (5% and 10% essential oil). | [140] | – Low cytotoxicity towards the normal cell line NIH3T3 (IC50 = 42.9 µg/mL) | [24] | ||
| – not cytotoxic at 200 μg/mL against tumor cell lines HepG2, Hep3B (hepatoma), A549, and breast MCF-7 and MDA-MB-231 cell lines | [139] | – Significant cytotoxic and genotoxic effects in peripheral blood lymphocytes at doses over 10 µg/mL, 1% (after 1 h exposure) and 0.1% (after 24 h exposure) | [24,74] | ||
| – Vapour and water soluble phases of the essential oil |
| [144,145] |
| [144,145] | |
| Extracts: | |||||
| – Essential oil extract (2%) loaded in a self-nanoemulsifying drug delivery system | – Breast MCF7 (10% cell survival) and pancreatic PANC1 (15% cell survival) cancer cell lines | [135] | N.d. ** | - | |
| – Methanol extract | – Cervical cancer cell line HeLa (IC50 = 265 μg/mL): a selectivity index towards normal kidney epithelial Vero cells SI > 3.8 (SI > 2 means selective toxicity) | [136] | – Low cytotoxicity on normal kidney epithelial Vero cells (IC50>1 g/mL) | [136] | |
| – 50% methanol extract | – Not cytotoxic at 2 μg/mL for 72 h towards U937 human lymphoma cell line. | [138] | N.d. | - | |
| – 50% ethanol extract of flowers | – Cervical cancer cells HeLa (IC50 (72 h) = 305.1 μg/mL, IC50 (48 h) = 1540 μg/mL, IC50 (24 h) = 2135 μg/mL for 24 h) | [73] | N.d. | [73] | |
| – 70% ethanol extract of rose petals | – Mice Ehrlich ascites carcinoma (EAC) cells (IC80 = 15 μg/mL, 80% growth inhibition at 200 μg/mL); | [142] | – Cytotoxic to mice peripheral blood leukocytes: 90% growth inhibition at 200 μg/mL | [142] | |
| – 50% hydroglycol extracts | – Epidermoid carcinoma cell line A431 (IC50 = 3220 μg/mL) | [143] | N.d. | - | |
| – Water extract of petals | – No cytotoxicity towards EAC cells | [142] | – Not cytotoxic to peripheral blood leukocytes | [142] | |
| Fractions: | |||||
| – Coumarin fractions of 70% ethanol extract | – Inhibition of EAC cells by approximately 50–70% | [142] | – About 30% inhibition of peripheral blood leukocytes for the monomer fraction | [142] | |
| – Phenol glycoside fractions of 70% ethanol extract | – Significant inhibition of EAC cells by approximately 85–95% for two of the fractions | [142] | – No significant inhibition of peripheral blood leukocytes | [142] | |
| Active substance | |||||
| – Isoprenylated aurone | – Acute myeloid leukemia NB4 (IC50 = 4.8 μM) and neuroblastoma SHSY5Y (IC50 = 3.4 μM) cell lines; less cytotoxic to lung A549, prostate PC3 and breast MCF7 cells | [137] | N.d. | - | |
| R. damascena Millervar. trigintipetala Dieck (Taif rose) | Essentialoils, concrete, and absolute |
| [75,146] | – Essential oil was cytotoxically and genotoxically safe at a dose of 10 μg/mL on normal human blood lymphocytes; concrete oil was even less toxic than absolute oil which showed significant antimutagenic activity at a dose of 10 μg/mL | [75] |
| Extracts: | |||||
| – Crude 80% methanol extract from fresh and dried roses | – HepG2 cells (fresh rose extract IC50 = 9 μg/mL ***, dry rose extract IC50 = 13 μg/mL ***) | [147] | – N.d. | - | |
| Fractions of crude 80% methanol extract from fresh and dried roses: | |||||
| – n-butanol | – HepG2 cells (fresh rose extract IC50 = 17 μg/mL ***, dry rose extract IC50 = 18 μg/mL ***) | [147] | – N.d. | - | |
| – aqueous | – HepG2 cells (fresh rose extract IC50 = 8 μg/mL ***, dry rose extract IC50 = 11 μg/mL ***) | ||||
| R. damascena “Alexandria” and R. gallica “Francesa” draft in R. canina | Methanol: water (80:20) extract (from petals) | – HeLa (IC50 = 308 μg/mL) and HepG2 (IC50 = 297 μg/mL) cellslarge cell lung carcinoma cell line NCI-H460 (IC50> 400 μg/mL) | [148] | – No hepatoxicity towards the non-tumor porcine liver primary culture (IC50 > 400 μg/mL). | [148] |
| Infusion (from petals) | – MCF-7 (IC50 = 377 μg/mL) and HepG2 (IC50 = 315 μg/mL) cellsNCI-H460 cells (IC50> 400 μg/mL) | [148] | – N.d. | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. https://doi.org/10.3390/biom11010127
Mileva M, Ilieva Y, Jovtchev G, Gateva S, Zaharieva MM, Georgieva A, Dimitrova L, Dobreva A, Angelova T, Vilhelmova-Ilieva N, et al. Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules. 2021; 11(1):127. https://doi.org/10.3390/biom11010127
Chicago/Turabian StyleMileva, Milka, Yana Ilieva, Gabriele Jovtchev, Svetla Gateva, Maya Margaritova Zaharieva, Almira Georgieva, Lyudmila Dimitrova, Ana Dobreva, Tsveta Angelova, Nelly Vilhelmova-Ilieva, and et al. 2021. "Rose Flowers—A Delicate Perfume or a Natural Healer?" Biomolecules 11, no. 1: 127. https://doi.org/10.3390/biom11010127
APA StyleMileva, M., Ilieva, Y., Jovtchev, G., Gateva, S., Zaharieva, M. M., Georgieva, A., Dimitrova, L., Dobreva, A., Angelova, T., Vilhelmova-Ilieva, N., Valcheva, V., & Najdenski, H. (2021). Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules, 11(1), 127. https://doi.org/10.3390/biom11010127